User login
Diagnostic value of the physical examination in patients with dyspnea
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
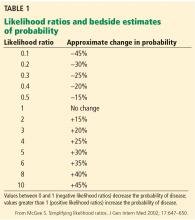
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
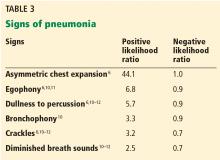
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
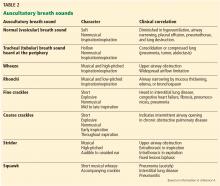
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
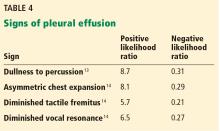
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
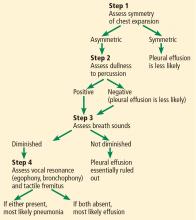
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
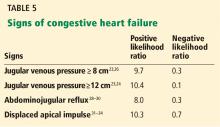
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
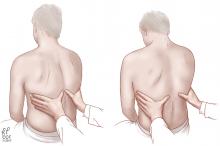
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
KEY POINTS
- Asymmetrical chest expansion, diminished breath sounds, egophony, bronchophony, and tactile fremitus can be used in combination to accurately diagnose pneumonia and pleural effusion.
- No physical sign performs with a high degree of accuracy for diagnosing early-stage chronic obstructive pulmonary disease.
- Inspiratory crackles, diminished breath sounds, and cardiac dullness have high diagnostic value for advanced obstructive airway disease.
- Congestive heart failure can be diagnosed at the bedside by examining the jugular veins and palpating the point of maximal intensity.