User login
Vulvar and gluteal manifestations of Crohn disease
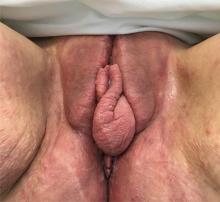
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
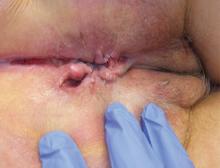
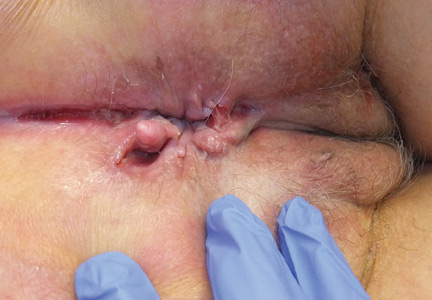
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
Women’s health 2019: Osteoporosis, breast cancer, contraception, and hormone therapy
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
KEY POINTS
- The US Preventive Services Task Force recommends screening bone density when the 10-year risk of major osteoporotic fracture is more than 8.4%.
- Women can be reassured that nonrifamycin antibiotics are unlikely to reduce efficacy of hormonal contraception.
- Hormone replacement therapy after prophylactic bilateral salpingo-oophorectomy does not increase breast cancer risk in women who carry the BRCA1 gene mutation.
- Hormonal contraception may increase the risk of breast cancer by 1 extra case per 7,690 women, although most studies suggest there is no increased risk.
- The use of digital breast tomosynthesis along with digital mammography can increase cancer detection in women with dense breast tissue, but it is not yet routinely recommended by most professional societies.