User login
Is Diabetes Distress on Your Radar Screen?
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
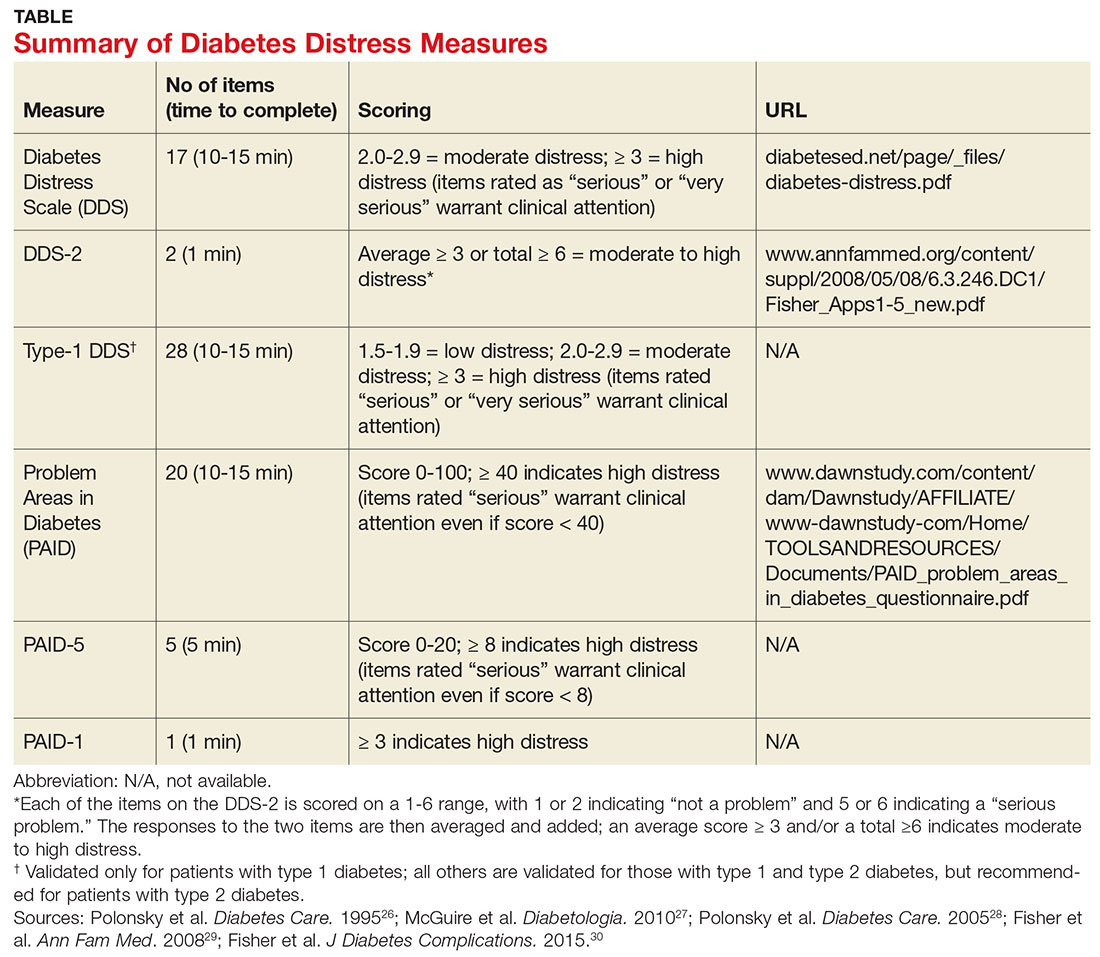
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.
Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.
Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of persons with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder; rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.13-15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings, this review is intended to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.19
DIABETES DISTRESS: WHAT IT IS, WHAT IT'S NOT
For patients with type 2 diabetes, diabetes distress centers around four main issues
- Frustration with the demands of self-care
- Apprehension about the future and the possibility of developing serious complications
- Concern about both the quality and the cost of required medical care
- Perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least five of the nine symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least two weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes, and the co-occurrence of a psychiatric disorder and diabetes distress is high.24,25Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that persons diagnosed with diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
SCREENING FOR DIABETES DISTRESS
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (see Table).26-30 Some of the measures are easily accessible online; others require a subscription to MEDLINE.
Problem Areas in Diabetes (PAID). There are three versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a five-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS). This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with two items and a 28-item scale specifically for patients with type 1 diabetes.29,30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the seven sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but also that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
HOW TO RESPOND TO DIABETES DISTRESS
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only two studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT).31 The trial had three arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress–specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), self-management behaviors, and A1C.
Participants in all three arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in A1C were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other two groups.31
The DIAMOS trial recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a two-arm RCT.32 One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, A1C, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, A1C, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function, the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors.36-38 Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care providers without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care providers to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Clinicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussions. (See “Directing Help Where It’s Most Needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educ. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psychol. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectr. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl):S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabet Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33:1080-1106.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatry. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diab Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Educ Couns. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectr. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed May 16, 2017.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Is diabetes distress on your radar screen?
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
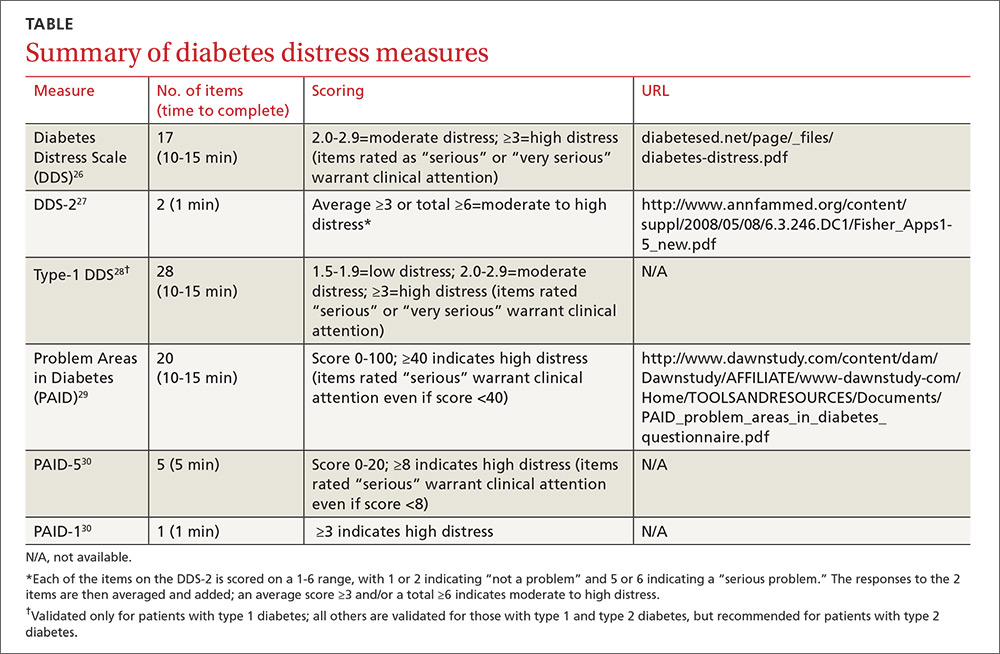
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
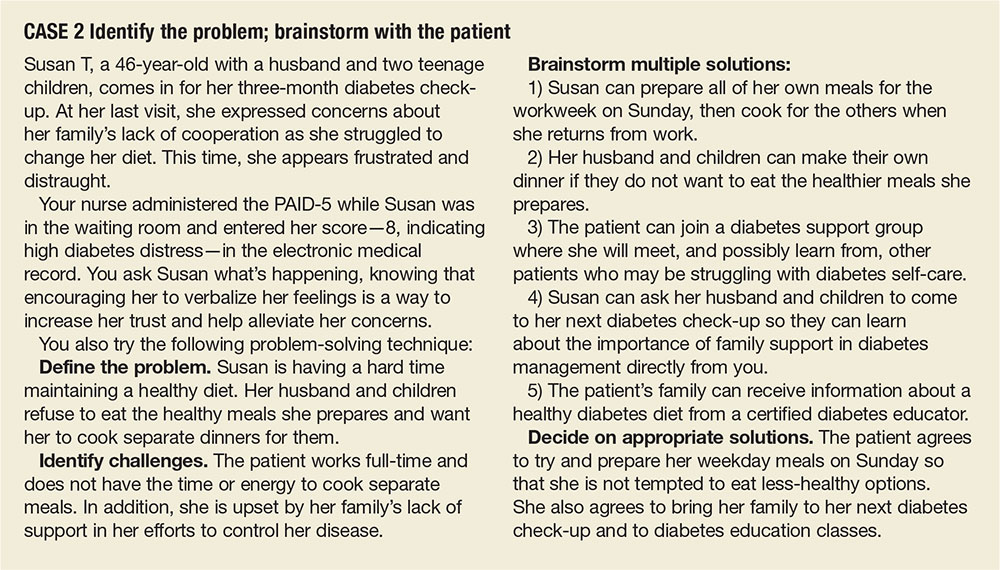
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; beverle1@ohio.edu.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; beverle1@ohio.edu.
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; beverle1@ohio.edu.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
PRACTICE RECOMMENDATIONS
› Educate patients about diabetes distress, explaining that diabetes is manageable and that neither complications nor diabetes distress is inevitable. C
› Empower patients to take an active role in self-management of diabetes, encouraging them to express their concerns and ask open-ended questions. A
› Support shared decision-making by inquiring about patients’ values and treatment preferences, presenting options, and reviewing the risks and benefits of each. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patient-Physician Communication and Diabetes Self-Care
Abstract
- Objective: To summarize the current literature, research findings, and interventions for self-care communication in the physician-patient relationship.
- Methods: Literature review.
- Results: Diabetes management requires patients to follow complex self-care recommendations for nutrition, physical activity, blood glucose monitoring, and medication. Adherence to these recommendations improves glycemic control and mitigates the risk of diabetes complications; however, many patients struggle to follow these behaviors in everyday life. In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. Physicians need to incorporate interpersonal and relational skills to establish a trusting relationship. Physician-level barriers to self-care communication include lack of time, lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for diabetes patients. Among patients, psychosocial barriers and health literacy may affect willingness to discuss self-care. Motivational interviewing techniques may be helpful for improving communication around patient self-management and promotion of healthy behaviors.
- Conclusion: Physicians can assist patients with their diabetes self-care by discussing self-care challenges during medical visits.
Diabetes is one of the most significant and growing chronic health problems in the world, affecting approximately 415 million people [1]. Diabetes is marked by the body’s inability to make insulin as well as the body’s inability to effectively use the insulin it produces [2]. Diagnosis of diabetes has increased sharply in recent decades and is expected to increase even more, with the largest increases in middle- and low-income countries [3]. Diabetes is a leading cause of blindness, kidney failure, myocardial infarction, stroke, and amputation [3], and in 2015 it accounted for 5 million deaths worldwide [1]. Further, diabetes’s costs to society represent 12% ($673 billion) of global health expenditures [1]. By 2040, models predict that 642 million people will be diagnosed with diabetes and costs will continue to grow as the population ages [1]. Thus, prevention of diabetes is the ultimate goal; however, more effective management for individuals already diagnosed with diabetes is critical to reduce the risk of complications and the economic burden of the disease.
Diabetes management requires patients to perform complex self-care regimens, including weight reduction, frequent blood glucose monitoring, taking oral and/or insulin medications, engaging in physical activity, adhering to diabetes nutrition guidelines, and attending clinic appointments [4–9]. These self-care behaviors are critically linked to improved glycemic control, however, integrating them into one’s daily life can be challenging [10–12]. Recent National Health and Nutrition Examination Survey (NHANES) data show that approximately half of adults with diabetes are not meeting recommended goals for diabetes care [13]. Physicians can assist patients with their diabetes self-care by scheduling frequent follow-up visits and discussing self-care challenges with their patients [14].
In this review, we discuss the current literature on physician-patient communication and diabetes self-care. First, we discuss the qualities of an effective physician-patient relationship followed by the importance of self-care communication in diabetes care. Next, we discuss barriers and facilitators to self-care communication. Finally, we review interventions for improving physician-patient communication in diabetes self-care.
Qualities of an Effective Physician-Patient Relationship
Successful diabetes care requires teamwork between physicians and patients [15]. Two components of successful teamwork are physician-patient communication and shared decision-making, both of which have been shown to improve patient satisfaction, adherence to treatment plans and health outcomes [16–23]. In shared decision-making, the physician and patient share medical information [24–26]. Specifically, the physician presents different treatment options to the patient and describes the risks and benefits of each option. Then the patient expresses his or her preferences for treatment to ensure that the care provided aligns with the patient’s values and needs [27]. Thus, shared decision-making in the treatment relationship is predicated on effective communication between the physician and patient [19].
Effective physician-patient communication is supported by continuous care [19,28], a secure attachment style [29, 30], shared goals [19], a mutual understanding of respective roles and tasks [15,31–33], and a bond characterized by liking, confidence, and trust [19,28,31]. Trust is paramount in physician-patient communication. Interpersonal trust and social trust are the 2 predominant types [34]. Interpersonal trust refers to the relationship the patient has with the physician, specifically the confidence the patient has in the physician as well as the responsibility, competence, compassion, and regard the physician has for the patient’s welfare [34–36]. For patients and physicians, interpersonal trust is developed over time with repeated interactions [34–36]. On the other hand, social trust refers to the beliefs of honesty, integrity, and reliability in others [36]. Social trust is influenced by social constructs, including the media and institutions of higher education [36].
In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. A patient’s trust can be acquired through multiple medical appointments with the physician. Further, how the patient is treated during these appointments as well as how much time and attention the physician invests in the patient’s care influences the level of interpersonal trust. A high level of trust in the relationship can lead to in improvements in adherence to self-care, continuity of care, physician-patient communication, and overall quality of the physician-patient relationship [37–39].
In the diabetes physician-patient relationship, minimal research has explored how trust in one’s physician impacts self-care communication. In a study by Beverly and colleagues, diabetes patients emphasized the importance of a trusting physician-patient relationship for diabetes care [27]. Another study by Ritholz and colleagues found that physicians and patients both stress the importance of developing trust to facilitate self-care communication [40]. Specifically, trust as well as acceptance from the physician contributes to open and honest self-care communication in the physician-patient diabetes relationship[40]. Additional research is needed to determine whether a high level of physician-patient trust is associated with increased self-care behaviors and improved diabetes outcomes over time.
Importance of Diabetes Self-Care Communication
Diabetes self-care communication in the physician-patient relationship increases patient satisfaction, improves adherence to treatment regimens, and leads to better clinical outcomes [22,41–43]. For physicians, effective self-care communication requires the performance of specific communication tasks and behaviors, including collecting a medical history, explaining a diagnosis and prognosis, and providing clear and concise therapeutic instructions [44]. In addition, physicians must incorporate interpersonal and relational skills to establish a trusting relationship [44,45]. Both physicians and patients agree that a trusting treatment relationship is a requirement for open and honest self-care communication [45]. For patients, effective communication necessitates the disclosure of self-care successes and failures [46]. Diabetes patients face challenging self-care regimens, and these challenges can interfere with glycemic control and increase the risk for diabetes complications [47,48]. For this reason, patients must feel comfortable discussing their self-are challenges so that their physician can individualize treatment prescriptions and recommendations, thereby increasing the likelihood of treatment success.
Barriers to Self-Care Communication
Physician-patient self-care communication is essential to improving patient adherence [29,49] yet numerous barriers exist that undermine effective physician-patient self-care communication. From the physician perspective, the most commonly cited barrier to self-care communication is time [50]. A recent study of family medicine practices found that the time physicians spent discussing self-care with their patients varied from 1 to 17 minutes, suggesting that time is a major barrier to self-care communication [51]. Other barriers include lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for patients with diabetes [50]. Relatedly, Beverly and colleagues [52] found that physicians often feel inadequately trained to address diabetes patients’ psychosocial issues and this perceived lack of expertise may contribute to physicians feeling overwhelmed and frustrated within the physician-patient relationship, which may hinder open self-care communication.
For patients, barriers tend to differ from those perceived by physicians. A qualitative study using semi-structured interviews with patients and clinicians, and direct observation of clinical encounters at an inner-city family practice training site, revealed different perceptions of the term “control” between physicians and patients. In practice, physicians used the term “control” to focus on the management of blood glucose levels rather than trying to understand the patients’ understanding of diabetes and subsequent treatment goals. Differing viewpoints contributed to frustration and hindered effective communication [53]. In another qualitative study with physicians and patients, both noted that patients were reluctant to discuss self-care for fear being judged or shamed about food intake and weight [45]. This finding was supported in a quantitative follow-up study assessing patient reluctance to discuss self-care. Thirty percent of surveyed patients reported reluctance to discussing self-care with their physicians for fear of being judged, not wanting to disappoint their doctors, guilt, and shame [14]. Interestingly, patients reporting elevated depressive symptoms were more likely to be reluctant to discuss their self-care [14]. Cognitive behavioral changes (eg, cognitive distortions, avoidance behavior, attention deficits) associated with major depression and depressive symptoms may impair patients’ ability to recall self-care information. Also, patients reporting more depressive symptoms may be more socially withdrawn during a medical appointment, and thus less willing to communicate with their physician about self-care.
Other studies found that psychosocial factors such as diabetes distress [54,55] and pessimistic attitudes [56–59], cultural differences [60–66], lack of family and social support [60,67–70], lack of readiness to change behavior [71], introversion and social isolation [72,73], hypo-glycemia fear [74,75] and ineffectual coping styles [76,77] interfere with self-care and glycemic control. Further, low health literacy is associated with difficulty adhering to self-care, particular medication regimens, and negative health outcomes [78].
In summary psychosocial barriers and health literacy may affect a patients’ willingness to discuss self-care during a medical visit. Therefore, routine assessment of psychosocial factors and health literacy may be necessary to address a patient’s barriers to self-care as well as to promote open and honest self-care communication. Interventions and evidenced-based approaches that address psychosocial factors, health literacy, and physician-patient self-care communication are needed.
Facilitators to Self-Care Communication
Despite numerous barriers to self-care communication, several factors promote self-care communication in the physician-patient relationship. For example, direct and non-accusatory communication from physicians as well as providing patients with hope for living with diabetes both support physician-patient self-care communication [45]. A recent systematic review by Sohal and colleagues [79] found that trust in physicians, the use of culturally appropriate exercise and dietary advice, and increasing family involvement improved physician-patient communication and diabetes self-care [79]. Lastly, a study by Schillinger and colleagues [80] found that physician assessment of patient recall and comprehension of new concepts during medical visits improved diabetes outcomes [80].
Patient-Physician Self-Care Communication Interventions
One of the more successful interventions for improving diabetes self-care and patient-physician communication is motivational interviewing (MI). MI is a non-judgmental communication style designed to explore a patient’s intrinsic motivation to change health behaviors [81]. Inherent to MI is the belief that motivation for change is malleable and that it can be transformed in the context of the patient-physician relationship [81]. MI is a patient-centered method designed to empower a patient’s ability and responsibility to make health-related decisions, with the physician supporting the patient’s autonomy in the process [82]. Recent meta-analyses and systematic reviews [83–87] showed that MI interventions improve self-care behaviors and glycemic control in the short-term; long-term effects of MI on self-care and glycemia remain inconclusive. More high-quality research is needed to evaluate the MI training content of these interventions in order to determine its long-term effectiveness and replicate outcomes in various healthcare settings [87].
Other studies not included in the meta-analyses and reviews found MI interventions improved self-care behaviors [88–90], glycemic control [90,91], and quality of life [91]. A qualitative study exploring diabetes patients’ experiences with MI and self-care behaviors revealed that patients’ appreciate when providers initiate discussions that result in new ways of thinking about self-care and promote a sense of well-being in patients [92]. New research utilizing patient navigators to connect diabetes patients’ to their primary care providers showed MI techniques improved patient self-efficacy and glycemic control [93]. Another study, an internet-based incentives study, found that the application of a brief MI interviewing session improved blood glucose monitoring in adolescents with type 1 diabetes [94]. Thus, creative strategies that employ MI techniques in collaboration with other members of the health care team (ie, patient navigation [93], telehealth [89], health coaching [95], internet-based tools [94]) hold promise for improving self-care and patient-physician communication. Increased collaboration with members of the health care team (eg, certified diabetes educators, nurses, dietitians, pharmacists, exercise physiologists), community health workers [96,97] and peer mentors [98,99] may help reinforce messages, promote shared decision-making, improve diabetes outcomes, increase patient satisfaction, and reduce medical costs [100].
Few other interventions have directly addressed physician-patient diabetes self-care communication. One older study examined the effectiveness of an intervention designed to increase of diabetes patients’ involvement in medical decision-making [16]. Patients randomized to the intervention arm participated in a 20-minute session prior to meeting with their physician, in which researchers reviewed their medical chart and used systematic prompts to encourage patients to negotiate medical decisions with their physician. Patients in the control arm received standard educational materials in a session of equal length. Patients in the intervention arm improved glycemia and elicited twice the amount of medical information from their physician compared to controls [16]. These findings suggest that brief interventions prior to medical appointments can improve patient communication, self-care behavior, and in turn, diabetes outcomes [16].
A recent study evaluated the effectiveness of a training program in communication skills for pediatric diabetes care providers in the UK [101, 102]. In this cluster randomized controlled trial, pediatric providers allocated to the Talking Diabetes intervention participated in web-based material and face-to-face seminars designed to prepare providers for constructive self-care conversations with patients as well as skills for promoting behavior change. The psychoeducational training emphasized shared decision-making and utilized motivational interviewing techniques [101]. Twenty-six centers and 693 young people with type 1 diabetes participated in the study [102]. At 12-month follow-up, the Talking Diabetes intervention did not demonstrate improvements in glycemic control. Further, the intervention had a negative effect on patients’ quality of life but a short-term improvement in coping [102]. Interestingly, parents of patients in the intervention arm reported greater continuity of care, which suggests that parents benefited more from the intervention than their children. Future communication interventions targeting the pediatric population should provide ongoing support to children of physicians exposed to interventions such as Talking Diabetes [102].
Currently, 3 ongoing studies aim to improve self-care and clinical outcomes via physician-patient communication interventions. A study by Ricci-Cabello and colleagues [103] aims to improve diabetes self-care by enhancing patient-physician communication in an underserved community of adults with uncontrolled type 2 diabetes. In this 3-arm randomized controlled trial, patients allocated to groups A and B received communication skills training and graphic feedback about glycosylated hemoglobin A1c levels; patients in group C received usual care. Patients in group B also received telephone reinforcement [103]. The second study, by Billimek and colleagues, aims to improve physician-patient communication about medication regimens via diabetes coaching [104]. In this intervention, 190 Mexican-American adult patients with type 2 diabetes were randomly assigned to complete a Coached Care visit with trained community health workers or a Coached Care visit plus the EMPATHy software toolkit, a computer-based activity with strategies and resources to overcome self-care barriers. The primary endpoints are (1) the development of care plan that addresses everyday barriers to medication adherence and (2) completion of a concrete behavioral goal [104]. Finally, the third study, by Grant and colleagues, aims to improve physician-patient communication via a pre-visit prioritization of diabetes concerns. In this controlled, cluster-randomized, multisite trial, primary care physicians were randomized to the Pre-Visit Prioritization for Complex Patients with Diabetes or the control group [105]. The Pre-Visit Prioritization IT-tool is designed to help patients identify one or two concerns prior to a medical visit and then send these priorities to the primary care physician via an electronic health record. The overall goal of the intervention is to improve communication of self-care concerns during a medical visit [105]. Findings from these 3 interventions are forthcoming; findings may provide evidence for validated interventions that improve physician-patient self-care communication in diabetes.
Techniques to Improve Self-Care Communication
Incorporating communication skills in continuing medical education and diabetes education may improve self-care communication in the physician-patient relationship. Educational programs that teach physicians how to provide consistent messages, repeat information, reinforce and offer feedback regarding specific self-care behaviors, and problem-solve self-care challenges may improve patients’ willingness to discuss self-care [14,106]. Most patients will remember only a small portion of the information given to them during medical visit. Studies that compare how much information patients retain versus how much information physicians provide show that patients forget 31% to 71% of information [107]. Therefore, physicians need techniques that promote open self-care communication during a visit. The following techniques can help physicians improve self-care communication [108]:
- Discuss the most important self-care information first; patients tend to remember the information that is presented first.
- Use the phrase “This is very important…” when discussing key points because patients will remember things that are perceived as important.
- Deliver simple, clear, and concrete instructions; patients are more likely to forget complex or confusing instructions. For example, “Check your blood glucose every morning within five minutes of waking up and before you eat breakfast” is more specific and easier to follow than “Check your blood glucose”.
- Ask open-ended questions to allow patients to verbalize feelings or concerns about their diabetes self-care.
- Employ MI techniques to help patients who are struggling to initiate and adhere to self-care behaviors. MI tools, such as the Readiness Ruler, Self-Evaluation Rulers, Decisional Balance Matrix, and Health Behavior Menu (Figure), may help patients and physicians discuss self-care behaviors during a medical visit [109].
– Express empathy by reflective listening and asking patients for permission before offering information or advice about diabetes self-care.
– Roll with resistance by engaging the patient in the process of problem solving rather than opposing a patient’s resistance to change behaviors.
– Develop discrepancy by helping the patient recognize that there is an inconsistency between his/her behavior and personal goals.
– Support self-efficacy by empowering the patient to believe that he/she can change behaviors.
6. Demonstrate active listening skills by reflecting and summarizing the patient’s statements. Reflecting and summarizing show the patient that the physician has been listening to concerns and understands what the patient is saying. This is also an opportunity to correct any miscommunications from the visit.
7. Write down instructions or provide handouts to the patient to help reinforce learning and information retention.
8. Ask patients to write a list of questions a few days prior to the medical appointment and bring it with them. Patients are more likely to remember information about issues they have previously considered that directly relate to them.
9. Consider collaborating with community health workers, patient navigators, peer mentors, and other members of the healthcare team to improve communication, diabetes outcomes, and patient satisfaction.
Summary
Physician-patient self-care communication is essential to achieving optimal diabetes outcomes [15,22,33,110] Patients’ ability to inform physicians about their self-care challenges [14], and physicians’ ability to respond to patients’ self-care reports directly and in non-accusatory language, are vital factors in effective diabetes care [45]. Interventions and education that promote open and honest conversations are particularly important given patients’ well-documented struggles achieving self-care and glycemic goals [111] and physicians’ feelings of inadequacy, frustration, and fatigue when they are not making an impact on patients’ outcomes [48,112]. More research is needed to determine the best strategies to improve self-care communication in the physician-patient relationship.
Corresponding author: Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, Athens, OH 45701, beverle1@ohio.edu.
Financial disclosures: None.
Author contributions: drafting of article, EAB, MFW, ABC, KEP, NNI; critical revision of the article, EAB, MFW, ABC, KEP, NNI.
1. Anderson E, Kian EM. Examining media contestation of masculinity and head trauma in the National Football League. J Men Masculinities 2011;1–22.
2. CDC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.
3. Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986.
4. Jacobson AM, Adler AG, Derby L, et al. Clinic attendance and glycemic control. Study of contrasting groups of patients with IDDM. Diabetes Care 1991;14:599–601.
5. Dyer PH, Lloyd CE, Lancashire RJ, et al. Factors associated with clinic non-attendance in adults with type 1 diabetes mellitus. Diab Med 1998;15:339–43.
6. Conn VS, Hafdahl AR, Mehr DR, et al. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia 2007;50:913–21.
7. Maiorana A, O’Driscoll G, Goodman C, et al. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diab Res Clin Pract 2002;56:115–23.
8. Pi-Sunyer FX, Maggio CA, McCarron DA, et al. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care 1999;22:191–7.
9. Delahanty LM, Halford BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care 1993;16:1453–8.
10. Gafarian CT, Heiby EM, Blair P, Singer F. The Diabetes Time Management Questionnaire. Diabetes Educ 1999;25:585–92.
11. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ 1997;23:558–62.
12. Rubin RR, Peyrot M. Psychological issues and treatment for people with diabetes. J Clin Psychol 2001;57:457–78.
13. Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999-2010. N Engl J Med 2013;369:287–8.
14. Beverly EA, Ganda OP, Ritholz MD, et al. Look who’s (not) talking: diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care 2012;35:1466–72.
15. Heisler M, Vijan S, Anderson RM, et al. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med 2003;18:893–902.
16. Greenfield S, Kaplan SH, Ware JE Jr, et al. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med 1988;3:448–57.
17. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med 1985;102:520–8.
18. Anderson RM, Funnell MM, Butler PM, et al. Patient empowerment. Results of a randomized controlled trial. Diabetes Care 1995;18:943–9.
19. Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med 1997;127:1097–102.
20. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high quality care in English general practice: observational study. BMJ 2001;323:784–7.
21. Bower P, Campbell S, Bojke C, Sibbald B. Team structure, team climate and the quality of care in primary care: an observational study. Qual Saf Health Care 2003;12:273–9.
22. Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med 2003;18:624-33.
23. Kerr EA, Smith DM, Kaplan SH, Hayward RA. The association between three different measures of health status and satisfaction among patients with diabetes. Med Care Res Rev 2003;60:158-77.
24. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6-8.
25. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780–1.
26. Truog RD. Patients and doctors--evolution of a relationship. N Engl J Med 2012;366:581–5.
27. Beverly EA, Wray LA, LaCoe CL, Gabbay R. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectrum 2014;27:44–9.
28. Bordin ES. The generalizability of the psychoanalytic concept of the working alliance. Psychotherapy 1979;26:252–60.
29. Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry 2001;158:29–35.
30. Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med 2004;66:720–8.
31. Jahng KH, Martin LR, Golin CE, DiMatteo MR. Preferences for medical collaboration: patient-physician congruence and patient outcomes. Patient Educ Couns 2005;57:308–14.
32. Street RL Jr, Krupat E, Bell RA, et al. Beliefs about control in the physician-patient relationship: effect on communication in medical encounters. J Gen Intern Med 2003;18:609–16.
33. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
34. Mechanic D. Changing medical organization and the erosion of trust. Milbank Q 1996;74:171–89.
35. Mechanic D, Schlesinger M. The impact of managed care on patients’ trust in medical care and their physicians. JAMA 1996;275:1693–7.
36. Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. J Gen Intern Med 2000;15:509–13.
37. Jones DE, Carson KA, Bleich SN, Cooper LA. Patient trust in physicians and adoption of lifestyle behaviors to control high blood pressure. Patient Educ Couns 2012;89:57–62.
38. Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. J Acquired Immun Def Syndr Hum Retrovir 1998;18:341–8.
39. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583–9.
40. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
41. Aikens JE, Bingham R, Piette JD. Patient-provider communication and self-care behavior among type 2 diabetes patients. Diabetes Educ 2005;31:681–90.
42. Bundesmann R, Kaplowitz SA. Provider communication and patient participation in diabetes self-care. Patient Educ Couns 2011;85:143–7.
43. Heisler M, Cole I, Weir D, et al. Does physician communication influence older patients’ diabetes self-management and glycemic control? Results from the Health and Retirement Study (HRS). J Gerontol A Biol Sci Med Sci 2007;62:1435–42.
44. Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med 2004;79:495–507.
45. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
46. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med 2006;63:3067–79.
47. Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
48. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diab Med 2005;22:1379–85.
49. DiMatteo MR, Linn LS, Chang BL, Cope DW. Affect and neutrality in physician behavior: a study of patients’ values and satisfaction. J Behav Med 1985;8:397–409.
50. Stuckey HL, Vallis M, Kovacs Burns K, et al. “I do my best to listen to patients”: qualitative insights into DAWN2 (Diabetes Psychosocial Care From the Perspective of Health Care Professionals in the Second Diabetes Attitudes, Wishes and Needs Study). Clin Ther 2015;37:1986–98.
51. Kruse RL, Olsberg JE, Oliver DP, et al. Patient-provider communication about diabetes self-care activities. Fam Med 2013;45:319–22.
52. Beverly EA, Hultgren BA, Brooks KM, et al. Understanding physicians’ challenges when treating type 2 diabetic patients’ social and emotional difficulties: a qualitative study. Diabetes Care 2011;34:1086–8.
53. Freeman J, Loewe R. Barriers to communication about diabetes mellitus. Patients’ and physicians’ different view of the disease. J Fam Pract 2000;49:507–12.
54. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia 2008;51:1822–5.
55. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–64.
56. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60.
57. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabetic Med 2003;20:69–72.
58. Weinger K, Kinsley BT, Bajaj M, et al. Diabetes-related emotional distress: A barrier to improving glycemic control during intensive diabetes treatment. Abstract. Diabetes 1997;46:Supp1:268A.
59. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns 2001;42:123–31.
60. Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care 2000;23:267–72.
61. Wen LK, Parchman ML, Shepherd MD. Family support and diet barriers among older Hispanic adults with type 2 diabetes. Fam Med 2004;36:423–30.
62. Chesla CA, Fisher L, Mullan JT, et al. Family and disease management in African-American patients with type 2 diabetes. Diabetes Care 2004;27:2850–5.
63. Brown SA, Harrist RB, Villagomez ET, et al. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ 2000;26:425–38.
64. Fisher L, Chesla CA, Chun KM, et al. Patient-appraised couple emotion management and disease management among Chinese American patients with type 2 diabetes. J Fam Psychol 2004;18:302–10.
65. Akimoto M, Fukunishi I, Kanno K, et al. Psychosocial predictors of relapse among diabetes patients: a 2-year follow-up after inpatient diabetes education. Psychosomatics 2004;45:343–9.
66. Samuel-Hodge CD, Headen SW, Skelly AH, et al. Influences on day-to-day self-management of type 2 diabetes among African-American women: spirituality, the multi-caregiver role, and other social context factors. Diabetes Care 2000;23:928–33.
67. Wing RR, Marcus MD, Epstein LH, Jawad A. A “family-based” approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156–62.
68. Trief PM, Ploutz-Snyder R, Britton KD, Weinstock RS. The relationship between marital quality and adherence to the diabetes care regimen. Ann Behav Med 2004;27:148–54.
69. Gleeson-Kreig J, Bernal H, Woolley S. The role of social support in the self-management of diabetes mellitus among a Hispanic population. Pub Health Nurs 2002;19:215–22.
70. Wen LK, Shepherd MD, Parchman ML. Family support, diet, and exercise among older Mexican Americans with type 2 diabetes. Diabetes Educ 2004;30:980–93.
71. Ruggiero L. Helping people with diabetes change behavior: from theory to practice. Diab Spectrum 2000;13:125–32.
72. Orr DP, Golden MP, Myers G, Marrero DG. Characteristics of adolescents with poorly controlled diabetes referred to a tertiary care center. Diabetes Care 1983;6:170–5.
73. Lane JD, Stabler B, Ross SL, et al. Psychological predictors of glucose control in patients with IDDM. Diabetes Care 1988;11:798–800.
74. Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychology 1992;11:135–8.
75. Irvine A, Cox D, Gonder-Frederick L. The fear of hypoglycemia scale. In: Bradley C, editor. Handbook of psychology and diabetes. Harwood Academic; 1994.
76. Peyrot MF, McMurry JF Jr. Stress buffering and glycemic control. The role of coping styles. Diabetes Care 1992;15:842–6.
77. Peyrot M, McMurry JF Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav 1999;40:141–58.
78. Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107.
79. Sohal S, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PloS One 2015:1–15.
80. Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003;163:83–90.
81. Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002.
82. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
83. Jones AJ, Gladstone BP, Lubeck M, et al. Motivational interventions in the management of HbA1c levels: A systematic review and meta-analysis. Prim Care Diabetes 2014;8:91–100.
84. Song D, Xu TZ, Sun QH. Effect of motivational interviewing on self-management in patients with type 2 diabetes mellitus: a meta-analysis. Intl J Nurs Sci 2014;1:291–7.
85. Clifford Mulimba A, Byron-Daniel J. Motivational interviewing-based interventions and diabetes mellitus. Br J Nurs 2014;23:8–14.
86. Noordman J, van der Weijden T, van Dulmen S. Communication-related behavior change techniques used in face-to-face lifestyle interventions in primary care: a systematic review of the literature. Patient Educ Couns 2012;89:227–44.
87. Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns 2011;84:16–26.
88. Kang SH, Kim BG, Lee GM. Justification of continuous packed-bed reactor for retroviral vector production from amphotropic PsiCRIP murine producer cell. Cytotechnology 2000;34(1–2):151–8.
89. Holmen H, Torbjornsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth 2014;2(4):e57.
90. Chlebowy DO, El-Mallakh P, Myers J, et al. Motivational interviewing to improve diabetes outcomes in African Americans adults with diabetes. West J Nurs Res 2015;37:566–80.
91. Kang HY, Gu MO. [Development and effects of a motivational interviewing self-management program for elderly patients with diabetes mellitus]. J Kor Acad Nurs 2015;45:533–43.
92. Brobeck E, Odencrants S, Bergh H, Hildingh C. Patients’ experiences of lifestyle discussions based on motivational interviewing: a qualitative study. BMC Nursing 2014;13:13.
93. Loskutova NY, Tsai AG, Fisher EB, et al. Patient navigators connecting patients to community resources to improve diabetes outcomes. J Am Board Fam Med 2016;29:78–89.
94. Raiff BR, Barry VB, Ridenour TA, Jitnarin N. Internet-based incentives increase blood glucose testing with a non-adherent, diverse sample of teens with type 1 diabetes mellitus: a randomized controlled Trial. Trans Behav Med 2016;6:179–88.
95. Sahlen KG, Johansson H, Nystrom L, Lindholm L. Health coaching to promote healthier lifestyle among older people at moderate risk for cardiovascular diseases, diabetes and depression: a study protocol for a randomized controlled trial in Sweden. BMC Public Health 2013;13:199.
96. Kane EP, Collinsworth AW, Schmidt KL, et al. Improving diabetes care and outcomes with community health workers. Fam Pract 2016;33:523–8.
97. Wagner JA, Bermudez-Millan A, Damio G, et al. A randomized, controlled trial of a stress management intervention for Latinos with type 2 diabetes delivered by community health workers: Outcomes for psychological wellbeing, glycemic control, and cortisol. Diabetes Res Clin Pract 2016;120:162–70.
98. Rogers EA, Hessler DM, Bodenheimer TS, et al. Diabetes peer coaching: do “better patients” make better coaches? Diabetes Educ 2014;40:107–15.
99. Long JA, Jahnle EC, Richardson DM, et al. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med 2012;156:416–24.
100. Mundt MP, Agneessens F, Tuan WJ, et al. Primary care team communication networks, team climate, quality of care, and medical costs for patients with diabetes: A cross-sectional study. Int J Nurs Stud 2016;58:1–11.
101. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–8.
102. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–6.
103. Ricci-Cabello I, Olry de Labry-Lima A, Bolivar-Munoz J, et al. Effectiveness of two interventions based on improving patient-practitioner communication on diabetes self-management in patients with low educational level: study protocol of a clustered randomized trial in primary care. BMC Health Serv Res 2013;13:433.
104. Billimek J, Guzman H, Angulo MA. Effectiveness and feasibility of a software tool to help patients communicate with doctors about problems they face with their medication regimen (EMPATHy): study protocol for a randomized controlled trial. Trials 2015;16:145.
105. Grant RW, Uratsu CS, Estacio KR, et al. Pre-Visit Prioritization for complex patients with diabetes: Randomized trial design and implementation within an integrated health care system. Contemp Clin Trials 2016;47:196–201.
106. Ritholz MD, Beverly EA, Abrahamson MJ, et al. Physicians’ perceptions of the type 2 diabetes multidisciplinary treatment team: a qualitative study. Diabetes Educ 2011.
107. Ley P. Satisfaction, compliance and communication. Br J Clin Psychol 1982;21:241–54.
108. Weinger K, Smaldone A, Beverly EA. Psychosocial and educational implications of diabetic foot complications. In: Veves A, Giurini JM, LoGerfo FW, editors. The diabetic foot: medical and surgical management. Boston: Springer; 2012:503–18.
109. Welch G, Rose G, Ernst D. Motivational interviewing and diabetes: what is it, how is it used, and does it work? Diabetes Spectr 2006;19:5–11.
110. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 1989;27(3 Suppl):S110–27.
111. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 2002;25:1722–8.
112. Wens J, Vermeire E, Royen PV, et al. GPs’ perspectives of type 2 diabetes patients’ adherence to treatment: A qualitative analysis of barriers and solutions. BMC Fam Pract 2005;6:20.
Abstract
- Objective: To summarize the current literature, research findings, and interventions for self-care communication in the physician-patient relationship.
- Methods: Literature review.
- Results: Diabetes management requires patients to follow complex self-care recommendations for nutrition, physical activity, blood glucose monitoring, and medication. Adherence to these recommendations improves glycemic control and mitigates the risk of diabetes complications; however, many patients struggle to follow these behaviors in everyday life. In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. Physicians need to incorporate interpersonal and relational skills to establish a trusting relationship. Physician-level barriers to self-care communication include lack of time, lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for diabetes patients. Among patients, psychosocial barriers and health literacy may affect willingness to discuss self-care. Motivational interviewing techniques may be helpful for improving communication around patient self-management and promotion of healthy behaviors.
- Conclusion: Physicians can assist patients with their diabetes self-care by discussing self-care challenges during medical visits.
Diabetes is one of the most significant and growing chronic health problems in the world, affecting approximately 415 million people [1]. Diabetes is marked by the body’s inability to make insulin as well as the body’s inability to effectively use the insulin it produces [2]. Diagnosis of diabetes has increased sharply in recent decades and is expected to increase even more, with the largest increases in middle- and low-income countries [3]. Diabetes is a leading cause of blindness, kidney failure, myocardial infarction, stroke, and amputation [3], and in 2015 it accounted for 5 million deaths worldwide [1]. Further, diabetes’s costs to society represent 12% ($673 billion) of global health expenditures [1]. By 2040, models predict that 642 million people will be diagnosed with diabetes and costs will continue to grow as the population ages [1]. Thus, prevention of diabetes is the ultimate goal; however, more effective management for individuals already diagnosed with diabetes is critical to reduce the risk of complications and the economic burden of the disease.
Diabetes management requires patients to perform complex self-care regimens, including weight reduction, frequent blood glucose monitoring, taking oral and/or insulin medications, engaging in physical activity, adhering to diabetes nutrition guidelines, and attending clinic appointments [4–9]. These self-care behaviors are critically linked to improved glycemic control, however, integrating them into one’s daily life can be challenging [10–12]. Recent National Health and Nutrition Examination Survey (NHANES) data show that approximately half of adults with diabetes are not meeting recommended goals for diabetes care [13]. Physicians can assist patients with their diabetes self-care by scheduling frequent follow-up visits and discussing self-care challenges with their patients [14].
In this review, we discuss the current literature on physician-patient communication and diabetes self-care. First, we discuss the qualities of an effective physician-patient relationship followed by the importance of self-care communication in diabetes care. Next, we discuss barriers and facilitators to self-care communication. Finally, we review interventions for improving physician-patient communication in diabetes self-care.
Qualities of an Effective Physician-Patient Relationship
Successful diabetes care requires teamwork between physicians and patients [15]. Two components of successful teamwork are physician-patient communication and shared decision-making, both of which have been shown to improve patient satisfaction, adherence to treatment plans and health outcomes [16–23]. In shared decision-making, the physician and patient share medical information [24–26]. Specifically, the physician presents different treatment options to the patient and describes the risks and benefits of each option. Then the patient expresses his or her preferences for treatment to ensure that the care provided aligns with the patient’s values and needs [27]. Thus, shared decision-making in the treatment relationship is predicated on effective communication between the physician and patient [19].
Effective physician-patient communication is supported by continuous care [19,28], a secure attachment style [29, 30], shared goals [19], a mutual understanding of respective roles and tasks [15,31–33], and a bond characterized by liking, confidence, and trust [19,28,31]. Trust is paramount in physician-patient communication. Interpersonal trust and social trust are the 2 predominant types [34]. Interpersonal trust refers to the relationship the patient has with the physician, specifically the confidence the patient has in the physician as well as the responsibility, competence, compassion, and regard the physician has for the patient’s welfare [34–36]. For patients and physicians, interpersonal trust is developed over time with repeated interactions [34–36]. On the other hand, social trust refers to the beliefs of honesty, integrity, and reliability in others [36]. Social trust is influenced by social constructs, including the media and institutions of higher education [36].
In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. A patient’s trust can be acquired through multiple medical appointments with the physician. Further, how the patient is treated during these appointments as well as how much time and attention the physician invests in the patient’s care influences the level of interpersonal trust. A high level of trust in the relationship can lead to in improvements in adherence to self-care, continuity of care, physician-patient communication, and overall quality of the physician-patient relationship [37–39].
In the diabetes physician-patient relationship, minimal research has explored how trust in one’s physician impacts self-care communication. In a study by Beverly and colleagues, diabetes patients emphasized the importance of a trusting physician-patient relationship for diabetes care [27]. Another study by Ritholz and colleagues found that physicians and patients both stress the importance of developing trust to facilitate self-care communication [40]. Specifically, trust as well as acceptance from the physician contributes to open and honest self-care communication in the physician-patient diabetes relationship[40]. Additional research is needed to determine whether a high level of physician-patient trust is associated with increased self-care behaviors and improved diabetes outcomes over time.
Importance of Diabetes Self-Care Communication
Diabetes self-care communication in the physician-patient relationship increases patient satisfaction, improves adherence to treatment regimens, and leads to better clinical outcomes [22,41–43]. For physicians, effective self-care communication requires the performance of specific communication tasks and behaviors, including collecting a medical history, explaining a diagnosis and prognosis, and providing clear and concise therapeutic instructions [44]. In addition, physicians must incorporate interpersonal and relational skills to establish a trusting relationship [44,45]. Both physicians and patients agree that a trusting treatment relationship is a requirement for open and honest self-care communication [45]. For patients, effective communication necessitates the disclosure of self-care successes and failures [46]. Diabetes patients face challenging self-care regimens, and these challenges can interfere with glycemic control and increase the risk for diabetes complications [47,48]. For this reason, patients must feel comfortable discussing their self-are challenges so that their physician can individualize treatment prescriptions and recommendations, thereby increasing the likelihood of treatment success.
Barriers to Self-Care Communication
Physician-patient self-care communication is essential to improving patient adherence [29,49] yet numerous barriers exist that undermine effective physician-patient self-care communication. From the physician perspective, the most commonly cited barrier to self-care communication is time [50]. A recent study of family medicine practices found that the time physicians spent discussing self-care with their patients varied from 1 to 17 minutes, suggesting that time is a major barrier to self-care communication [51]. Other barriers include lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for patients with diabetes [50]. Relatedly, Beverly and colleagues [52] found that physicians often feel inadequately trained to address diabetes patients’ psychosocial issues and this perceived lack of expertise may contribute to physicians feeling overwhelmed and frustrated within the physician-patient relationship, which may hinder open self-care communication.
For patients, barriers tend to differ from those perceived by physicians. A qualitative study using semi-structured interviews with patients and clinicians, and direct observation of clinical encounters at an inner-city family practice training site, revealed different perceptions of the term “control” between physicians and patients. In practice, physicians used the term “control” to focus on the management of blood glucose levels rather than trying to understand the patients’ understanding of diabetes and subsequent treatment goals. Differing viewpoints contributed to frustration and hindered effective communication [53]. In another qualitative study with physicians and patients, both noted that patients were reluctant to discuss self-care for fear being judged or shamed about food intake and weight [45]. This finding was supported in a quantitative follow-up study assessing patient reluctance to discuss self-care. Thirty percent of surveyed patients reported reluctance to discussing self-care with their physicians for fear of being judged, not wanting to disappoint their doctors, guilt, and shame [14]. Interestingly, patients reporting elevated depressive symptoms were more likely to be reluctant to discuss their self-care [14]. Cognitive behavioral changes (eg, cognitive distortions, avoidance behavior, attention deficits) associated with major depression and depressive symptoms may impair patients’ ability to recall self-care information. Also, patients reporting more depressive symptoms may be more socially withdrawn during a medical appointment, and thus less willing to communicate with their physician about self-care.
Other studies found that psychosocial factors such as diabetes distress [54,55] and pessimistic attitudes [56–59], cultural differences [60–66], lack of family and social support [60,67–70], lack of readiness to change behavior [71], introversion and social isolation [72,73], hypo-glycemia fear [74,75] and ineffectual coping styles [76,77] interfere with self-care and glycemic control. Further, low health literacy is associated with difficulty adhering to self-care, particular medication regimens, and negative health outcomes [78].
In summary psychosocial barriers and health literacy may affect a patients’ willingness to discuss self-care during a medical visit. Therefore, routine assessment of psychosocial factors and health literacy may be necessary to address a patient’s barriers to self-care as well as to promote open and honest self-care communication. Interventions and evidenced-based approaches that address psychosocial factors, health literacy, and physician-patient self-care communication are needed.
Facilitators to Self-Care Communication
Despite numerous barriers to self-care communication, several factors promote self-care communication in the physician-patient relationship. For example, direct and non-accusatory communication from physicians as well as providing patients with hope for living with diabetes both support physician-patient self-care communication [45]. A recent systematic review by Sohal and colleagues [79] found that trust in physicians, the use of culturally appropriate exercise and dietary advice, and increasing family involvement improved physician-patient communication and diabetes self-care [79]. Lastly, a study by Schillinger and colleagues [80] found that physician assessment of patient recall and comprehension of new concepts during medical visits improved diabetes outcomes [80].
Patient-Physician Self-Care Communication Interventions
One of the more successful interventions for improving diabetes self-care and patient-physician communication is motivational interviewing (MI). MI is a non-judgmental communication style designed to explore a patient’s intrinsic motivation to change health behaviors [81]. Inherent to MI is the belief that motivation for change is malleable and that it can be transformed in the context of the patient-physician relationship [81]. MI is a patient-centered method designed to empower a patient’s ability and responsibility to make health-related decisions, with the physician supporting the patient’s autonomy in the process [82]. Recent meta-analyses and systematic reviews [83–87] showed that MI interventions improve self-care behaviors and glycemic control in the short-term; long-term effects of MI on self-care and glycemia remain inconclusive. More high-quality research is needed to evaluate the MI training content of these interventions in order to determine its long-term effectiveness and replicate outcomes in various healthcare settings [87].
Other studies not included in the meta-analyses and reviews found MI interventions improved self-care behaviors [88–90], glycemic control [90,91], and quality of life [91]. A qualitative study exploring diabetes patients’ experiences with MI and self-care behaviors revealed that patients’ appreciate when providers initiate discussions that result in new ways of thinking about self-care and promote a sense of well-being in patients [92]. New research utilizing patient navigators to connect diabetes patients’ to their primary care providers showed MI techniques improved patient self-efficacy and glycemic control [93]. Another study, an internet-based incentives study, found that the application of a brief MI interviewing session improved blood glucose monitoring in adolescents with type 1 diabetes [94]. Thus, creative strategies that employ MI techniques in collaboration with other members of the health care team (ie, patient navigation [93], telehealth [89], health coaching [95], internet-based tools [94]) hold promise for improving self-care and patient-physician communication. Increased collaboration with members of the health care team (eg, certified diabetes educators, nurses, dietitians, pharmacists, exercise physiologists), community health workers [96,97] and peer mentors [98,99] may help reinforce messages, promote shared decision-making, improve diabetes outcomes, increase patient satisfaction, and reduce medical costs [100].
Few other interventions have directly addressed physician-patient diabetes self-care communication. One older study examined the effectiveness of an intervention designed to increase of diabetes patients’ involvement in medical decision-making [16]. Patients randomized to the intervention arm participated in a 20-minute session prior to meeting with their physician, in which researchers reviewed their medical chart and used systematic prompts to encourage patients to negotiate medical decisions with their physician. Patients in the control arm received standard educational materials in a session of equal length. Patients in the intervention arm improved glycemia and elicited twice the amount of medical information from their physician compared to controls [16]. These findings suggest that brief interventions prior to medical appointments can improve patient communication, self-care behavior, and in turn, diabetes outcomes [16].
A recent study evaluated the effectiveness of a training program in communication skills for pediatric diabetes care providers in the UK [101, 102]. In this cluster randomized controlled trial, pediatric providers allocated to the Talking Diabetes intervention participated in web-based material and face-to-face seminars designed to prepare providers for constructive self-care conversations with patients as well as skills for promoting behavior change. The psychoeducational training emphasized shared decision-making and utilized motivational interviewing techniques [101]. Twenty-six centers and 693 young people with type 1 diabetes participated in the study [102]. At 12-month follow-up, the Talking Diabetes intervention did not demonstrate improvements in glycemic control. Further, the intervention had a negative effect on patients’ quality of life but a short-term improvement in coping [102]. Interestingly, parents of patients in the intervention arm reported greater continuity of care, which suggests that parents benefited more from the intervention than their children. Future communication interventions targeting the pediatric population should provide ongoing support to children of physicians exposed to interventions such as Talking Diabetes [102].
Currently, 3 ongoing studies aim to improve self-care and clinical outcomes via physician-patient communication interventions. A study by Ricci-Cabello and colleagues [103] aims to improve diabetes self-care by enhancing patient-physician communication in an underserved community of adults with uncontrolled type 2 diabetes. In this 3-arm randomized controlled trial, patients allocated to groups A and B received communication skills training and graphic feedback about glycosylated hemoglobin A1c levels; patients in group C received usual care. Patients in group B also received telephone reinforcement [103]. The second study, by Billimek and colleagues, aims to improve physician-patient communication about medication regimens via diabetes coaching [104]. In this intervention, 190 Mexican-American adult patients with type 2 diabetes were randomly assigned to complete a Coached Care visit with trained community health workers or a Coached Care visit plus the EMPATHy software toolkit, a computer-based activity with strategies and resources to overcome self-care barriers. The primary endpoints are (1) the development of care plan that addresses everyday barriers to medication adherence and (2) completion of a concrete behavioral goal [104]. Finally, the third study, by Grant and colleagues, aims to improve physician-patient communication via a pre-visit prioritization of diabetes concerns. In this controlled, cluster-randomized, multisite trial, primary care physicians were randomized to the Pre-Visit Prioritization for Complex Patients with Diabetes or the control group [105]. The Pre-Visit Prioritization IT-tool is designed to help patients identify one or two concerns prior to a medical visit and then send these priorities to the primary care physician via an electronic health record. The overall goal of the intervention is to improve communication of self-care concerns during a medical visit [105]. Findings from these 3 interventions are forthcoming; findings may provide evidence for validated interventions that improve physician-patient self-care communication in diabetes.
Techniques to Improve Self-Care Communication
Incorporating communication skills in continuing medical education and diabetes education may improve self-care communication in the physician-patient relationship. Educational programs that teach physicians how to provide consistent messages, repeat information, reinforce and offer feedback regarding specific self-care behaviors, and problem-solve self-care challenges may improve patients’ willingness to discuss self-care [14,106]. Most patients will remember only a small portion of the information given to them during medical visit. Studies that compare how much information patients retain versus how much information physicians provide show that patients forget 31% to 71% of information [107]. Therefore, physicians need techniques that promote open self-care communication during a visit. The following techniques can help physicians improve self-care communication [108]:
- Discuss the most important self-care information first; patients tend to remember the information that is presented first.
- Use the phrase “This is very important…” when discussing key points because patients will remember things that are perceived as important.
- Deliver simple, clear, and concrete instructions; patients are more likely to forget complex or confusing instructions. For example, “Check your blood glucose every morning within five minutes of waking up and before you eat breakfast” is more specific and easier to follow than “Check your blood glucose”.
- Ask open-ended questions to allow patients to verbalize feelings or concerns about their diabetes self-care.
- Employ MI techniques to help patients who are struggling to initiate and adhere to self-care behaviors. MI tools, such as the Readiness Ruler, Self-Evaluation Rulers, Decisional Balance Matrix, and Health Behavior Menu (Figure), may help patients and physicians discuss self-care behaviors during a medical visit [109].
– Express empathy by reflective listening and asking patients for permission before offering information or advice about diabetes self-care.
– Roll with resistance by engaging the patient in the process of problem solving rather than opposing a patient’s resistance to change behaviors.
– Develop discrepancy by helping the patient recognize that there is an inconsistency between his/her behavior and personal goals.
– Support self-efficacy by empowering the patient to believe that he/she can change behaviors.
6. Demonstrate active listening skills by reflecting and summarizing the patient’s statements. Reflecting and summarizing show the patient that the physician has been listening to concerns and understands what the patient is saying. This is also an opportunity to correct any miscommunications from the visit.
7. Write down instructions or provide handouts to the patient to help reinforce learning and information retention.
8. Ask patients to write a list of questions a few days prior to the medical appointment and bring it with them. Patients are more likely to remember information about issues they have previously considered that directly relate to them.
9. Consider collaborating with community health workers, patient navigators, peer mentors, and other members of the healthcare team to improve communication, diabetes outcomes, and patient satisfaction.
Summary
Physician-patient self-care communication is essential to achieving optimal diabetes outcomes [15,22,33,110] Patients’ ability to inform physicians about their self-care challenges [14], and physicians’ ability to respond to patients’ self-care reports directly and in non-accusatory language, are vital factors in effective diabetes care [45]. Interventions and education that promote open and honest conversations are particularly important given patients’ well-documented struggles achieving self-care and glycemic goals [111] and physicians’ feelings of inadequacy, frustration, and fatigue when they are not making an impact on patients’ outcomes [48,112]. More research is needed to determine the best strategies to improve self-care communication in the physician-patient relationship.
Corresponding author: Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, Athens, OH 45701, beverle1@ohio.edu.
Financial disclosures: None.
Author contributions: drafting of article, EAB, MFW, ABC, KEP, NNI; critical revision of the article, EAB, MFW, ABC, KEP, NNI.
Abstract
- Objective: To summarize the current literature, research findings, and interventions for self-care communication in the physician-patient relationship.
- Methods: Literature review.
- Results: Diabetes management requires patients to follow complex self-care recommendations for nutrition, physical activity, blood glucose monitoring, and medication. Adherence to these recommendations improves glycemic control and mitigates the risk of diabetes complications; however, many patients struggle to follow these behaviors in everyday life. In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. Physicians need to incorporate interpersonal and relational skills to establish a trusting relationship. Physician-level barriers to self-care communication include lack of time, lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for diabetes patients. Among patients, psychosocial barriers and health literacy may affect willingness to discuss self-care. Motivational interviewing techniques may be helpful for improving communication around patient self-management and promotion of healthy behaviors.
- Conclusion: Physicians can assist patients with their diabetes self-care by discussing self-care challenges during medical visits.
Diabetes is one of the most significant and growing chronic health problems in the world, affecting approximately 415 million people [1]. Diabetes is marked by the body’s inability to make insulin as well as the body’s inability to effectively use the insulin it produces [2]. Diagnosis of diabetes has increased sharply in recent decades and is expected to increase even more, with the largest increases in middle- and low-income countries [3]. Diabetes is a leading cause of blindness, kidney failure, myocardial infarction, stroke, and amputation [3], and in 2015 it accounted for 5 million deaths worldwide [1]. Further, diabetes’s costs to society represent 12% ($673 billion) of global health expenditures [1]. By 2040, models predict that 642 million people will be diagnosed with diabetes and costs will continue to grow as the population ages [1]. Thus, prevention of diabetes is the ultimate goal; however, more effective management for individuals already diagnosed with diabetes is critical to reduce the risk of complications and the economic burden of the disease.
Diabetes management requires patients to perform complex self-care regimens, including weight reduction, frequent blood glucose monitoring, taking oral and/or insulin medications, engaging in physical activity, adhering to diabetes nutrition guidelines, and attending clinic appointments [4–9]. These self-care behaviors are critically linked to improved glycemic control, however, integrating them into one’s daily life can be challenging [10–12]. Recent National Health and Nutrition Examination Survey (NHANES) data show that approximately half of adults with diabetes are not meeting recommended goals for diabetes care [13]. Physicians can assist patients with their diabetes self-care by scheduling frequent follow-up visits and discussing self-care challenges with their patients [14].
In this review, we discuss the current literature on physician-patient communication and diabetes self-care. First, we discuss the qualities of an effective physician-patient relationship followed by the importance of self-care communication in diabetes care. Next, we discuss barriers and facilitators to self-care communication. Finally, we review interventions for improving physician-patient communication in diabetes self-care.
Qualities of an Effective Physician-Patient Relationship
Successful diabetes care requires teamwork between physicians and patients [15]. Two components of successful teamwork are physician-patient communication and shared decision-making, both of which have been shown to improve patient satisfaction, adherence to treatment plans and health outcomes [16–23]. In shared decision-making, the physician and patient share medical information [24–26]. Specifically, the physician presents different treatment options to the patient and describes the risks and benefits of each option. Then the patient expresses his or her preferences for treatment to ensure that the care provided aligns with the patient’s values and needs [27]. Thus, shared decision-making in the treatment relationship is predicated on effective communication between the physician and patient [19].
Effective physician-patient communication is supported by continuous care [19,28], a secure attachment style [29, 30], shared goals [19], a mutual understanding of respective roles and tasks [15,31–33], and a bond characterized by liking, confidence, and trust [19,28,31]. Trust is paramount in physician-patient communication. Interpersonal trust and social trust are the 2 predominant types [34]. Interpersonal trust refers to the relationship the patient has with the physician, specifically the confidence the patient has in the physician as well as the responsibility, competence, compassion, and regard the physician has for the patient’s welfare [34–36]. For patients and physicians, interpersonal trust is developed over time with repeated interactions [34–36]. On the other hand, social trust refers to the beliefs of honesty, integrity, and reliability in others [36]. Social trust is influenced by social constructs, including the media and institutions of higher education [36].
In the physician-patient relationship, self-care communication is largely influenced by interpersonal trust. A patient’s trust can be acquired through multiple medical appointments with the physician. Further, how the patient is treated during these appointments as well as how much time and attention the physician invests in the patient’s care influences the level of interpersonal trust. A high level of trust in the relationship can lead to in improvements in adherence to self-care, continuity of care, physician-patient communication, and overall quality of the physician-patient relationship [37–39].
In the diabetes physician-patient relationship, minimal research has explored how trust in one’s physician impacts self-care communication. In a study by Beverly and colleagues, diabetes patients emphasized the importance of a trusting physician-patient relationship for diabetes care [27]. Another study by Ritholz and colleagues found that physicians and patients both stress the importance of developing trust to facilitate self-care communication [40]. Specifically, trust as well as acceptance from the physician contributes to open and honest self-care communication in the physician-patient diabetes relationship[40]. Additional research is needed to determine whether a high level of physician-patient trust is associated with increased self-care behaviors and improved diabetes outcomes over time.
Importance of Diabetes Self-Care Communication
Diabetes self-care communication in the physician-patient relationship increases patient satisfaction, improves adherence to treatment regimens, and leads to better clinical outcomes [22,41–43]. For physicians, effective self-care communication requires the performance of specific communication tasks and behaviors, including collecting a medical history, explaining a diagnosis and prognosis, and providing clear and concise therapeutic instructions [44]. In addition, physicians must incorporate interpersonal and relational skills to establish a trusting relationship [44,45]. Both physicians and patients agree that a trusting treatment relationship is a requirement for open and honest self-care communication [45]. For patients, effective communication necessitates the disclosure of self-care successes and failures [46]. Diabetes patients face challenging self-care regimens, and these challenges can interfere with glycemic control and increase the risk for diabetes complications [47,48]. For this reason, patients must feel comfortable discussing their self-are challenges so that their physician can individualize treatment prescriptions and recommendations, thereby increasing the likelihood of treatment success.
Barriers to Self-Care Communication
Physician-patient self-care communication is essential to improving patient adherence [29,49] yet numerous barriers exist that undermine effective physician-patient self-care communication. From the physician perspective, the most commonly cited barrier to self-care communication is time [50]. A recent study of family medicine practices found that the time physicians spent discussing self-care with their patients varied from 1 to 17 minutes, suggesting that time is a major barrier to self-care communication [51]. Other barriers include lack of collaboration and teamwork among health care providers, lack of patients’ access to resources, and lack of psychosocial support for patients with diabetes [50]. Relatedly, Beverly and colleagues [52] found that physicians often feel inadequately trained to address diabetes patients’ psychosocial issues and this perceived lack of expertise may contribute to physicians feeling overwhelmed and frustrated within the physician-patient relationship, which may hinder open self-care communication.
For patients, barriers tend to differ from those perceived by physicians. A qualitative study using semi-structured interviews with patients and clinicians, and direct observation of clinical encounters at an inner-city family practice training site, revealed different perceptions of the term “control” between physicians and patients. In practice, physicians used the term “control” to focus on the management of blood glucose levels rather than trying to understand the patients’ understanding of diabetes and subsequent treatment goals. Differing viewpoints contributed to frustration and hindered effective communication [53]. In another qualitative study with physicians and patients, both noted that patients were reluctant to discuss self-care for fear being judged or shamed about food intake and weight [45]. This finding was supported in a quantitative follow-up study assessing patient reluctance to discuss self-care. Thirty percent of surveyed patients reported reluctance to discussing self-care with their physicians for fear of being judged, not wanting to disappoint their doctors, guilt, and shame [14]. Interestingly, patients reporting elevated depressive symptoms were more likely to be reluctant to discuss their self-care [14]. Cognitive behavioral changes (eg, cognitive distortions, avoidance behavior, attention deficits) associated with major depression and depressive symptoms may impair patients’ ability to recall self-care information. Also, patients reporting more depressive symptoms may be more socially withdrawn during a medical appointment, and thus less willing to communicate with their physician about self-care.
Other studies found that psychosocial factors such as diabetes distress [54,55] and pessimistic attitudes [56–59], cultural differences [60–66], lack of family and social support [60,67–70], lack of readiness to change behavior [71], introversion and social isolation [72,73], hypo-glycemia fear [74,75] and ineffectual coping styles [76,77] interfere with self-care and glycemic control. Further, low health literacy is associated with difficulty adhering to self-care, particular medication regimens, and negative health outcomes [78].
In summary psychosocial barriers and health literacy may affect a patients’ willingness to discuss self-care during a medical visit. Therefore, routine assessment of psychosocial factors and health literacy may be necessary to address a patient’s barriers to self-care as well as to promote open and honest self-care communication. Interventions and evidenced-based approaches that address psychosocial factors, health literacy, and physician-patient self-care communication are needed.
Facilitators to Self-Care Communication
Despite numerous barriers to self-care communication, several factors promote self-care communication in the physician-patient relationship. For example, direct and non-accusatory communication from physicians as well as providing patients with hope for living with diabetes both support physician-patient self-care communication [45]. A recent systematic review by Sohal and colleagues [79] found that trust in physicians, the use of culturally appropriate exercise and dietary advice, and increasing family involvement improved physician-patient communication and diabetes self-care [79]. Lastly, a study by Schillinger and colleagues [80] found that physician assessment of patient recall and comprehension of new concepts during medical visits improved diabetes outcomes [80].
Patient-Physician Self-Care Communication Interventions
One of the more successful interventions for improving diabetes self-care and patient-physician communication is motivational interviewing (MI). MI is a non-judgmental communication style designed to explore a patient’s intrinsic motivation to change health behaviors [81]. Inherent to MI is the belief that motivation for change is malleable and that it can be transformed in the context of the patient-physician relationship [81]. MI is a patient-centered method designed to empower a patient’s ability and responsibility to make health-related decisions, with the physician supporting the patient’s autonomy in the process [82]. Recent meta-analyses and systematic reviews [83–87] showed that MI interventions improve self-care behaviors and glycemic control in the short-term; long-term effects of MI on self-care and glycemia remain inconclusive. More high-quality research is needed to evaluate the MI training content of these interventions in order to determine its long-term effectiveness and replicate outcomes in various healthcare settings [87].
Other studies not included in the meta-analyses and reviews found MI interventions improved self-care behaviors [88–90], glycemic control [90,91], and quality of life [91]. A qualitative study exploring diabetes patients’ experiences with MI and self-care behaviors revealed that patients’ appreciate when providers initiate discussions that result in new ways of thinking about self-care and promote a sense of well-being in patients [92]. New research utilizing patient navigators to connect diabetes patients’ to their primary care providers showed MI techniques improved patient self-efficacy and glycemic control [93]. Another study, an internet-based incentives study, found that the application of a brief MI interviewing session improved blood glucose monitoring in adolescents with type 1 diabetes [94]. Thus, creative strategies that employ MI techniques in collaboration with other members of the health care team (ie, patient navigation [93], telehealth [89], health coaching [95], internet-based tools [94]) hold promise for improving self-care and patient-physician communication. Increased collaboration with members of the health care team (eg, certified diabetes educators, nurses, dietitians, pharmacists, exercise physiologists), community health workers [96,97] and peer mentors [98,99] may help reinforce messages, promote shared decision-making, improve diabetes outcomes, increase patient satisfaction, and reduce medical costs [100].
Few other interventions have directly addressed physician-patient diabetes self-care communication. One older study examined the effectiveness of an intervention designed to increase of diabetes patients’ involvement in medical decision-making [16]. Patients randomized to the intervention arm participated in a 20-minute session prior to meeting with their physician, in which researchers reviewed their medical chart and used systematic prompts to encourage patients to negotiate medical decisions with their physician. Patients in the control arm received standard educational materials in a session of equal length. Patients in the intervention arm improved glycemia and elicited twice the amount of medical information from their physician compared to controls [16]. These findings suggest that brief interventions prior to medical appointments can improve patient communication, self-care behavior, and in turn, diabetes outcomes [16].
A recent study evaluated the effectiveness of a training program in communication skills for pediatric diabetes care providers in the UK [101, 102]. In this cluster randomized controlled trial, pediatric providers allocated to the Talking Diabetes intervention participated in web-based material and face-to-face seminars designed to prepare providers for constructive self-care conversations with patients as well as skills for promoting behavior change. The psychoeducational training emphasized shared decision-making and utilized motivational interviewing techniques [101]. Twenty-six centers and 693 young people with type 1 diabetes participated in the study [102]. At 12-month follow-up, the Talking Diabetes intervention did not demonstrate improvements in glycemic control. Further, the intervention had a negative effect on patients’ quality of life but a short-term improvement in coping [102]. Interestingly, parents of patients in the intervention arm reported greater continuity of care, which suggests that parents benefited more from the intervention than their children. Future communication interventions targeting the pediatric population should provide ongoing support to children of physicians exposed to interventions such as Talking Diabetes [102].
Currently, 3 ongoing studies aim to improve self-care and clinical outcomes via physician-patient communication interventions. A study by Ricci-Cabello and colleagues [103] aims to improve diabetes self-care by enhancing patient-physician communication in an underserved community of adults with uncontrolled type 2 diabetes. In this 3-arm randomized controlled trial, patients allocated to groups A and B received communication skills training and graphic feedback about glycosylated hemoglobin A1c levels; patients in group C received usual care. Patients in group B also received telephone reinforcement [103]. The second study, by Billimek and colleagues, aims to improve physician-patient communication about medication regimens via diabetes coaching [104]. In this intervention, 190 Mexican-American adult patients with type 2 diabetes were randomly assigned to complete a Coached Care visit with trained community health workers or a Coached Care visit plus the EMPATHy software toolkit, a computer-based activity with strategies and resources to overcome self-care barriers. The primary endpoints are (1) the development of care plan that addresses everyday barriers to medication adherence and (2) completion of a concrete behavioral goal [104]. Finally, the third study, by Grant and colleagues, aims to improve physician-patient communication via a pre-visit prioritization of diabetes concerns. In this controlled, cluster-randomized, multisite trial, primary care physicians were randomized to the Pre-Visit Prioritization for Complex Patients with Diabetes or the control group [105]. The Pre-Visit Prioritization IT-tool is designed to help patients identify one or two concerns prior to a medical visit and then send these priorities to the primary care physician via an electronic health record. The overall goal of the intervention is to improve communication of self-care concerns during a medical visit [105]. Findings from these 3 interventions are forthcoming; findings may provide evidence for validated interventions that improve physician-patient self-care communication in diabetes.
Techniques to Improve Self-Care Communication
Incorporating communication skills in continuing medical education and diabetes education may improve self-care communication in the physician-patient relationship. Educational programs that teach physicians how to provide consistent messages, repeat information, reinforce and offer feedback regarding specific self-care behaviors, and problem-solve self-care challenges may improve patients’ willingness to discuss self-care [14,106]. Most patients will remember only a small portion of the information given to them during medical visit. Studies that compare how much information patients retain versus how much information physicians provide show that patients forget 31% to 71% of information [107]. Therefore, physicians need techniques that promote open self-care communication during a visit. The following techniques can help physicians improve self-care communication [108]:
- Discuss the most important self-care information first; patients tend to remember the information that is presented first.
- Use the phrase “This is very important…” when discussing key points because patients will remember things that are perceived as important.
- Deliver simple, clear, and concrete instructions; patients are more likely to forget complex or confusing instructions. For example, “Check your blood glucose every morning within five minutes of waking up and before you eat breakfast” is more specific and easier to follow than “Check your blood glucose”.
- Ask open-ended questions to allow patients to verbalize feelings or concerns about their diabetes self-care.
- Employ MI techniques to help patients who are struggling to initiate and adhere to self-care behaviors. MI tools, such as the Readiness Ruler, Self-Evaluation Rulers, Decisional Balance Matrix, and Health Behavior Menu (Figure), may help patients and physicians discuss self-care behaviors during a medical visit [109].
– Express empathy by reflective listening and asking patients for permission before offering information or advice about diabetes self-care.
– Roll with resistance by engaging the patient in the process of problem solving rather than opposing a patient’s resistance to change behaviors.
– Develop discrepancy by helping the patient recognize that there is an inconsistency between his/her behavior and personal goals.
– Support self-efficacy by empowering the patient to believe that he/she can change behaviors.
6. Demonstrate active listening skills by reflecting and summarizing the patient’s statements. Reflecting and summarizing show the patient that the physician has been listening to concerns and understands what the patient is saying. This is also an opportunity to correct any miscommunications from the visit.
7. Write down instructions or provide handouts to the patient to help reinforce learning and information retention.
8. Ask patients to write a list of questions a few days prior to the medical appointment and bring it with them. Patients are more likely to remember information about issues they have previously considered that directly relate to them.
9. Consider collaborating with community health workers, patient navigators, peer mentors, and other members of the healthcare team to improve communication, diabetes outcomes, and patient satisfaction.
Summary
Physician-patient self-care communication is essential to achieving optimal diabetes outcomes [15,22,33,110] Patients’ ability to inform physicians about their self-care challenges [14], and physicians’ ability to respond to patients’ self-care reports directly and in non-accusatory language, are vital factors in effective diabetes care [45]. Interventions and education that promote open and honest conversations are particularly important given patients’ well-documented struggles achieving self-care and glycemic goals [111] and physicians’ feelings of inadequacy, frustration, and fatigue when they are not making an impact on patients’ outcomes [48,112]. More research is needed to determine the best strategies to improve self-care communication in the physician-patient relationship.
Corresponding author: Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, Athens, OH 45701, beverle1@ohio.edu.
Financial disclosures: None.
Author contributions: drafting of article, EAB, MFW, ABC, KEP, NNI; critical revision of the article, EAB, MFW, ABC, KEP, NNI.
1. Anderson E, Kian EM. Examining media contestation of masculinity and head trauma in the National Football League. J Men Masculinities 2011;1–22.
2. CDC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.
3. Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986.
4. Jacobson AM, Adler AG, Derby L, et al. Clinic attendance and glycemic control. Study of contrasting groups of patients with IDDM. Diabetes Care 1991;14:599–601.
5. Dyer PH, Lloyd CE, Lancashire RJ, et al. Factors associated with clinic non-attendance in adults with type 1 diabetes mellitus. Diab Med 1998;15:339–43.
6. Conn VS, Hafdahl AR, Mehr DR, et al. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia 2007;50:913–21.
7. Maiorana A, O’Driscoll G, Goodman C, et al. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diab Res Clin Pract 2002;56:115–23.
8. Pi-Sunyer FX, Maggio CA, McCarron DA, et al. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care 1999;22:191–7.
9. Delahanty LM, Halford BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care 1993;16:1453–8.
10. Gafarian CT, Heiby EM, Blair P, Singer F. The Diabetes Time Management Questionnaire. Diabetes Educ 1999;25:585–92.
11. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ 1997;23:558–62.
12. Rubin RR, Peyrot M. Psychological issues and treatment for people with diabetes. J Clin Psychol 2001;57:457–78.
13. Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999-2010. N Engl J Med 2013;369:287–8.
14. Beverly EA, Ganda OP, Ritholz MD, et al. Look who’s (not) talking: diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care 2012;35:1466–72.
15. Heisler M, Vijan S, Anderson RM, et al. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med 2003;18:893–902.
16. Greenfield S, Kaplan SH, Ware JE Jr, et al. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med 1988;3:448–57.
17. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med 1985;102:520–8.
18. Anderson RM, Funnell MM, Butler PM, et al. Patient empowerment. Results of a randomized controlled trial. Diabetes Care 1995;18:943–9.
19. Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med 1997;127:1097–102.
20. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high quality care in English general practice: observational study. BMJ 2001;323:784–7.
21. Bower P, Campbell S, Bojke C, Sibbald B. Team structure, team climate and the quality of care in primary care: an observational study. Qual Saf Health Care 2003;12:273–9.
22. Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med 2003;18:624-33.
23. Kerr EA, Smith DM, Kaplan SH, Hayward RA. The association between three different measures of health status and satisfaction among patients with diabetes. Med Care Res Rev 2003;60:158-77.
24. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6-8.
25. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780–1.
26. Truog RD. Patients and doctors--evolution of a relationship. N Engl J Med 2012;366:581–5.
27. Beverly EA, Wray LA, LaCoe CL, Gabbay R. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectrum 2014;27:44–9.
28. Bordin ES. The generalizability of the psychoanalytic concept of the working alliance. Psychotherapy 1979;26:252–60.
29. Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry 2001;158:29–35.
30. Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med 2004;66:720–8.
31. Jahng KH, Martin LR, Golin CE, DiMatteo MR. Preferences for medical collaboration: patient-physician congruence and patient outcomes. Patient Educ Couns 2005;57:308–14.
32. Street RL Jr, Krupat E, Bell RA, et al. Beliefs about control in the physician-patient relationship: effect on communication in medical encounters. J Gen Intern Med 2003;18:609–16.
33. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
34. Mechanic D. Changing medical organization and the erosion of trust. Milbank Q 1996;74:171–89.
35. Mechanic D, Schlesinger M. The impact of managed care on patients’ trust in medical care and their physicians. JAMA 1996;275:1693–7.
36. Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. J Gen Intern Med 2000;15:509–13.
37. Jones DE, Carson KA, Bleich SN, Cooper LA. Patient trust in physicians and adoption of lifestyle behaviors to control high blood pressure. Patient Educ Couns 2012;89:57–62.
38. Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. J Acquired Immun Def Syndr Hum Retrovir 1998;18:341–8.
39. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583–9.
40. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
41. Aikens JE, Bingham R, Piette JD. Patient-provider communication and self-care behavior among type 2 diabetes patients. Diabetes Educ 2005;31:681–90.
42. Bundesmann R, Kaplowitz SA. Provider communication and patient participation in diabetes self-care. Patient Educ Couns 2011;85:143–7.
43. Heisler M, Cole I, Weir D, et al. Does physician communication influence older patients’ diabetes self-management and glycemic control? Results from the Health and Retirement Study (HRS). J Gerontol A Biol Sci Med Sci 2007;62:1435–42.
44. Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med 2004;79:495–507.
45. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
46. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med 2006;63:3067–79.
47. Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
48. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diab Med 2005;22:1379–85.
49. DiMatteo MR, Linn LS, Chang BL, Cope DW. Affect and neutrality in physician behavior: a study of patients’ values and satisfaction. J Behav Med 1985;8:397–409.
50. Stuckey HL, Vallis M, Kovacs Burns K, et al. “I do my best to listen to patients”: qualitative insights into DAWN2 (Diabetes Psychosocial Care From the Perspective of Health Care Professionals in the Second Diabetes Attitudes, Wishes and Needs Study). Clin Ther 2015;37:1986–98.
51. Kruse RL, Olsberg JE, Oliver DP, et al. Patient-provider communication about diabetes self-care activities. Fam Med 2013;45:319–22.
52. Beverly EA, Hultgren BA, Brooks KM, et al. Understanding physicians’ challenges when treating type 2 diabetic patients’ social and emotional difficulties: a qualitative study. Diabetes Care 2011;34:1086–8.
53. Freeman J, Loewe R. Barriers to communication about diabetes mellitus. Patients’ and physicians’ different view of the disease. J Fam Pract 2000;49:507–12.
54. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia 2008;51:1822–5.
55. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–64.
56. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60.
57. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabetic Med 2003;20:69–72.
58. Weinger K, Kinsley BT, Bajaj M, et al. Diabetes-related emotional distress: A barrier to improving glycemic control during intensive diabetes treatment. Abstract. Diabetes 1997;46:Supp1:268A.
59. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns 2001;42:123–31.
60. Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care 2000;23:267–72.
61. Wen LK, Parchman ML, Shepherd MD. Family support and diet barriers among older Hispanic adults with type 2 diabetes. Fam Med 2004;36:423–30.
62. Chesla CA, Fisher L, Mullan JT, et al. Family and disease management in African-American patients with type 2 diabetes. Diabetes Care 2004;27:2850–5.
63. Brown SA, Harrist RB, Villagomez ET, et al. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ 2000;26:425–38.
64. Fisher L, Chesla CA, Chun KM, et al. Patient-appraised couple emotion management and disease management among Chinese American patients with type 2 diabetes. J Fam Psychol 2004;18:302–10.
65. Akimoto M, Fukunishi I, Kanno K, et al. Psychosocial predictors of relapse among diabetes patients: a 2-year follow-up after inpatient diabetes education. Psychosomatics 2004;45:343–9.
66. Samuel-Hodge CD, Headen SW, Skelly AH, et al. Influences on day-to-day self-management of type 2 diabetes among African-American women: spirituality, the multi-caregiver role, and other social context factors. Diabetes Care 2000;23:928–33.
67. Wing RR, Marcus MD, Epstein LH, Jawad A. A “family-based” approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156–62.
68. Trief PM, Ploutz-Snyder R, Britton KD, Weinstock RS. The relationship between marital quality and adherence to the diabetes care regimen. Ann Behav Med 2004;27:148–54.
69. Gleeson-Kreig J, Bernal H, Woolley S. The role of social support in the self-management of diabetes mellitus among a Hispanic population. Pub Health Nurs 2002;19:215–22.
70. Wen LK, Shepherd MD, Parchman ML. Family support, diet, and exercise among older Mexican Americans with type 2 diabetes. Diabetes Educ 2004;30:980–93.
71. Ruggiero L. Helping people with diabetes change behavior: from theory to practice. Diab Spectrum 2000;13:125–32.
72. Orr DP, Golden MP, Myers G, Marrero DG. Characteristics of adolescents with poorly controlled diabetes referred to a tertiary care center. Diabetes Care 1983;6:170–5.
73. Lane JD, Stabler B, Ross SL, et al. Psychological predictors of glucose control in patients with IDDM. Diabetes Care 1988;11:798–800.
74. Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychology 1992;11:135–8.
75. Irvine A, Cox D, Gonder-Frederick L. The fear of hypoglycemia scale. In: Bradley C, editor. Handbook of psychology and diabetes. Harwood Academic; 1994.
76. Peyrot MF, McMurry JF Jr. Stress buffering and glycemic control. The role of coping styles. Diabetes Care 1992;15:842–6.
77. Peyrot M, McMurry JF Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav 1999;40:141–58.
78. Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107.
79. Sohal S, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PloS One 2015:1–15.
80. Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003;163:83–90.
81. Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002.
82. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
83. Jones AJ, Gladstone BP, Lubeck M, et al. Motivational interventions in the management of HbA1c levels: A systematic review and meta-analysis. Prim Care Diabetes 2014;8:91–100.
84. Song D, Xu TZ, Sun QH. Effect of motivational interviewing on self-management in patients with type 2 diabetes mellitus: a meta-analysis. Intl J Nurs Sci 2014;1:291–7.
85. Clifford Mulimba A, Byron-Daniel J. Motivational interviewing-based interventions and diabetes mellitus. Br J Nurs 2014;23:8–14.
86. Noordman J, van der Weijden T, van Dulmen S. Communication-related behavior change techniques used in face-to-face lifestyle interventions in primary care: a systematic review of the literature. Patient Educ Couns 2012;89:227–44.
87. Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns 2011;84:16–26.
88. Kang SH, Kim BG, Lee GM. Justification of continuous packed-bed reactor for retroviral vector production from amphotropic PsiCRIP murine producer cell. Cytotechnology 2000;34(1–2):151–8.
89. Holmen H, Torbjornsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth 2014;2(4):e57.
90. Chlebowy DO, El-Mallakh P, Myers J, et al. Motivational interviewing to improve diabetes outcomes in African Americans adults with diabetes. West J Nurs Res 2015;37:566–80.
91. Kang HY, Gu MO. [Development and effects of a motivational interviewing self-management program for elderly patients with diabetes mellitus]. J Kor Acad Nurs 2015;45:533–43.
92. Brobeck E, Odencrants S, Bergh H, Hildingh C. Patients’ experiences of lifestyle discussions based on motivational interviewing: a qualitative study. BMC Nursing 2014;13:13.
93. Loskutova NY, Tsai AG, Fisher EB, et al. Patient navigators connecting patients to community resources to improve diabetes outcomes. J Am Board Fam Med 2016;29:78–89.
94. Raiff BR, Barry VB, Ridenour TA, Jitnarin N. Internet-based incentives increase blood glucose testing with a non-adherent, diverse sample of teens with type 1 diabetes mellitus: a randomized controlled Trial. Trans Behav Med 2016;6:179–88.
95. Sahlen KG, Johansson H, Nystrom L, Lindholm L. Health coaching to promote healthier lifestyle among older people at moderate risk for cardiovascular diseases, diabetes and depression: a study protocol for a randomized controlled trial in Sweden. BMC Public Health 2013;13:199.
96. Kane EP, Collinsworth AW, Schmidt KL, et al. Improving diabetes care and outcomes with community health workers. Fam Pract 2016;33:523–8.
97. Wagner JA, Bermudez-Millan A, Damio G, et al. A randomized, controlled trial of a stress management intervention for Latinos with type 2 diabetes delivered by community health workers: Outcomes for psychological wellbeing, glycemic control, and cortisol. Diabetes Res Clin Pract 2016;120:162–70.
98. Rogers EA, Hessler DM, Bodenheimer TS, et al. Diabetes peer coaching: do “better patients” make better coaches? Diabetes Educ 2014;40:107–15.
99. Long JA, Jahnle EC, Richardson DM, et al. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med 2012;156:416–24.
100. Mundt MP, Agneessens F, Tuan WJ, et al. Primary care team communication networks, team climate, quality of care, and medical costs for patients with diabetes: A cross-sectional study. Int J Nurs Stud 2016;58:1–11.
101. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–8.
102. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–6.
103. Ricci-Cabello I, Olry de Labry-Lima A, Bolivar-Munoz J, et al. Effectiveness of two interventions based on improving patient-practitioner communication on diabetes self-management in patients with low educational level: study protocol of a clustered randomized trial in primary care. BMC Health Serv Res 2013;13:433.
104. Billimek J, Guzman H, Angulo MA. Effectiveness and feasibility of a software tool to help patients communicate with doctors about problems they face with their medication regimen (EMPATHy): study protocol for a randomized controlled trial. Trials 2015;16:145.
105. Grant RW, Uratsu CS, Estacio KR, et al. Pre-Visit Prioritization for complex patients with diabetes: Randomized trial design and implementation within an integrated health care system. Contemp Clin Trials 2016;47:196–201.
106. Ritholz MD, Beverly EA, Abrahamson MJ, et al. Physicians’ perceptions of the type 2 diabetes multidisciplinary treatment team: a qualitative study. Diabetes Educ 2011.
107. Ley P. Satisfaction, compliance and communication. Br J Clin Psychol 1982;21:241–54.
108. Weinger K, Smaldone A, Beverly EA. Psychosocial and educational implications of diabetic foot complications. In: Veves A, Giurini JM, LoGerfo FW, editors. The diabetic foot: medical and surgical management. Boston: Springer; 2012:503–18.
109. Welch G, Rose G, Ernst D. Motivational interviewing and diabetes: what is it, how is it used, and does it work? Diabetes Spectr 2006;19:5–11.
110. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 1989;27(3 Suppl):S110–27.
111. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 2002;25:1722–8.
112. Wens J, Vermeire E, Royen PV, et al. GPs’ perspectives of type 2 diabetes patients’ adherence to treatment: A qualitative analysis of barriers and solutions. BMC Fam Pract 2005;6:20.
1. Anderson E, Kian EM. Examining media contestation of masculinity and head trauma in the National Football League. J Men Masculinities 2011;1–22.
2. CDC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.
3. Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986.
4. Jacobson AM, Adler AG, Derby L, et al. Clinic attendance and glycemic control. Study of contrasting groups of patients with IDDM. Diabetes Care 1991;14:599–601.
5. Dyer PH, Lloyd CE, Lancashire RJ, et al. Factors associated with clinic non-attendance in adults with type 1 diabetes mellitus. Diab Med 1998;15:339–43.
6. Conn VS, Hafdahl AR, Mehr DR, et al. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia 2007;50:913–21.
7. Maiorana A, O’Driscoll G, Goodman C, et al. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diab Res Clin Pract 2002;56:115–23.
8. Pi-Sunyer FX, Maggio CA, McCarron DA, et al. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care 1999;22:191–7.
9. Delahanty LM, Halford BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care 1993;16:1453–8.
10. Gafarian CT, Heiby EM, Blair P, Singer F. The Diabetes Time Management Questionnaire. Diabetes Educ 1999;25:585–92.
11. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ 1997;23:558–62.
12. Rubin RR, Peyrot M. Psychological issues and treatment for people with diabetes. J Clin Psychol 2001;57:457–78.
13. Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999-2010. N Engl J Med 2013;369:287–8.
14. Beverly EA, Ganda OP, Ritholz MD, et al. Look who’s (not) talking: diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care 2012;35:1466–72.
15. Heisler M, Vijan S, Anderson RM, et al. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med 2003;18:893–902.
16. Greenfield S, Kaplan SH, Ware JE Jr, et al. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med 1988;3:448–57.
17. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med 1985;102:520–8.
18. Anderson RM, Funnell MM, Butler PM, et al. Patient empowerment. Results of a randomized controlled trial. Diabetes Care 1995;18:943–9.
19. Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med 1997;127:1097–102.
20. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high quality care in English general practice: observational study. BMJ 2001;323:784–7.
21. Bower P, Campbell S, Bojke C, Sibbald B. Team structure, team climate and the quality of care in primary care: an observational study. Qual Saf Health Care 2003;12:273–9.
22. Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med 2003;18:624-33.
23. Kerr EA, Smith DM, Kaplan SH, Hayward RA. The association between three different measures of health status and satisfaction among patients with diabetes. Med Care Res Rev 2003;60:158-77.
24. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6-8.
25. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366:780–1.
26. Truog RD. Patients and doctors--evolution of a relationship. N Engl J Med 2012;366:581–5.
27. Beverly EA, Wray LA, LaCoe CL, Gabbay R. Listening to older adults’ values and preferences for type 2 diabetes care: a qualitative study. Diabetes Spectrum 2014;27:44–9.
28. Bordin ES. The generalizability of the psychoanalytic concept of the working alliance. Psychotherapy 1979;26:252–60.
29. Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry 2001;158:29–35.
30. Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med 2004;66:720–8.
31. Jahng KH, Martin LR, Golin CE, DiMatteo MR. Preferences for medical collaboration: patient-physician congruence and patient outcomes. Patient Educ Couns 2005;57:308–14.
32. Street RL Jr, Krupat E, Bell RA, et al. Beliefs about control in the physician-patient relationship: effect on communication in medical encounters. J Gen Intern Med 2003;18:609–16.
33. Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 2002;17:243–52.
34. Mechanic D. Changing medical organization and the erosion of trust. Milbank Q 1996;74:171–89.
35. Mechanic D, Schlesinger M. The impact of managed care on patients’ trust in medical care and their physicians. JAMA 1996;275:1693–7.
36. Pearson SD, Raeke LH. Patients’ trust in physicians: many theories, few measures, and little data. J Gen Intern Med 2000;15:509–13.
37. Jones DE, Carson KA, Bleich SN, Cooper LA. Patient trust in physicians and adoption of lifestyle behaviors to control high blood pressure. Patient Educ Couns 2012;89:57–62.
38. Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. J Acquired Immun Def Syndr Hum Retrovir 1998;18:341–8.
39. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583–9.
40. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
41. Aikens JE, Bingham R, Piette JD. Patient-provider communication and self-care behavior among type 2 diabetes patients. Diabetes Educ 2005;31:681–90.
42. Bundesmann R, Kaplowitz SA. Provider communication and patient participation in diabetes self-care. Patient Educ Couns 2011;85:143–7.
43. Heisler M, Cole I, Weir D, et al. Does physician communication influence older patients’ diabetes self-management and glycemic control? Results from the Health and Retirement Study (HRS). J Gerontol A Biol Sci Med Sci 2007;62:1435–42.
44. Duffy FD, Gordon GH, Whelan G, et al. Assessing competence in communication and interpersonal skills: the Kalamazoo II report. Acad Med 2004;79:495–507.
45. Ritholz MD, Beverly EA, Brooks KM, et al. Barriers and facilitators to self-care communication during medical appointments in the United States for adults with type 2 diabetes. Chronic Illn 2014;10:303–13.
46. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med 2006;63:3067–79.
47. Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–86.
48. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diab Med 2005;22:1379–85.
49. DiMatteo MR, Linn LS, Chang BL, Cope DW. Affect and neutrality in physician behavior: a study of patients’ values and satisfaction. J Behav Med 1985;8:397–409.
50. Stuckey HL, Vallis M, Kovacs Burns K, et al. “I do my best to listen to patients”: qualitative insights into DAWN2 (Diabetes Psychosocial Care From the Perspective of Health Care Professionals in the Second Diabetes Attitudes, Wishes and Needs Study). Clin Ther 2015;37:1986–98.
51. Kruse RL, Olsberg JE, Oliver DP, et al. Patient-provider communication about diabetes self-care activities. Fam Med 2013;45:319–22.
52. Beverly EA, Hultgren BA, Brooks KM, et al. Understanding physicians’ challenges when treating type 2 diabetic patients’ social and emotional difficulties: a qualitative study. Diabetes Care 2011;34:1086–8.
53. Freeman J, Loewe R. Barriers to communication about diabetes mellitus. Patients’ and physicians’ different view of the disease. J Fam Pract 2000;49:507–12.
54. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia 2008;51:1822–5.
55. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–64.
56. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60.
57. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabetic Med 2003;20:69–72.
58. Weinger K, Kinsley BT, Bajaj M, et al. Diabetes-related emotional distress: A barrier to improving glycemic control during intensive diabetes treatment. Abstract. Diabetes 1997;46:Supp1:268A.
59. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns 2001;42:123–31.
60. Fisher L, Chesla CA, Skaff MM, et al. The family and disease management in Hispanic and European-American patients with type 2 diabetes. Diabetes Care 2000;23:267–72.
61. Wen LK, Parchman ML, Shepherd MD. Family support and diet barriers among older Hispanic adults with type 2 diabetes. Fam Med 2004;36:423–30.
62. Chesla CA, Fisher L, Mullan JT, et al. Family and disease management in African-American patients with type 2 diabetes. Diabetes Care 2004;27:2850–5.
63. Brown SA, Harrist RB, Villagomez ET, et al. Gender and treatment differences in knowledge, health beliefs, and metabolic control in Mexican Americans with type 2 diabetes. Diabetes Educ 2000;26:425–38.
64. Fisher L, Chesla CA, Chun KM, et al. Patient-appraised couple emotion management and disease management among Chinese American patients with type 2 diabetes. J Fam Psychol 2004;18:302–10.
65. Akimoto M, Fukunishi I, Kanno K, et al. Psychosocial predictors of relapse among diabetes patients: a 2-year follow-up after inpatient diabetes education. Psychosomatics 2004;45:343–9.
66. Samuel-Hodge CD, Headen SW, Skelly AH, et al. Influences on day-to-day self-management of type 2 diabetes among African-American women: spirituality, the multi-caregiver role, and other social context factors. Diabetes Care 2000;23:928–33.
67. Wing RR, Marcus MD, Epstein LH, Jawad A. A “family-based” approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156–62.
68. Trief PM, Ploutz-Snyder R, Britton KD, Weinstock RS. The relationship between marital quality and adherence to the diabetes care regimen. Ann Behav Med 2004;27:148–54.
69. Gleeson-Kreig J, Bernal H, Woolley S. The role of social support in the self-management of diabetes mellitus among a Hispanic population. Pub Health Nurs 2002;19:215–22.
70. Wen LK, Shepherd MD, Parchman ML. Family support, diet, and exercise among older Mexican Americans with type 2 diabetes. Diabetes Educ 2004;30:980–93.
71. Ruggiero L. Helping people with diabetes change behavior: from theory to practice. Diab Spectrum 2000;13:125–32.
72. Orr DP, Golden MP, Myers G, Marrero DG. Characteristics of adolescents with poorly controlled diabetes referred to a tertiary care center. Diabetes Care 1983;6:170–5.
73. Lane JD, Stabler B, Ross SL, et al. Psychological predictors of glucose control in patients with IDDM. Diabetes Care 1988;11:798–800.
74. Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychology 1992;11:135–8.
75. Irvine A, Cox D, Gonder-Frederick L. The fear of hypoglycemia scale. In: Bradley C, editor. Handbook of psychology and diabetes. Harwood Academic; 1994.
76. Peyrot MF, McMurry JF Jr. Stress buffering and glycemic control. The role of coping styles. Diabetes Care 1992;15:842–6.
77. Peyrot M, McMurry JF Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav 1999;40:141–58.
78. Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107.
79. Sohal S, Sohal P, King-Shier KM, Khan NA. Barriers and facilitators for type-2 diabetes management in South Asians: a systematic review. PloS One 2015:1–15.
80. Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003;163:83–90.
81. Miller WR, Rollnick S. Motivational interviewing: preparing people for change. New York: Guilford Press; 2002.
82. Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–30.
83. Jones AJ, Gladstone BP, Lubeck M, et al. Motivational interventions in the management of HbA1c levels: A systematic review and meta-analysis. Prim Care Diabetes 2014;8:91–100.
84. Song D, Xu TZ, Sun QH. Effect of motivational interviewing on self-management in patients with type 2 diabetes mellitus: a meta-analysis. Intl J Nurs Sci 2014;1:291–7.
85. Clifford Mulimba A, Byron-Daniel J. Motivational interviewing-based interventions and diabetes mellitus. Br J Nurs 2014;23:8–14.
86. Noordman J, van der Weijden T, van Dulmen S. Communication-related behavior change techniques used in face-to-face lifestyle interventions in primary care: a systematic review of the literature. Patient Educ Couns 2012;89:227–44.
87. Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns 2011;84:16–26.
88. Kang SH, Kim BG, Lee GM. Justification of continuous packed-bed reactor for retroviral vector production from amphotropic PsiCRIP murine producer cell. Cytotechnology 2000;34(1–2):151–8.
89. Holmen H, Torbjornsen A, Wahl AK, et al. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth 2014;2(4):e57.
90. Chlebowy DO, El-Mallakh P, Myers J, et al. Motivational interviewing to improve diabetes outcomes in African Americans adults with diabetes. West J Nurs Res 2015;37:566–80.
91. Kang HY, Gu MO. [Development and effects of a motivational interviewing self-management program for elderly patients with diabetes mellitus]. J Kor Acad Nurs 2015;45:533–43.
92. Brobeck E, Odencrants S, Bergh H, Hildingh C. Patients’ experiences of lifestyle discussions based on motivational interviewing: a qualitative study. BMC Nursing 2014;13:13.
93. Loskutova NY, Tsai AG, Fisher EB, et al. Patient navigators connecting patients to community resources to improve diabetes outcomes. J Am Board Fam Med 2016;29:78–89.
94. Raiff BR, Barry VB, Ridenour TA, Jitnarin N. Internet-based incentives increase blood glucose testing with a non-adherent, diverse sample of teens with type 1 diabetes mellitus: a randomized controlled Trial. Trans Behav Med 2016;6:179–88.
95. Sahlen KG, Johansson H, Nystrom L, Lindholm L. Health coaching to promote healthier lifestyle among older people at moderate risk for cardiovascular diseases, diabetes and depression: a study protocol for a randomized controlled trial in Sweden. BMC Public Health 2013;13:199.
96. Kane EP, Collinsworth AW, Schmidt KL, et al. Improving diabetes care and outcomes with community health workers. Fam Pract 2016;33:523–8.
97. Wagner JA, Bermudez-Millan A, Damio G, et al. A randomized, controlled trial of a stress management intervention for Latinos with type 2 diabetes delivered by community health workers: Outcomes for psychological wellbeing, glycemic control, and cortisol. Diabetes Res Clin Pract 2016;120:162–70.
98. Rogers EA, Hessler DM, Bodenheimer TS, et al. Diabetes peer coaching: do “better patients” make better coaches? Diabetes Educ 2014;40:107–15.
99. Long JA, Jahnle EC, Richardson DM, et al. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med 2012;156:416–24.
100. Mundt MP, Agneessens F, Tuan WJ, et al. Primary care team communication networks, team climate, quality of care, and medical costs for patients with diabetes: A cross-sectional study. Int J Nurs Stud 2016;58:1–11.
101. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–8.
102. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–6.
103. Ricci-Cabello I, Olry de Labry-Lima A, Bolivar-Munoz J, et al. Effectiveness of two interventions based on improving patient-practitioner communication on diabetes self-management in patients with low educational level: study protocol of a clustered randomized trial in primary care. BMC Health Serv Res 2013;13:433.
104. Billimek J, Guzman H, Angulo MA. Effectiveness and feasibility of a software tool to help patients communicate with doctors about problems they face with their medication regimen (EMPATHy): study protocol for a randomized controlled trial. Trials 2015;16:145.
105. Grant RW, Uratsu CS, Estacio KR, et al. Pre-Visit Prioritization for complex patients with diabetes: Randomized trial design and implementation within an integrated health care system. Contemp Clin Trials 2016;47:196–201.
106. Ritholz MD, Beverly EA, Abrahamson MJ, et al. Physicians’ perceptions of the type 2 diabetes multidisciplinary treatment team: a qualitative study. Diabetes Educ 2011.
107. Ley P. Satisfaction, compliance and communication. Br J Clin Psychol 1982;21:241–54.
108. Weinger K, Smaldone A, Beverly EA. Psychosocial and educational implications of diabetic foot complications. In: Veves A, Giurini JM, LoGerfo FW, editors. The diabetic foot: medical and surgical management. Boston: Springer; 2012:503–18.
109. Welch G, Rose G, Ernst D. Motivational interviewing and diabetes: what is it, how is it used, and does it work? Diabetes Spectr 2006;19:5–11.
110. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 1989;27(3 Suppl):S110–27.
111. Nelson KM, Reiber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 2002;25:1722–8.
112. Wens J, Vermeire E, Royen PV, et al. GPs’ perspectives of type 2 diabetes patients’ adherence to treatment: A qualitative analysis of barriers and solutions. BMC Fam Pract 2005;6:20.