User login
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
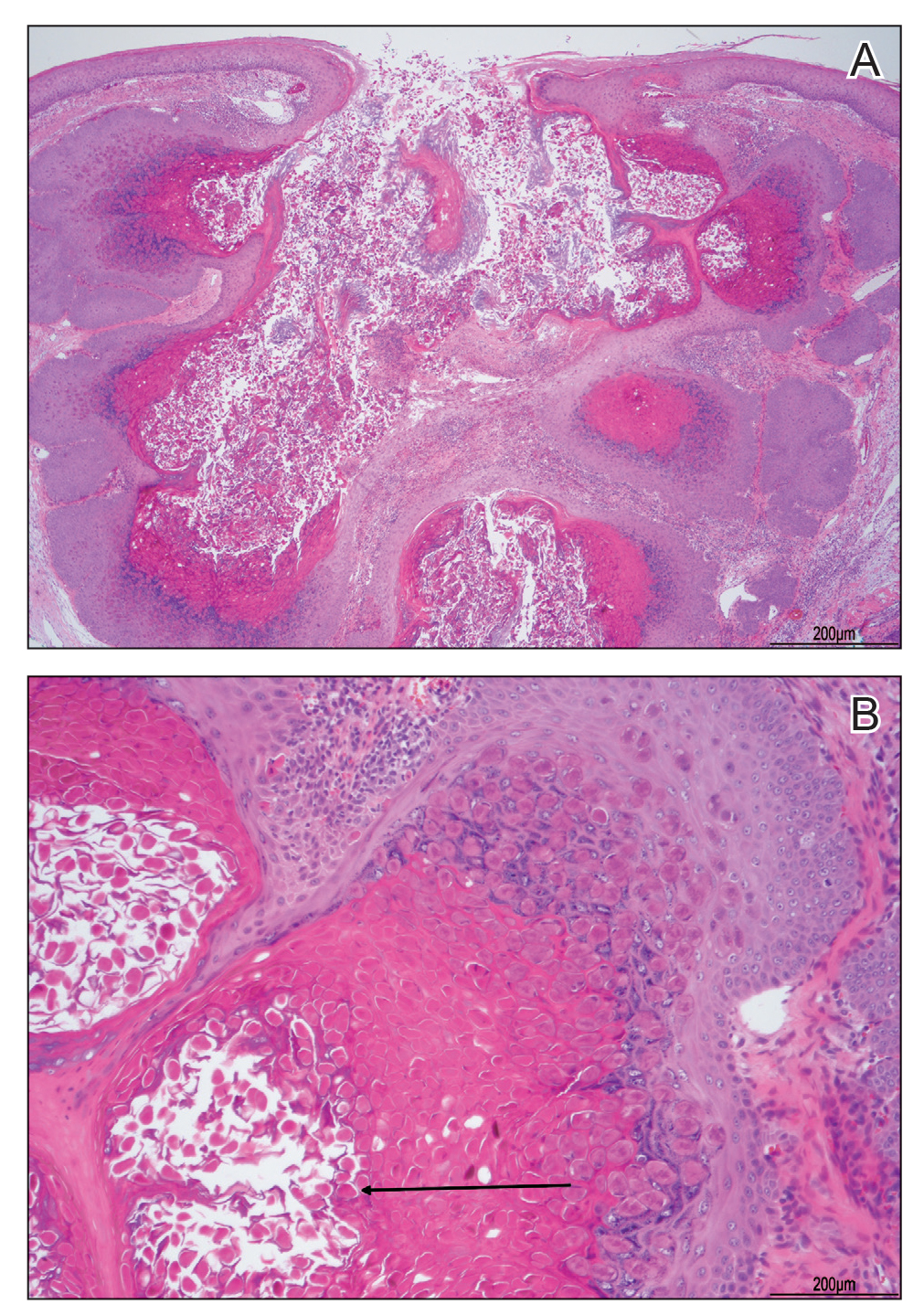
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
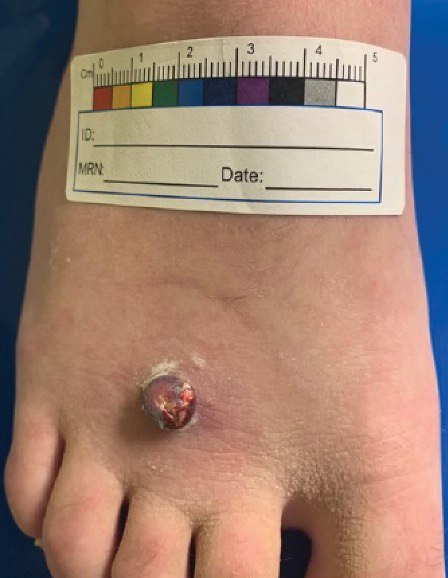
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
Painful ulcers on gingiva, tongue, and buccal mucosa
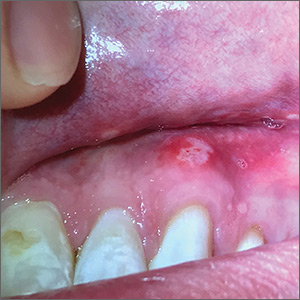
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; rbrodell@umc.edu
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; rbrodell@umc.edu
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; rbrodell@umc.edu
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.