User login
Circumscribed Nodule in a Renal Transplant Patient
The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
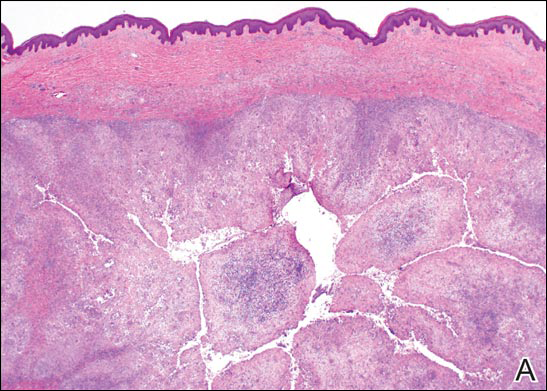
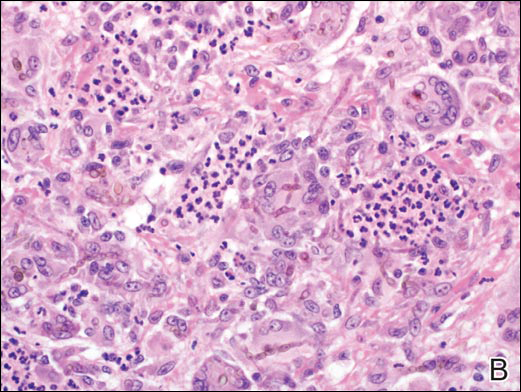
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.
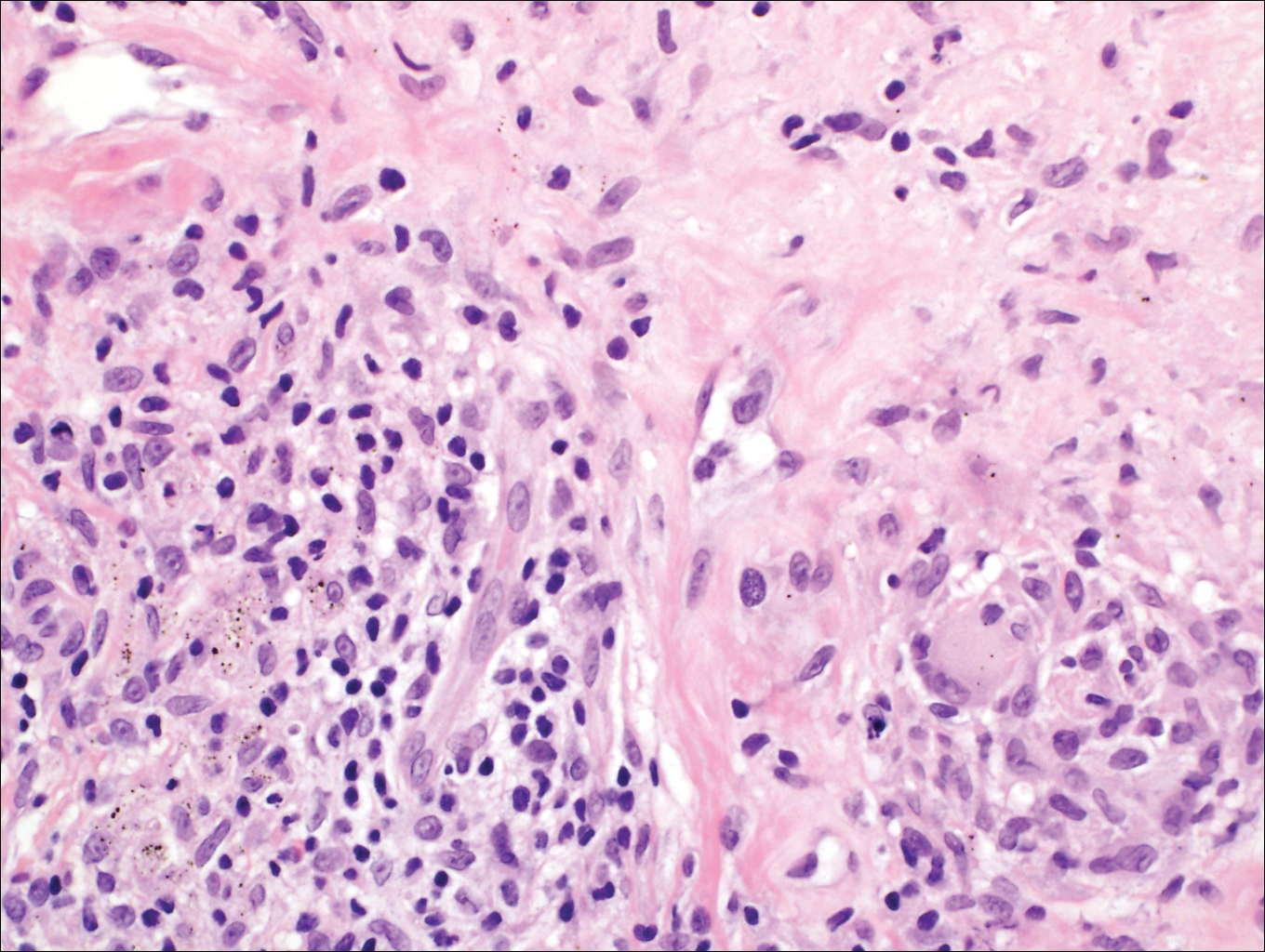
Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.

Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.

Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 63-year-old man on immunosuppressive therapy following renal transplantation 5 years prior presented with a nontender circumscribed nodule above the left knee of 6 months’ duration. The patient denied any trauma or injury to the site.