User login
Osteoporosis is underdiagnosed and undertreated,1 even though it is common and causes serious problems, and even though effective treatments are available. The US Surgeon General has challenged the health care profession to close “the gap between clinical knowledge and its application in the community.”2
This review describes current shortcomings in the care of patients with osteoporosis and suggests strategies for health care providers to improve clinical outcomes.
COMMON AND SERIOUS
Approximately 44 million American men and women, representing 55% of the population age 50 and over, have osteoporosis or low bone density that can lead to fractures.2 An estimated 2 million osteoporosis-related fractures were reported in the United States in 2005, with a direct health care cost of about $17 billion.3 By 2025, more than 3 million osteoporosis-related fractures per year are expected, with an annual cost of more than $25 billion.3
Fractures of the spine and hip are associated with chronic pain, deformity, depression, disability, and death. About 50% of patients with a hip fracture are left permanently unable to walk without assistance, and 25% require long-term care.4 The death rate 5 years after a hip fracture or a clinical vertebral fracture is about 20% higher than expected.5
DIAGNOSING OSTEOPOROSIS BEFORE A FRACTURE OCCURS
World Health Organization classification
The World Health Organization6 classifies bone mineral density on the basis of the T-score, ie, the difference, in standard deviations, between the patient’s bone mineral density, measured by dual-energy x-ray absorptiometry (DXA), and the mean bone mineral density of a young adult reference population:
- Normal (a T-score of −1.0 or higher)
- Osteopenia (a T-score of less than −1.0 but higher than −2.5)
- Osteoporosis (a T-score of −2.5 or less)
- Severe osteoporosis (a T-score of −2.5 or less with a fragility fracture).
The International Society for Clinical Densitometry has established indications for bone density measurement, quality control, acquisition, analysis, interpretation, reporting, and nomenclature.7 The Society states, for example, that bone mineral density may be classified according to the lowest T-score of the lumbar spine, total hip, femoral neck, or distal one-third (33%) radius (if measured), using a white female reference database in women and a white male reference database in men.
Why test bone mineral density?
Bone density testing allows a physician to diagnose osteoporosis before a fracture occurs and to intervene early to reduce the risk of fracture. A clinical diagnosis of osteoporosis can be made in a patient who has had a fragility fracture, independently of bone mineral density, although this is less desirable than diagnosing osteoporosis before the first fracture.
While one can argue that fracture risk assessment is of greater clinical importance than diagnostic classification (ie, normal, osteopenia, osteoporosis), a diagnosis of osteoporosis conveys a clear message to the patient and health care providers about the presence of a disease that requires evaluation and treatment. Also, in the United States, diagnostic classification is necessary to select a numerical code for insurance billing and sometimes to determine eligibility for insurance coverage of drug therapy.
Osteoporosis is underdiagnosed, even after fractures
Osteoporosis is underdiagnosed.1 Data from Medicare claims for 1999 to 2000 showed that only 30% of eligible women age 65 and older had a bone density test,8 despite recognition by many organizations that fracture risk is high and DXA is indicated in this population.7,9,10
An adult with any fracture,11 even one due to trauma,12 may have osteoporosis, may be at risk of future fractures, and should be considered for further evaluation. Vertebral fractures, the most prevalent type of osteoporotic fracture, are commonly underrecognized and underreported,13,14 thereby missing an opportunity to identify and treat a patient at high risk.
Clinical vertebral fractures are those that come to clinical attention because of symptoms and then are appropriately diagnosed, while morphometric vertebral fractures are those detected by an imaging study regardless of symptoms. Only about one-third of all vertebral fractures are clinically apparent.15
In 2005, Foley et al16 reported that only 10.2% of women age 67 and older with a fracture were tested for osteoporosis within the following 6 months. Patients discharged from the hospital after hip fractures are commonly not diagnosed with or treated for osteoporosis,17,18 although the risk of future fractures is very high.19 Inpatient consultation with a medical specialist has not consistently improved osteoporosis care, with some reports of no effect17 and others suggesting a modest benefit.20,21
Many factors are responsible for underdiagnosis, and no single specialty is to blame. Primary care physicians are often overburdened with clinical, administrative, and regulatory responsibilities that leave little time to consider a silent disease that increases the risk of an event that may occur far in the future. Acute fractures are often treated by an orthopedist or emergency department specialist who is not responsible for long-term care and prevention of future fractures. The primary care physician may not become aware of the fracture until long after it has occurred.
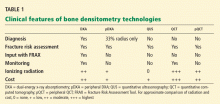
DXA is the gold standard test
Although all of these tests provide results that correlate with fracture risk, DXA is the only one that can be used for diagnostic classification7 and the only one that can be used with the Fracture Risk Assessment Tool, or FRAX (more about this below).22 DXA is also the most clinically useful way to monitor the effects of therapy, with a correlation, albeit an imperfect one, between changes in bone mineral density with therapy and reduction in fracture risk.23
For these reasons, DXA is generally considered the gold standard for measuring bone mineral density.24
Using the wrong technology for the clinical need25 or performing poor-quality testing26,27 may result in inappropriate patient care decisions and wastes limited health care resources.
Medicare coverage for DXA has been cut
Recent cuts in Medicare reimbursement for DXA in the United States have been so severe that payment is now less than the cost of providing the service at many facilities.28 With further reductions in reimbursement expected, it is projected that most outpatient DXA centers—ie, about two-thirds of all DXA facilities in the United States—will no longer be operating by 2010.29
The anticipated consequences: fewer patients will be diagnosed with osteoporosis, fewer patients will be treated, and more fractures will occur, with fracture-related health care expenses far exceeding the savings from fewer DXA tests and fewer prescriptions for drugs to reduce fracture risk. I have characterized this as a “crisis in osteoporosis care,”30 and it is in stark contrast to the mandate of the US Surgeon General to improve osteoporosis care.1
Reports from several large health care systems support the proposition that more rather than fewer patients should undergo bone density testing. Data from the Kaiser Southern California and the Geisinger Health Plan show that when more patients undergo DXA and more are treated for osteoporosis, fewer have fractures, and money is saved.31,32
STRATEGIES FOR IMPROVING DIAGNOSIS
Appoint an advocate
The first step in the early diagnosis of osteoporosis is to select appropriate patients for bone density testing by recognizing high-risk populations.
Given the many demands placed on primary care physicians, who may not be focused on osteoporosis, it may be helpful to appoint one or more office staff as “advocates” for skeletal health. This could be a medical assistant, nurse, or health care educator who is charged with alerting the physician when bone mineral density testing is needed, or who could perhaps be given the authority to order the test within prespecified parameters. Other responsibilities might include patient education on nutrition, lifestyle, fall prevention, and drug administration, and follow-up by phone or in the office to ensure compliance with therapy.
Set up a disease-management program
Changing the health care system may be a more effective way to improve clinical outcomes than changing the actions of individual physicians. Disease-management programs that institutionalize pathways of care for osteoporosis have shown promise,32,33 and postfracture intervention programs may provide an opportunity to better manage patients at very high risk of future fractures.34,35
Lobby your legislators
To assure patient access to diagnostic services for assessment of skeletal health, advocates are focusing on legislation to restore DXA reimbursement to a level that would allow outpatient DXA facilities to avoid financial losses and continue operating.28 This possibility may be aided by grassroots support from concerned physicians and from patients likely to be harmed by limited access to DXA testing because of fewer instruments in operation and greater distances to travel to reach them. The largest US patient advocate organization for osteoporosis care, the National Osteoporosis Foundation (www.nof.org), is spearheading a drive to educate legislators on the value of bone density testing and to pass corrective legislation.
ASSESSING FRACTURE RISK WITH FRAX
The patients who get the greatest reduction in fracture risk with drug therapy are those who have the highest baseline risk of fracture.6 An estimate of fracture risk is therefore important in determining which patients to treat.
While bone mineral density is an excellent predictor of fracture risk, density combined with clinical risk factors for fracture is a better predictor than density or clinical risk factors alone.
FRAX22 is an electronic clinical tool (www.shef.ac.uk/FRAX/) for calculating fracture risk on the basis of the bone mineral density of the femoral neck; the patient’s age, sex, height, and weight; and seven clinical risk factors (previous fracture, having a parent who had a hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, and ingestion of three or more units of alcohol daily). One enters this information plus the brand of DXA machine used (Hologic, GE Lunar, or Norland), and the algorithm estimates the 10-year probability of a major osteoporotic fracture (hip, spine, proximal humerus, or distal forearm), and the 10-year probability of hip fracture
The FRAX model was developed through an analysis of almost 60,000 men and women in 12 population-based cohorts with about 250,000 person-years of observation, and externally validated in an additional 11 cohorts with 230,000 men and women and more than 1.2 million person-years of observation.8 The analysis of these extraordinarily robust databases was a mammoth project undertaken by the World Health Organization, under the direction of Professor John Kanis and with the support of many other organizations and professional societies. Criteria for inclusion of a clinical risk factor in FRAX included international validation, independence from bone mineral density in predicting fractures, ease of collecting the information in clinical practice, and the potential for modification with drug therapy. Falls were not considered as clinical risk factors, since it is not clear that pharmacologic intervention can significantly change the risk of falls.
FRAX remains a work in progress, with continuing updates expected as new information becomes available on country-specific fracture rates and potential additional clinical risk factors. Future versions of FRAX may also include input from skeletal sites other than the femoral neck and bone density measurement with technologies other than DXA.
Benefits and limitations of FRAX
To use FRAX, one needs to understand its benefits and limitations.
Benefits. FRAX can be used to estimate fracture risk in untreated women and men from age 40 to 90,22 although the National Osteoporosis Foundation guidelines recommend that it be used to make treatment decisions only in untreated postmenopausal women and men age 50 and older with osteopenia who do not otherwise qualify for treatment.9
Expressing fracture risk as a 10-year probability is more clinically useful than expressing it as a relative risk. For example, if the relative risk of fracture is five times that of a comparator population in which the risk is close to zero, then the patient’s risk is low, although a physician might feel compelled to treat upon learning that the relative risk is 5. A 50-year-old woman and an 80-year-old woman with identical T-scores of −2.5 have the same relative risk of fracture,36 even though the 10-year probability of fracture is far greater for the older woman.37
Limitations. FRAX has not been validated in treated patients, in women and men outside the specified age range, or in children. In the United States, the use of FRAX is limited to four ethnic groups—white, black, Hispanic, and Asian. FRAX has not been validated in patients of mixed ethnicity or of other ethnic groups in the United States.
The seven clinical risk factors in FRAX are entered as yes-or-no responses, whereas the actual risk in an individual patient may depend on the dose or severity of the risk factor. For example, a patient who was treated with the glucocorticoid prednisone 5 mg per day for 4 months many years ago has a much lower risk than a similar patient who has been taking prednisone 10 mg per day for the past 10 years, even though the FRAX input (“yes” for glucocorticoid therapy) is the same and the FRAX estimation of fracture risk is the same.
Only the bone mineral density in the femoral neck is used in FRAX, although in some patients, the density at another skeletal site may be better correlated with fracture risk (eg, low lumbar spine density may be associated with high fracture risk even when femoral neck density is not low).
Other important risk factors, such as falling, rate of bone loss, and high bone turnover are not part of the FRAX algorithm.
These limitations of FRAX may lead to overestimation or underestimation of actual fracture risk when used in some clinical circumstances, with an uncertain range of error for the calculated 10-year fracture probability.
Using FRAX appropriately
Clinicians must recognize when FRAX is likely to overestimate or underestimate fracture risk (see above).
FRAX also requires appropriate patient selection and a thorough understanding of its role in patient care decisions. Although it can be used to estimate fracture risk for a postmenopausal woman with osteoporosis, this use is not necessary and may be confusing; the National Osteoporosis Foundation guidelines recommend treatment for such a patient regardless of what FRAX says, while the FRAX calculation might result in a value that is below the treatment threshold.
Since the main clinical utility of FRAX is to help in making treatment decisions, strategies for using FRAX in the United States are discussed in association with the National Osteoporosis Foundation treatment guidelines in the section that follows.
NATIONAL OSTEOPOROSIS FOUNDATION GUIDELINES FOR TREATMENT
Many medical organizations have issued clinical practice guidelines for treating osteoporosis, with some recommendations that differ and therefore confuse more than enlighten.38
In an effort to unify these disparate recommendations, the National Osteoporosis Foundation, with the support and endorsement of numerous professional societies, developed the Clinician’s Guide to Prevention and Treatment of Osteoporosis.9 This document addresses postmenopausal women and men age 50 and older of all ethnic groups in the United States and is intended for use by clinicians in making decisions in the care of individual patients. The recommendations should not be taken as rigid standards of practice but rather as a framework for making clinical decisions with consideration of the needs of each individual patient.
Recommendations for all patients
- A daily intake of elemental calcium of at least 1,200 mg with diet plus supplements, if needed (with no more than 500–600 mg of calcium supplementation in a single dose due to limited absorption of higher doses)
- Vitamin D3 800–1,000 IU per day, with more needed in some patients to bring the serum 25-hydroxyvitamin D to a desirable level of 30 ng/mL (75 nmol/L) or higher
- Regular weight-bearing exercise
- Fall prevention
- Avoidance of tobacco use and excessive alcohol intake.
Who should be tested?
The National Osteoporosis Foundation recommends bone density testing in patients at risk of osteoporosis according to indications that are almost identical to those of the International Society for Clinical Densitometry, ie:
- Women age 65 and older
- Postmenopausal women under age 65 with risk factors for fracture
- Women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or use of high-risk drugs such as glucocorticoids
- Men age 70 and older
- Men under age 70 with clinical risk factors for fracture
- Adults with a fragility fracture
- Adults with a disease or condition associated with low bone mass or bone loss
- Adults taking drugs associated with low bone mass or bone loss
- Anyone being considered for pharmacologic therapy
- Anyone being treated, to monitor treatment effect
- Anyone not receiving therapy in whom evidence of bone loss would lead to treatment.
Women discontinuing estrogen should be considered for bone density testing according to the indications listed above.
All patients with osteoporosis should have a skeletal-related history and physical examination, with appropriate laboratory testing to evaluate for contributing factors.
Who should be treated?
The National Osteoporosis Foundation recommends considering starting drug therapy in postmenopausal women and men age 50 and older who have any of the following:
- A hip or vertebral (clinical or morphometric) fracture
- A T-score of −2.5 or less at the femoral neck or spine after appropriate evaluation to exclude secondary causes
- Low bone mass (a T-score between −1.0 and −2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of 3% or more or a 10-year probability of a major osteoporosis-related fracture of 20% or more, based on the US version of FRAX (patient preferences may indicate treatment for people with 10-year fracture probabilities above or below these levels).
Economic assumptions behind the guidelines
The National Osteoporosis Foundation based its recommendations on cost-effectiveness modeling39 and the US version of FRAX.40 The fracture risk algorithm was calibrated to US fracture and death rates, with economic assumptions that included the following: 5 years of drug therapy with 100% compliance and persistent use of a drug that costs $600 per year and results in a 35% reduction in fracture risk, followed by discontinuation of the drug associated with a linear offset of effect over the next 5 years, with a societal willingness to pay up to $60,000 per quality-adjusted life-year gained.39
FRAX is used to decide on treatment only in those with osteopenia
FRAX is used for making treatment decisions only in patients with osteopenia. Those with a densitometric diagnosis of osteoporosis according to a T-score value of −2.5 or less or a clinical diagnosis of osteoporosis by virtue of having had a fracture of the hip or spine should be treated regardless of FRAX, and those with normal T-scores are not recommended for treatment regardless of FRAX. By providing a quantitative estimation of fracture probability, FRAX allows clinicians to distinguish patients with osteopenia who are at high fracture risk from those who are not, and thereby to treat those most likely to benefit.
Since approximately one-half of patients who have fragility fractures do not have T-scores in the osteoporosis range,41,42 there is great clinical utility in identifying the subset of osteopenic patients who are candidates for treatment. It is likely that the use of FRAX will result in fewer early postmenopausal women and more older women with osteopenia being treated with drugs, since age is an important risk factor for fracture that is independent of bone mineral density.
Which drugs to use?
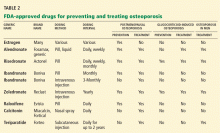
The drugs currently approved in the United States for preventing and treating osteoporosis are:
- Alendronate (Fosamax)
- Risedronate (Actonel)
- Ibandronate (Boniva)
- Zoledronate (Reclast)
- Salmon calcitonin (Miacalcin, Fortical)
- Raloxifene (Evista)
- Teriparatide (Forteo) (Table 2).
It is not known with certainty whether any of these drugs is more effective than any other, because no head-to-head clinical trials have been done using fracture as the primary end point.
Selecting a drug requires assessing its benefits and risks for each patient. The National Osteoporosis Foundation’s intervention thresholds do not consider nonskeletal benefits and risks, such as reduction in the risk of invasive breast cancer and increase in the risk of thromboembolic events with raloxifene.
For patients on glucocorticoids
Chronic glucocorticoid therapy is a special category of fracture risk. Rapid bone loss can occur at the start of therapy43 and the adverse effects on bone strength are at least partially independent of bone mineral density.44 US Food and Drug Administration indications for drugs for prevention and treatment of glucocorticoid-induced osteoporosis are distinct from those for postmenopausal osteoporosis and osteoporosis in men (Table 2). Since FRAX may underestimate the fracture risk in some patients on glucocorticoid therapy, the National Osteoporosis Foundation recommendations may leave out some patients who could benefit from therapy.
The American College of Rheumatology recommends prescribing a bisphosphonate (with caution in premenopausal women) for patients beginning therapy with prednisone 5 mg per day or higher (or its equivalent) if the corticosteroid therapy is planned to continue for 3 months or longer, and for patients who have been receiving this dose long-term who have low bone mineral density (T-score < −1.0).45
STRATEGIES FOR IMPROVING TREATMENT
Look for causes of secondary osteoporosis
All patients with osteoporosis should undergo an evaluation for factors other than postmenopausal status or aging that may adversely affect skeletal health or the choice of treatment. Causes of secondary osteoporosis are common,46 occurring in about two-thirds of men and about one-fifth of postmenopausal women,47 and unless they are recognized and treated, they may block or attenuate the response to therapy.
Particular attention must be paid to the adequacy of calcium and vitamin D intake and absorption. While there is no standard laboratory evaluation, one study suggests that cost-effective testing in a referral practice includes measurement of serum calcium, serum 25-hydroxyvitamin D, serum parathyroid hormone, 24-hour urinary calcium, and thyroidstimulating hormone if on thyroid replacement in all women with osteoporosis.48,49
Other helpful tests are a complete blood count, alkaline phosphatase, serum creatinine, serum phosphorus, serum protein electrophoresis in elderly patients, and serum testosterone in men; additional evaluation may be indicated, depending on clinical circumstances.
Think about special indications for or contraindications to specific drugs
If you have determined that drug therapy is indicated to reduce fracture risk, then a particular drug must be selected that is likely to be effective, safe, and affordable for the individual patient. Consideration must be given to what is known about the skeletal and non-skeletal benefits and risks, as well as patient factors such as other medical conditions, past drug experiences, and beliefs. For example:
- An oral bisphosphonate should not be given to a patient with a history of esophageal stricture, but an intravenous bisphosphonate may be very helpful.
- Raloxifene may be attractive for a patient at high risk of invasive breast cancer but not if there is a history of thromboembolic disease.
- Generic alendronate may be the first choice for a patient whose primary concern is keeping cost to a minimum, but risedronate or ibandronate may be best for a patient who prefers the convenience of monthly oral dosing.
Educate patients to improve compliance
Many patients who are prescribed medication do not start taking it, take it incorrectly, or stop taking it before obtaining benefit.
Some patients may not understand the goal of therapy (reduction of fracture risk) or the serious consequences of fractures. The lay press and medical journals contain much information on potential adverse effects of drug therapy (eg, osteonecrosis of the jaw, atrial fibrillation, mid-shaft femur fractures, esophageal cancer with bisphosphonates), often presented without consideration of the benefit-risk ratio. Patients may fear real or perceived adverse effects, and physicians may not be sufficiently knowledgeable to allay those fears.
For drugs that are complex to administer, such as oral bisphosphonates, patients may not fully understand the requirements or their rationale and importance.
For these reasons, a patient who is prescribed a drug must also be educated about the importance of filling the prescription, taking it regularly and correctly (particularly important for oral bisphosphonates), and taking it long enough to benefit.
Keep in touch with the patient
The causes of poor compliance and persistence are many, and effective interventions to help are few.50 One approach that has been shown to be helpful is regular contact with a health care provider.51
Patients often stop treatment when they develop an adverse effect (real or perceived), or when a news release of a new medical report raises the fear of an adverse effect. Without contact with a health care provider, these issues cannot be recognized and addressed.
Monitor for effectiveness
Monitoring for effectiveness of therapy, usually with DXA 1 to 2 years after starting therapy, provides useful clinical information.
Stability or an increase in bone mineral density is considered a good response that is associated with a reduction in fracture risk.7 A significant loss of bone mineral density is cause for concern and consideration of evaluation for secondary causes.52,53
Allowable intervals for insurance coverage of DXA may vary according to Medicare jurisdiction and health plan.
SHOULD BISPHOSPHONATES BE STOPPED AFTER LONG-TERM USE?
The concept of a “drug holiday” has arisen because bisphosphonates have a prolonged but waning antiresorptive effect with discontinuation after long-term use. A drug holiday, for a period of 1 year or perhaps longer, may be considered for patients on alendronate who are no longer or never were at high risk of fracture. On the other hand, given the evidence of increased risk of clinical vertebral fracture54 and hip fracture55 after bisphosphonates are discontinued, a drug holiday is probably not a reasonable choice in patients at high risk of fracture.
For bisphosphonates with very long dosing intervals, such as zoledronic acid given intravenously every 12 months, there may be opportunities for extending the dosing interval, but the lack of data means that no recommendations can be made at this time.
WHEN TO REFER
Most patients with osteoporosis can be effectively managed by the primary care provider. Referral to an osteoporosis specialist should be considered when the evaluation or treatment is beyond the comfort zone or level of expertise of the provider. Examples of situations in which referral may be appropriate are young patients with fragility fractures, patients with normal bone mineral density and fragility fractures, unusual laboratory findings on the evaluation for secondary osteoporosis, poor response to therapy (declining bone mineral density, failure of bone turnover markers to change as expected, fractures), and inability to tolerate therapy.
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004.
- National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995; 17( suppl 5):505S–511S.
- Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993; 137:1001–1005.
- Kanis JA; on behalf of the World Health Organization Scientific Group 2007. Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK, 2008.
- Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 2008; 11:75–91.
- Curtis JR, Carbone L, Cheng H, et al. Longitudinal patterns in bone mass measurement among U.S. Medicare beneficiaries [abstract]. J Bone Miner Res 2007; 22( suppl 1):S193.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285:785–795.
- van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 2002; 13:624–629.
- Cummings SR, Stone KL, Lui LL, et al. Are traumatic fractures osteoporotic? [abstract] J Bone Miner Res 2002; 17(suppl 1):S175.
- Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 2005; 20:557–563.
- Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int 2000; 11:577–582.
- Cooper C, O’Neill T, Silman A. The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 1993; 14( suppl 1):S89–S97.
- Foley KA, Foster SA, Meadows ES, Baser O, Long SR. Assessment of the clinical management of fragility fractures and implications for the new HEDIS osteoporosis measure. Med Care 2007; 45:902–906.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000; 109:326–328.
- Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002; 162:2217–2222.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15:721–739.
- Quintos-Macasa AM, Quinet R, Spady M, et al. Implementation of a mandatory rheumatology osteoporosis consultation in patients with low-impact hip fracture. J Clin Rheumatol 2007; 13:70–72.
- Streeten EA, Mohamed A, Gandhi A, et al. The inpatient consultation approach to osteoporosis treatment in patients with a fracture. Is automatic consultation needed? J Bone Joint Surg Am 2006; 88:1968–1974.
- World Health Organization. FRAX WHO Fracture Risk Assessment Tool. World Health Organization 2008. www.shef.ac.uk/FRAX/. Accessed 6/28/2008.
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000; 85:231–236.
- Lewiecki EM, Borges JL. Bone density testing in clinical practice. Arq Bras Endocrinol Metabol 2006; 50:586–595.
- Lewiecki EM, Richmond B, Miller PD. Uses and misuses of quantitative ultrasonography in managing osteoporosis. Cleve Clin J Med 2006; 73:742–752.
- Lewiecki EM, Binkley N, Petak S. Impact of DXA quality on patient care: clinician and technologist perceptions [abstract]. J Bone Miner Res 2006; 21( suppl 1):S355.
- Lewiecki EM, Binkley N, Petak SM. DXA quality matters. J Clin Densitom 2006; 9:388–392.
- Lewiecki EM, Baim S, Siris ES. Osteoporosis care at risk in the United States. Osteoporos Int 2008; 19:1505–1509.
- The Lewin Group. Assessing the costs of performing DXA services in the office-based setting. Final Report. www.aace.com/advocacy/leg/pdfs/DXAExecutiveSummary.pdf. Accessed 6/28.2009.
- Lewiecki EM. Crisis in osteoporosis care. The Female Patient 2009; 34:1–2.
- Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008; 90(suppl 4):188–194.
- Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood GC. Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int 2003; 14:146–151.
- Ayoub WT, Newman ED, Blosky MA, Stewart WF, Wood GC. Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int 2009; 20:37–42.
- Harrington JT, Barash HL, Day S, Lease J. Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum 2005; 53:198–204.
- McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 2003; 14:1028–1034.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312:1254–1259.
- Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001; 12:417–427.
- Lewiecki EM. Review of guidelines for bone mineral density testing and treatment of osteoporosis. Curr Osteoporos Rep 2005; 3:75–83.
- Tosteson AN, Melton LJ, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 2008; 19:437–447.
- Dawson-Hughes B, Tosteson AN, Melton LJ, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 2008; 19:449–458.
- Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005; 90:2787–2793.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004; 164:1108–1112.
- Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 1999; 14:1061–1066.
- van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc 2002; 77:453–468.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Luckey MM, Tannenbaum C. Authors' response: Recommended testing in patients with low bone density. J Clin Endocrinol Metab 2003; 88:1405.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007; 18:1023–1031.
- Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2004; 89:1117–1123.
- Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int 2008; 19:1363–1368.
- Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom 2003; 6:307–314.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
Osteoporosis is underdiagnosed and undertreated,1 even though it is common and causes serious problems, and even though effective treatments are available. The US Surgeon General has challenged the health care profession to close “the gap between clinical knowledge and its application in the community.”2
This review describes current shortcomings in the care of patients with osteoporosis and suggests strategies for health care providers to improve clinical outcomes.
COMMON AND SERIOUS
Approximately 44 million American men and women, representing 55% of the population age 50 and over, have osteoporosis or low bone density that can lead to fractures.2 An estimated 2 million osteoporosis-related fractures were reported in the United States in 2005, with a direct health care cost of about $17 billion.3 By 2025, more than 3 million osteoporosis-related fractures per year are expected, with an annual cost of more than $25 billion.3
Fractures of the spine and hip are associated with chronic pain, deformity, depression, disability, and death. About 50% of patients with a hip fracture are left permanently unable to walk without assistance, and 25% require long-term care.4 The death rate 5 years after a hip fracture or a clinical vertebral fracture is about 20% higher than expected.5
DIAGNOSING OSTEOPOROSIS BEFORE A FRACTURE OCCURS
World Health Organization classification
The World Health Organization6 classifies bone mineral density on the basis of the T-score, ie, the difference, in standard deviations, between the patient’s bone mineral density, measured by dual-energy x-ray absorptiometry (DXA), and the mean bone mineral density of a young adult reference population:
- Normal (a T-score of −1.0 or higher)
- Osteopenia (a T-score of less than −1.0 but higher than −2.5)
- Osteoporosis (a T-score of −2.5 or less)
- Severe osteoporosis (a T-score of −2.5 or less with a fragility fracture).
The International Society for Clinical Densitometry has established indications for bone density measurement, quality control, acquisition, analysis, interpretation, reporting, and nomenclature.7 The Society states, for example, that bone mineral density may be classified according to the lowest T-score of the lumbar spine, total hip, femoral neck, or distal one-third (33%) radius (if measured), using a white female reference database in women and a white male reference database in men.
Why test bone mineral density?
Bone density testing allows a physician to diagnose osteoporosis before a fracture occurs and to intervene early to reduce the risk of fracture. A clinical diagnosis of osteoporosis can be made in a patient who has had a fragility fracture, independently of bone mineral density, although this is less desirable than diagnosing osteoporosis before the first fracture.
While one can argue that fracture risk assessment is of greater clinical importance than diagnostic classification (ie, normal, osteopenia, osteoporosis), a diagnosis of osteoporosis conveys a clear message to the patient and health care providers about the presence of a disease that requires evaluation and treatment. Also, in the United States, diagnostic classification is necessary to select a numerical code for insurance billing and sometimes to determine eligibility for insurance coverage of drug therapy.
Osteoporosis is underdiagnosed, even after fractures
Osteoporosis is underdiagnosed.1 Data from Medicare claims for 1999 to 2000 showed that only 30% of eligible women age 65 and older had a bone density test,8 despite recognition by many organizations that fracture risk is high and DXA is indicated in this population.7,9,10
An adult with any fracture,11 even one due to trauma,12 may have osteoporosis, may be at risk of future fractures, and should be considered for further evaluation. Vertebral fractures, the most prevalent type of osteoporotic fracture, are commonly underrecognized and underreported,13,14 thereby missing an opportunity to identify and treat a patient at high risk.
Clinical vertebral fractures are those that come to clinical attention because of symptoms and then are appropriately diagnosed, while morphometric vertebral fractures are those detected by an imaging study regardless of symptoms. Only about one-third of all vertebral fractures are clinically apparent.15
In 2005, Foley et al16 reported that only 10.2% of women age 67 and older with a fracture were tested for osteoporosis within the following 6 months. Patients discharged from the hospital after hip fractures are commonly not diagnosed with or treated for osteoporosis,17,18 although the risk of future fractures is very high.19 Inpatient consultation with a medical specialist has not consistently improved osteoporosis care, with some reports of no effect17 and others suggesting a modest benefit.20,21
Many factors are responsible for underdiagnosis, and no single specialty is to blame. Primary care physicians are often overburdened with clinical, administrative, and regulatory responsibilities that leave little time to consider a silent disease that increases the risk of an event that may occur far in the future. Acute fractures are often treated by an orthopedist or emergency department specialist who is not responsible for long-term care and prevention of future fractures. The primary care physician may not become aware of the fracture until long after it has occurred.
DXA is the gold standard test
Although all of these tests provide results that correlate with fracture risk, DXA is the only one that can be used for diagnostic classification7 and the only one that can be used with the Fracture Risk Assessment Tool, or FRAX (more about this below).22 DXA is also the most clinically useful way to monitor the effects of therapy, with a correlation, albeit an imperfect one, between changes in bone mineral density with therapy and reduction in fracture risk.23
For these reasons, DXA is generally considered the gold standard for measuring bone mineral density.24
Using the wrong technology for the clinical need25 or performing poor-quality testing26,27 may result in inappropriate patient care decisions and wastes limited health care resources.
Medicare coverage for DXA has been cut
Recent cuts in Medicare reimbursement for DXA in the United States have been so severe that payment is now less than the cost of providing the service at many facilities.28 With further reductions in reimbursement expected, it is projected that most outpatient DXA centers—ie, about two-thirds of all DXA facilities in the United States—will no longer be operating by 2010.29
The anticipated consequences: fewer patients will be diagnosed with osteoporosis, fewer patients will be treated, and more fractures will occur, with fracture-related health care expenses far exceeding the savings from fewer DXA tests and fewer prescriptions for drugs to reduce fracture risk. I have characterized this as a “crisis in osteoporosis care,”30 and it is in stark contrast to the mandate of the US Surgeon General to improve osteoporosis care.1
Reports from several large health care systems support the proposition that more rather than fewer patients should undergo bone density testing. Data from the Kaiser Southern California and the Geisinger Health Plan show that when more patients undergo DXA and more are treated for osteoporosis, fewer have fractures, and money is saved.31,32
STRATEGIES FOR IMPROVING DIAGNOSIS
Appoint an advocate
The first step in the early diagnosis of osteoporosis is to select appropriate patients for bone density testing by recognizing high-risk populations.
Given the many demands placed on primary care physicians, who may not be focused on osteoporosis, it may be helpful to appoint one or more office staff as “advocates” for skeletal health. This could be a medical assistant, nurse, or health care educator who is charged with alerting the physician when bone mineral density testing is needed, or who could perhaps be given the authority to order the test within prespecified parameters. Other responsibilities might include patient education on nutrition, lifestyle, fall prevention, and drug administration, and follow-up by phone or in the office to ensure compliance with therapy.
Set up a disease-management program
Changing the health care system may be a more effective way to improve clinical outcomes than changing the actions of individual physicians. Disease-management programs that institutionalize pathways of care for osteoporosis have shown promise,32,33 and postfracture intervention programs may provide an opportunity to better manage patients at very high risk of future fractures.34,35
Lobby your legislators
To assure patient access to diagnostic services for assessment of skeletal health, advocates are focusing on legislation to restore DXA reimbursement to a level that would allow outpatient DXA facilities to avoid financial losses and continue operating.28 This possibility may be aided by grassroots support from concerned physicians and from patients likely to be harmed by limited access to DXA testing because of fewer instruments in operation and greater distances to travel to reach them. The largest US patient advocate organization for osteoporosis care, the National Osteoporosis Foundation (www.nof.org), is spearheading a drive to educate legislators on the value of bone density testing and to pass corrective legislation.
ASSESSING FRACTURE RISK WITH FRAX
The patients who get the greatest reduction in fracture risk with drug therapy are those who have the highest baseline risk of fracture.6 An estimate of fracture risk is therefore important in determining which patients to treat.
While bone mineral density is an excellent predictor of fracture risk, density combined with clinical risk factors for fracture is a better predictor than density or clinical risk factors alone.
FRAX22 is an electronic clinical tool (www.shef.ac.uk/FRAX/) for calculating fracture risk on the basis of the bone mineral density of the femoral neck; the patient’s age, sex, height, and weight; and seven clinical risk factors (previous fracture, having a parent who had a hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, and ingestion of three or more units of alcohol daily). One enters this information plus the brand of DXA machine used (Hologic, GE Lunar, or Norland), and the algorithm estimates the 10-year probability of a major osteoporotic fracture (hip, spine, proximal humerus, or distal forearm), and the 10-year probability of hip fracture
The FRAX model was developed through an analysis of almost 60,000 men and women in 12 population-based cohorts with about 250,000 person-years of observation, and externally validated in an additional 11 cohorts with 230,000 men and women and more than 1.2 million person-years of observation.8 The analysis of these extraordinarily robust databases was a mammoth project undertaken by the World Health Organization, under the direction of Professor John Kanis and with the support of many other organizations and professional societies. Criteria for inclusion of a clinical risk factor in FRAX included international validation, independence from bone mineral density in predicting fractures, ease of collecting the information in clinical practice, and the potential for modification with drug therapy. Falls were not considered as clinical risk factors, since it is not clear that pharmacologic intervention can significantly change the risk of falls.
FRAX remains a work in progress, with continuing updates expected as new information becomes available on country-specific fracture rates and potential additional clinical risk factors. Future versions of FRAX may also include input from skeletal sites other than the femoral neck and bone density measurement with technologies other than DXA.
Benefits and limitations of FRAX
To use FRAX, one needs to understand its benefits and limitations.
Benefits. FRAX can be used to estimate fracture risk in untreated women and men from age 40 to 90,22 although the National Osteoporosis Foundation guidelines recommend that it be used to make treatment decisions only in untreated postmenopausal women and men age 50 and older with osteopenia who do not otherwise qualify for treatment.9
Expressing fracture risk as a 10-year probability is more clinically useful than expressing it as a relative risk. For example, if the relative risk of fracture is five times that of a comparator population in which the risk is close to zero, then the patient’s risk is low, although a physician might feel compelled to treat upon learning that the relative risk is 5. A 50-year-old woman and an 80-year-old woman with identical T-scores of −2.5 have the same relative risk of fracture,36 even though the 10-year probability of fracture is far greater for the older woman.37
Limitations. FRAX has not been validated in treated patients, in women and men outside the specified age range, or in children. In the United States, the use of FRAX is limited to four ethnic groups—white, black, Hispanic, and Asian. FRAX has not been validated in patients of mixed ethnicity or of other ethnic groups in the United States.
The seven clinical risk factors in FRAX are entered as yes-or-no responses, whereas the actual risk in an individual patient may depend on the dose or severity of the risk factor. For example, a patient who was treated with the glucocorticoid prednisone 5 mg per day for 4 months many years ago has a much lower risk than a similar patient who has been taking prednisone 10 mg per day for the past 10 years, even though the FRAX input (“yes” for glucocorticoid therapy) is the same and the FRAX estimation of fracture risk is the same.
Only the bone mineral density in the femoral neck is used in FRAX, although in some patients, the density at another skeletal site may be better correlated with fracture risk (eg, low lumbar spine density may be associated with high fracture risk even when femoral neck density is not low).
Other important risk factors, such as falling, rate of bone loss, and high bone turnover are not part of the FRAX algorithm.
These limitations of FRAX may lead to overestimation or underestimation of actual fracture risk when used in some clinical circumstances, with an uncertain range of error for the calculated 10-year fracture probability.
Using FRAX appropriately
Clinicians must recognize when FRAX is likely to overestimate or underestimate fracture risk (see above).
FRAX also requires appropriate patient selection and a thorough understanding of its role in patient care decisions. Although it can be used to estimate fracture risk for a postmenopausal woman with osteoporosis, this use is not necessary and may be confusing; the National Osteoporosis Foundation guidelines recommend treatment for such a patient regardless of what FRAX says, while the FRAX calculation might result in a value that is below the treatment threshold.
Since the main clinical utility of FRAX is to help in making treatment decisions, strategies for using FRAX in the United States are discussed in association with the National Osteoporosis Foundation treatment guidelines in the section that follows.
NATIONAL OSTEOPOROSIS FOUNDATION GUIDELINES FOR TREATMENT
Many medical organizations have issued clinical practice guidelines for treating osteoporosis, with some recommendations that differ and therefore confuse more than enlighten.38
In an effort to unify these disparate recommendations, the National Osteoporosis Foundation, with the support and endorsement of numerous professional societies, developed the Clinician’s Guide to Prevention and Treatment of Osteoporosis.9 This document addresses postmenopausal women and men age 50 and older of all ethnic groups in the United States and is intended for use by clinicians in making decisions in the care of individual patients. The recommendations should not be taken as rigid standards of practice but rather as a framework for making clinical decisions with consideration of the needs of each individual patient.
Recommendations for all patients
- A daily intake of elemental calcium of at least 1,200 mg with diet plus supplements, if needed (with no more than 500–600 mg of calcium supplementation in a single dose due to limited absorption of higher doses)
- Vitamin D3 800–1,000 IU per day, with more needed in some patients to bring the serum 25-hydroxyvitamin D to a desirable level of 30 ng/mL (75 nmol/L) or higher
- Regular weight-bearing exercise
- Fall prevention
- Avoidance of tobacco use and excessive alcohol intake.
Who should be tested?
The National Osteoporosis Foundation recommends bone density testing in patients at risk of osteoporosis according to indications that are almost identical to those of the International Society for Clinical Densitometry, ie:
- Women age 65 and older
- Postmenopausal women under age 65 with risk factors for fracture
- Women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or use of high-risk drugs such as glucocorticoids
- Men age 70 and older
- Men under age 70 with clinical risk factors for fracture
- Adults with a fragility fracture
- Adults with a disease or condition associated with low bone mass or bone loss
- Adults taking drugs associated with low bone mass or bone loss
- Anyone being considered for pharmacologic therapy
- Anyone being treated, to monitor treatment effect
- Anyone not receiving therapy in whom evidence of bone loss would lead to treatment.
Women discontinuing estrogen should be considered for bone density testing according to the indications listed above.
All patients with osteoporosis should have a skeletal-related history and physical examination, with appropriate laboratory testing to evaluate for contributing factors.
Who should be treated?
The National Osteoporosis Foundation recommends considering starting drug therapy in postmenopausal women and men age 50 and older who have any of the following:
- A hip or vertebral (clinical or morphometric) fracture
- A T-score of −2.5 or less at the femoral neck or spine after appropriate evaluation to exclude secondary causes
- Low bone mass (a T-score between −1.0 and −2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of 3% or more or a 10-year probability of a major osteoporosis-related fracture of 20% or more, based on the US version of FRAX (patient preferences may indicate treatment for people with 10-year fracture probabilities above or below these levels).
Economic assumptions behind the guidelines
The National Osteoporosis Foundation based its recommendations on cost-effectiveness modeling39 and the US version of FRAX.40 The fracture risk algorithm was calibrated to US fracture and death rates, with economic assumptions that included the following: 5 years of drug therapy with 100% compliance and persistent use of a drug that costs $600 per year and results in a 35% reduction in fracture risk, followed by discontinuation of the drug associated with a linear offset of effect over the next 5 years, with a societal willingness to pay up to $60,000 per quality-adjusted life-year gained.39
FRAX is used to decide on treatment only in those with osteopenia
FRAX is used for making treatment decisions only in patients with osteopenia. Those with a densitometric diagnosis of osteoporosis according to a T-score value of −2.5 or less or a clinical diagnosis of osteoporosis by virtue of having had a fracture of the hip or spine should be treated regardless of FRAX, and those with normal T-scores are not recommended for treatment regardless of FRAX. By providing a quantitative estimation of fracture probability, FRAX allows clinicians to distinguish patients with osteopenia who are at high fracture risk from those who are not, and thereby to treat those most likely to benefit.
Since approximately one-half of patients who have fragility fractures do not have T-scores in the osteoporosis range,41,42 there is great clinical utility in identifying the subset of osteopenic patients who are candidates for treatment. It is likely that the use of FRAX will result in fewer early postmenopausal women and more older women with osteopenia being treated with drugs, since age is an important risk factor for fracture that is independent of bone mineral density.
Which drugs to use?
The drugs currently approved in the United States for preventing and treating osteoporosis are:
- Alendronate (Fosamax)
- Risedronate (Actonel)
- Ibandronate (Boniva)
- Zoledronate (Reclast)
- Salmon calcitonin (Miacalcin, Fortical)
- Raloxifene (Evista)
- Teriparatide (Forteo) (Table 2).
It is not known with certainty whether any of these drugs is more effective than any other, because no head-to-head clinical trials have been done using fracture as the primary end point.
Selecting a drug requires assessing its benefits and risks for each patient. The National Osteoporosis Foundation’s intervention thresholds do not consider nonskeletal benefits and risks, such as reduction in the risk of invasive breast cancer and increase in the risk of thromboembolic events with raloxifene.
For patients on glucocorticoids
Chronic glucocorticoid therapy is a special category of fracture risk. Rapid bone loss can occur at the start of therapy43 and the adverse effects on bone strength are at least partially independent of bone mineral density.44 US Food and Drug Administration indications for drugs for prevention and treatment of glucocorticoid-induced osteoporosis are distinct from those for postmenopausal osteoporosis and osteoporosis in men (Table 2). Since FRAX may underestimate the fracture risk in some patients on glucocorticoid therapy, the National Osteoporosis Foundation recommendations may leave out some patients who could benefit from therapy.
The American College of Rheumatology recommends prescribing a bisphosphonate (with caution in premenopausal women) for patients beginning therapy with prednisone 5 mg per day or higher (or its equivalent) if the corticosteroid therapy is planned to continue for 3 months or longer, and for patients who have been receiving this dose long-term who have low bone mineral density (T-score < −1.0).45
STRATEGIES FOR IMPROVING TREATMENT
Look for causes of secondary osteoporosis
All patients with osteoporosis should undergo an evaluation for factors other than postmenopausal status or aging that may adversely affect skeletal health or the choice of treatment. Causes of secondary osteoporosis are common,46 occurring in about two-thirds of men and about one-fifth of postmenopausal women,47 and unless they are recognized and treated, they may block or attenuate the response to therapy.
Particular attention must be paid to the adequacy of calcium and vitamin D intake and absorption. While there is no standard laboratory evaluation, one study suggests that cost-effective testing in a referral practice includes measurement of serum calcium, serum 25-hydroxyvitamin D, serum parathyroid hormone, 24-hour urinary calcium, and thyroidstimulating hormone if on thyroid replacement in all women with osteoporosis.48,49
Other helpful tests are a complete blood count, alkaline phosphatase, serum creatinine, serum phosphorus, serum protein electrophoresis in elderly patients, and serum testosterone in men; additional evaluation may be indicated, depending on clinical circumstances.
Think about special indications for or contraindications to specific drugs
If you have determined that drug therapy is indicated to reduce fracture risk, then a particular drug must be selected that is likely to be effective, safe, and affordable for the individual patient. Consideration must be given to what is known about the skeletal and non-skeletal benefits and risks, as well as patient factors such as other medical conditions, past drug experiences, and beliefs. For example:
- An oral bisphosphonate should not be given to a patient with a history of esophageal stricture, but an intravenous bisphosphonate may be very helpful.
- Raloxifene may be attractive for a patient at high risk of invasive breast cancer but not if there is a history of thromboembolic disease.
- Generic alendronate may be the first choice for a patient whose primary concern is keeping cost to a minimum, but risedronate or ibandronate may be best for a patient who prefers the convenience of monthly oral dosing.
Educate patients to improve compliance
Many patients who are prescribed medication do not start taking it, take it incorrectly, or stop taking it before obtaining benefit.
Some patients may not understand the goal of therapy (reduction of fracture risk) or the serious consequences of fractures. The lay press and medical journals contain much information on potential adverse effects of drug therapy (eg, osteonecrosis of the jaw, atrial fibrillation, mid-shaft femur fractures, esophageal cancer with bisphosphonates), often presented without consideration of the benefit-risk ratio. Patients may fear real or perceived adverse effects, and physicians may not be sufficiently knowledgeable to allay those fears.
For drugs that are complex to administer, such as oral bisphosphonates, patients may not fully understand the requirements or their rationale and importance.
For these reasons, a patient who is prescribed a drug must also be educated about the importance of filling the prescription, taking it regularly and correctly (particularly important for oral bisphosphonates), and taking it long enough to benefit.
Keep in touch with the patient
The causes of poor compliance and persistence are many, and effective interventions to help are few.50 One approach that has been shown to be helpful is regular contact with a health care provider.51
Patients often stop treatment when they develop an adverse effect (real or perceived), or when a news release of a new medical report raises the fear of an adverse effect. Without contact with a health care provider, these issues cannot be recognized and addressed.
Monitor for effectiveness
Monitoring for effectiveness of therapy, usually with DXA 1 to 2 years after starting therapy, provides useful clinical information.
Stability or an increase in bone mineral density is considered a good response that is associated with a reduction in fracture risk.7 A significant loss of bone mineral density is cause for concern and consideration of evaluation for secondary causes.52,53
Allowable intervals for insurance coverage of DXA may vary according to Medicare jurisdiction and health plan.
SHOULD BISPHOSPHONATES BE STOPPED AFTER LONG-TERM USE?
The concept of a “drug holiday” has arisen because bisphosphonates have a prolonged but waning antiresorptive effect with discontinuation after long-term use. A drug holiday, for a period of 1 year or perhaps longer, may be considered for patients on alendronate who are no longer or never were at high risk of fracture. On the other hand, given the evidence of increased risk of clinical vertebral fracture54 and hip fracture55 after bisphosphonates are discontinued, a drug holiday is probably not a reasonable choice in patients at high risk of fracture.
For bisphosphonates with very long dosing intervals, such as zoledronic acid given intravenously every 12 months, there may be opportunities for extending the dosing interval, but the lack of data means that no recommendations can be made at this time.
WHEN TO REFER
Most patients with osteoporosis can be effectively managed by the primary care provider. Referral to an osteoporosis specialist should be considered when the evaluation or treatment is beyond the comfort zone or level of expertise of the provider. Examples of situations in which referral may be appropriate are young patients with fragility fractures, patients with normal bone mineral density and fragility fractures, unusual laboratory findings on the evaluation for secondary osteoporosis, poor response to therapy (declining bone mineral density, failure of bone turnover markers to change as expected, fractures), and inability to tolerate therapy.
Osteoporosis is underdiagnosed and undertreated,1 even though it is common and causes serious problems, and even though effective treatments are available. The US Surgeon General has challenged the health care profession to close “the gap between clinical knowledge and its application in the community.”2
This review describes current shortcomings in the care of patients with osteoporosis and suggests strategies for health care providers to improve clinical outcomes.
COMMON AND SERIOUS
Approximately 44 million American men and women, representing 55% of the population age 50 and over, have osteoporosis or low bone density that can lead to fractures.2 An estimated 2 million osteoporosis-related fractures were reported in the United States in 2005, with a direct health care cost of about $17 billion.3 By 2025, more than 3 million osteoporosis-related fractures per year are expected, with an annual cost of more than $25 billion.3
Fractures of the spine and hip are associated with chronic pain, deformity, depression, disability, and death. About 50% of patients with a hip fracture are left permanently unable to walk without assistance, and 25% require long-term care.4 The death rate 5 years after a hip fracture or a clinical vertebral fracture is about 20% higher than expected.5
DIAGNOSING OSTEOPOROSIS BEFORE A FRACTURE OCCURS
World Health Organization classification
The World Health Organization6 classifies bone mineral density on the basis of the T-score, ie, the difference, in standard deviations, between the patient’s bone mineral density, measured by dual-energy x-ray absorptiometry (DXA), and the mean bone mineral density of a young adult reference population:
- Normal (a T-score of −1.0 or higher)
- Osteopenia (a T-score of less than −1.0 but higher than −2.5)
- Osteoporosis (a T-score of −2.5 or less)
- Severe osteoporosis (a T-score of −2.5 or less with a fragility fracture).
The International Society for Clinical Densitometry has established indications for bone density measurement, quality control, acquisition, analysis, interpretation, reporting, and nomenclature.7 The Society states, for example, that bone mineral density may be classified according to the lowest T-score of the lumbar spine, total hip, femoral neck, or distal one-third (33%) radius (if measured), using a white female reference database in women and a white male reference database in men.
Why test bone mineral density?
Bone density testing allows a physician to diagnose osteoporosis before a fracture occurs and to intervene early to reduce the risk of fracture. A clinical diagnosis of osteoporosis can be made in a patient who has had a fragility fracture, independently of bone mineral density, although this is less desirable than diagnosing osteoporosis before the first fracture.
While one can argue that fracture risk assessment is of greater clinical importance than diagnostic classification (ie, normal, osteopenia, osteoporosis), a diagnosis of osteoporosis conveys a clear message to the patient and health care providers about the presence of a disease that requires evaluation and treatment. Also, in the United States, diagnostic classification is necessary to select a numerical code for insurance billing and sometimes to determine eligibility for insurance coverage of drug therapy.
Osteoporosis is underdiagnosed, even after fractures
Osteoporosis is underdiagnosed.1 Data from Medicare claims for 1999 to 2000 showed that only 30% of eligible women age 65 and older had a bone density test,8 despite recognition by many organizations that fracture risk is high and DXA is indicated in this population.7,9,10
An adult with any fracture,11 even one due to trauma,12 may have osteoporosis, may be at risk of future fractures, and should be considered for further evaluation. Vertebral fractures, the most prevalent type of osteoporotic fracture, are commonly underrecognized and underreported,13,14 thereby missing an opportunity to identify and treat a patient at high risk.
Clinical vertebral fractures are those that come to clinical attention because of symptoms and then are appropriately diagnosed, while morphometric vertebral fractures are those detected by an imaging study regardless of symptoms. Only about one-third of all vertebral fractures are clinically apparent.15
In 2005, Foley et al16 reported that only 10.2% of women age 67 and older with a fracture were tested for osteoporosis within the following 6 months. Patients discharged from the hospital after hip fractures are commonly not diagnosed with or treated for osteoporosis,17,18 although the risk of future fractures is very high.19 Inpatient consultation with a medical specialist has not consistently improved osteoporosis care, with some reports of no effect17 and others suggesting a modest benefit.20,21
Many factors are responsible for underdiagnosis, and no single specialty is to blame. Primary care physicians are often overburdened with clinical, administrative, and regulatory responsibilities that leave little time to consider a silent disease that increases the risk of an event that may occur far in the future. Acute fractures are often treated by an orthopedist or emergency department specialist who is not responsible for long-term care and prevention of future fractures. The primary care physician may not become aware of the fracture until long after it has occurred.
DXA is the gold standard test
Although all of these tests provide results that correlate with fracture risk, DXA is the only one that can be used for diagnostic classification7 and the only one that can be used with the Fracture Risk Assessment Tool, or FRAX (more about this below).22 DXA is also the most clinically useful way to monitor the effects of therapy, with a correlation, albeit an imperfect one, between changes in bone mineral density with therapy and reduction in fracture risk.23
For these reasons, DXA is generally considered the gold standard for measuring bone mineral density.24
Using the wrong technology for the clinical need25 or performing poor-quality testing26,27 may result in inappropriate patient care decisions and wastes limited health care resources.
Medicare coverage for DXA has been cut
Recent cuts in Medicare reimbursement for DXA in the United States have been so severe that payment is now less than the cost of providing the service at many facilities.28 With further reductions in reimbursement expected, it is projected that most outpatient DXA centers—ie, about two-thirds of all DXA facilities in the United States—will no longer be operating by 2010.29
The anticipated consequences: fewer patients will be diagnosed with osteoporosis, fewer patients will be treated, and more fractures will occur, with fracture-related health care expenses far exceeding the savings from fewer DXA tests and fewer prescriptions for drugs to reduce fracture risk. I have characterized this as a “crisis in osteoporosis care,”30 and it is in stark contrast to the mandate of the US Surgeon General to improve osteoporosis care.1
Reports from several large health care systems support the proposition that more rather than fewer patients should undergo bone density testing. Data from the Kaiser Southern California and the Geisinger Health Plan show that when more patients undergo DXA and more are treated for osteoporosis, fewer have fractures, and money is saved.31,32
STRATEGIES FOR IMPROVING DIAGNOSIS
Appoint an advocate
The first step in the early diagnosis of osteoporosis is to select appropriate patients for bone density testing by recognizing high-risk populations.
Given the many demands placed on primary care physicians, who may not be focused on osteoporosis, it may be helpful to appoint one or more office staff as “advocates” for skeletal health. This could be a medical assistant, nurse, or health care educator who is charged with alerting the physician when bone mineral density testing is needed, or who could perhaps be given the authority to order the test within prespecified parameters. Other responsibilities might include patient education on nutrition, lifestyle, fall prevention, and drug administration, and follow-up by phone or in the office to ensure compliance with therapy.
Set up a disease-management program
Changing the health care system may be a more effective way to improve clinical outcomes than changing the actions of individual physicians. Disease-management programs that institutionalize pathways of care for osteoporosis have shown promise,32,33 and postfracture intervention programs may provide an opportunity to better manage patients at very high risk of future fractures.34,35
Lobby your legislators
To assure patient access to diagnostic services for assessment of skeletal health, advocates are focusing on legislation to restore DXA reimbursement to a level that would allow outpatient DXA facilities to avoid financial losses and continue operating.28 This possibility may be aided by grassroots support from concerned physicians and from patients likely to be harmed by limited access to DXA testing because of fewer instruments in operation and greater distances to travel to reach them. The largest US patient advocate organization for osteoporosis care, the National Osteoporosis Foundation (www.nof.org), is spearheading a drive to educate legislators on the value of bone density testing and to pass corrective legislation.
ASSESSING FRACTURE RISK WITH FRAX
The patients who get the greatest reduction in fracture risk with drug therapy are those who have the highest baseline risk of fracture.6 An estimate of fracture risk is therefore important in determining which patients to treat.
While bone mineral density is an excellent predictor of fracture risk, density combined with clinical risk factors for fracture is a better predictor than density or clinical risk factors alone.
FRAX22 is an electronic clinical tool (www.shef.ac.uk/FRAX/) for calculating fracture risk on the basis of the bone mineral density of the femoral neck; the patient’s age, sex, height, and weight; and seven clinical risk factors (previous fracture, having a parent who had a hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, and ingestion of three or more units of alcohol daily). One enters this information plus the brand of DXA machine used (Hologic, GE Lunar, or Norland), and the algorithm estimates the 10-year probability of a major osteoporotic fracture (hip, spine, proximal humerus, or distal forearm), and the 10-year probability of hip fracture
The FRAX model was developed through an analysis of almost 60,000 men and women in 12 population-based cohorts with about 250,000 person-years of observation, and externally validated in an additional 11 cohorts with 230,000 men and women and more than 1.2 million person-years of observation.8 The analysis of these extraordinarily robust databases was a mammoth project undertaken by the World Health Organization, under the direction of Professor John Kanis and with the support of many other organizations and professional societies. Criteria for inclusion of a clinical risk factor in FRAX included international validation, independence from bone mineral density in predicting fractures, ease of collecting the information in clinical practice, and the potential for modification with drug therapy. Falls were not considered as clinical risk factors, since it is not clear that pharmacologic intervention can significantly change the risk of falls.
FRAX remains a work in progress, with continuing updates expected as new information becomes available on country-specific fracture rates and potential additional clinical risk factors. Future versions of FRAX may also include input from skeletal sites other than the femoral neck and bone density measurement with technologies other than DXA.
Benefits and limitations of FRAX
To use FRAX, one needs to understand its benefits and limitations.
Benefits. FRAX can be used to estimate fracture risk in untreated women and men from age 40 to 90,22 although the National Osteoporosis Foundation guidelines recommend that it be used to make treatment decisions only in untreated postmenopausal women and men age 50 and older with osteopenia who do not otherwise qualify for treatment.9
Expressing fracture risk as a 10-year probability is more clinically useful than expressing it as a relative risk. For example, if the relative risk of fracture is five times that of a comparator population in which the risk is close to zero, then the patient’s risk is low, although a physician might feel compelled to treat upon learning that the relative risk is 5. A 50-year-old woman and an 80-year-old woman with identical T-scores of −2.5 have the same relative risk of fracture,36 even though the 10-year probability of fracture is far greater for the older woman.37
Limitations. FRAX has not been validated in treated patients, in women and men outside the specified age range, or in children. In the United States, the use of FRAX is limited to four ethnic groups—white, black, Hispanic, and Asian. FRAX has not been validated in patients of mixed ethnicity or of other ethnic groups in the United States.
The seven clinical risk factors in FRAX are entered as yes-or-no responses, whereas the actual risk in an individual patient may depend on the dose or severity of the risk factor. For example, a patient who was treated with the glucocorticoid prednisone 5 mg per day for 4 months many years ago has a much lower risk than a similar patient who has been taking prednisone 10 mg per day for the past 10 years, even though the FRAX input (“yes” for glucocorticoid therapy) is the same and the FRAX estimation of fracture risk is the same.
Only the bone mineral density in the femoral neck is used in FRAX, although in some patients, the density at another skeletal site may be better correlated with fracture risk (eg, low lumbar spine density may be associated with high fracture risk even when femoral neck density is not low).
Other important risk factors, such as falling, rate of bone loss, and high bone turnover are not part of the FRAX algorithm.
These limitations of FRAX may lead to overestimation or underestimation of actual fracture risk when used in some clinical circumstances, with an uncertain range of error for the calculated 10-year fracture probability.
Using FRAX appropriately
Clinicians must recognize when FRAX is likely to overestimate or underestimate fracture risk (see above).
FRAX also requires appropriate patient selection and a thorough understanding of its role in patient care decisions. Although it can be used to estimate fracture risk for a postmenopausal woman with osteoporosis, this use is not necessary and may be confusing; the National Osteoporosis Foundation guidelines recommend treatment for such a patient regardless of what FRAX says, while the FRAX calculation might result in a value that is below the treatment threshold.
Since the main clinical utility of FRAX is to help in making treatment decisions, strategies for using FRAX in the United States are discussed in association with the National Osteoporosis Foundation treatment guidelines in the section that follows.
NATIONAL OSTEOPOROSIS FOUNDATION GUIDELINES FOR TREATMENT
Many medical organizations have issued clinical practice guidelines for treating osteoporosis, with some recommendations that differ and therefore confuse more than enlighten.38
In an effort to unify these disparate recommendations, the National Osteoporosis Foundation, with the support and endorsement of numerous professional societies, developed the Clinician’s Guide to Prevention and Treatment of Osteoporosis.9 This document addresses postmenopausal women and men age 50 and older of all ethnic groups in the United States and is intended for use by clinicians in making decisions in the care of individual patients. The recommendations should not be taken as rigid standards of practice but rather as a framework for making clinical decisions with consideration of the needs of each individual patient.
Recommendations for all patients
- A daily intake of elemental calcium of at least 1,200 mg with diet plus supplements, if needed (with no more than 500–600 mg of calcium supplementation in a single dose due to limited absorption of higher doses)
- Vitamin D3 800–1,000 IU per day, with more needed in some patients to bring the serum 25-hydroxyvitamin D to a desirable level of 30 ng/mL (75 nmol/L) or higher
- Regular weight-bearing exercise
- Fall prevention
- Avoidance of tobacco use and excessive alcohol intake.
Who should be tested?
The National Osteoporosis Foundation recommends bone density testing in patients at risk of osteoporosis according to indications that are almost identical to those of the International Society for Clinical Densitometry, ie:
- Women age 65 and older
- Postmenopausal women under age 65 with risk factors for fracture
- Women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture, or use of high-risk drugs such as glucocorticoids
- Men age 70 and older
- Men under age 70 with clinical risk factors for fracture
- Adults with a fragility fracture
- Adults with a disease or condition associated with low bone mass or bone loss
- Adults taking drugs associated with low bone mass or bone loss
- Anyone being considered for pharmacologic therapy
- Anyone being treated, to monitor treatment effect
- Anyone not receiving therapy in whom evidence of bone loss would lead to treatment.
Women discontinuing estrogen should be considered for bone density testing according to the indications listed above.
All patients with osteoporosis should have a skeletal-related history and physical examination, with appropriate laboratory testing to evaluate for contributing factors.
Who should be treated?
The National Osteoporosis Foundation recommends considering starting drug therapy in postmenopausal women and men age 50 and older who have any of the following:
- A hip or vertebral (clinical or morphometric) fracture
- A T-score of −2.5 or less at the femoral neck or spine after appropriate evaluation to exclude secondary causes
- Low bone mass (a T-score between −1.0 and −2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture of 3% or more or a 10-year probability of a major osteoporosis-related fracture of 20% or more, based on the US version of FRAX (patient preferences may indicate treatment for people with 10-year fracture probabilities above or below these levels).
Economic assumptions behind the guidelines
The National Osteoporosis Foundation based its recommendations on cost-effectiveness modeling39 and the US version of FRAX.40 The fracture risk algorithm was calibrated to US fracture and death rates, with economic assumptions that included the following: 5 years of drug therapy with 100% compliance and persistent use of a drug that costs $600 per year and results in a 35% reduction in fracture risk, followed by discontinuation of the drug associated with a linear offset of effect over the next 5 years, with a societal willingness to pay up to $60,000 per quality-adjusted life-year gained.39
FRAX is used to decide on treatment only in those with osteopenia
FRAX is used for making treatment decisions only in patients with osteopenia. Those with a densitometric diagnosis of osteoporosis according to a T-score value of −2.5 or less or a clinical diagnosis of osteoporosis by virtue of having had a fracture of the hip or spine should be treated regardless of FRAX, and those with normal T-scores are not recommended for treatment regardless of FRAX. By providing a quantitative estimation of fracture probability, FRAX allows clinicians to distinguish patients with osteopenia who are at high fracture risk from those who are not, and thereby to treat those most likely to benefit.
Since approximately one-half of patients who have fragility fractures do not have T-scores in the osteoporosis range,41,42 there is great clinical utility in identifying the subset of osteopenic patients who are candidates for treatment. It is likely that the use of FRAX will result in fewer early postmenopausal women and more older women with osteopenia being treated with drugs, since age is an important risk factor for fracture that is independent of bone mineral density.
Which drugs to use?
The drugs currently approved in the United States for preventing and treating osteoporosis are:
- Alendronate (Fosamax)
- Risedronate (Actonel)
- Ibandronate (Boniva)
- Zoledronate (Reclast)
- Salmon calcitonin (Miacalcin, Fortical)
- Raloxifene (Evista)
- Teriparatide (Forteo) (Table 2).
It is not known with certainty whether any of these drugs is more effective than any other, because no head-to-head clinical trials have been done using fracture as the primary end point.
Selecting a drug requires assessing its benefits and risks for each patient. The National Osteoporosis Foundation’s intervention thresholds do not consider nonskeletal benefits and risks, such as reduction in the risk of invasive breast cancer and increase in the risk of thromboembolic events with raloxifene.
For patients on glucocorticoids
Chronic glucocorticoid therapy is a special category of fracture risk. Rapid bone loss can occur at the start of therapy43 and the adverse effects on bone strength are at least partially independent of bone mineral density.44 US Food and Drug Administration indications for drugs for prevention and treatment of glucocorticoid-induced osteoporosis are distinct from those for postmenopausal osteoporosis and osteoporosis in men (Table 2). Since FRAX may underestimate the fracture risk in some patients on glucocorticoid therapy, the National Osteoporosis Foundation recommendations may leave out some patients who could benefit from therapy.
The American College of Rheumatology recommends prescribing a bisphosphonate (with caution in premenopausal women) for patients beginning therapy with prednisone 5 mg per day or higher (or its equivalent) if the corticosteroid therapy is planned to continue for 3 months or longer, and for patients who have been receiving this dose long-term who have low bone mineral density (T-score < −1.0).45
STRATEGIES FOR IMPROVING TREATMENT
Look for causes of secondary osteoporosis
All patients with osteoporosis should undergo an evaluation for factors other than postmenopausal status or aging that may adversely affect skeletal health or the choice of treatment. Causes of secondary osteoporosis are common,46 occurring in about two-thirds of men and about one-fifth of postmenopausal women,47 and unless they are recognized and treated, they may block or attenuate the response to therapy.
Particular attention must be paid to the adequacy of calcium and vitamin D intake and absorption. While there is no standard laboratory evaluation, one study suggests that cost-effective testing in a referral practice includes measurement of serum calcium, serum 25-hydroxyvitamin D, serum parathyroid hormone, 24-hour urinary calcium, and thyroidstimulating hormone if on thyroid replacement in all women with osteoporosis.48,49
Other helpful tests are a complete blood count, alkaline phosphatase, serum creatinine, serum phosphorus, serum protein electrophoresis in elderly patients, and serum testosterone in men; additional evaluation may be indicated, depending on clinical circumstances.
Think about special indications for or contraindications to specific drugs
If you have determined that drug therapy is indicated to reduce fracture risk, then a particular drug must be selected that is likely to be effective, safe, and affordable for the individual patient. Consideration must be given to what is known about the skeletal and non-skeletal benefits and risks, as well as patient factors such as other medical conditions, past drug experiences, and beliefs. For example:
- An oral bisphosphonate should not be given to a patient with a history of esophageal stricture, but an intravenous bisphosphonate may be very helpful.
- Raloxifene may be attractive for a patient at high risk of invasive breast cancer but not if there is a history of thromboembolic disease.
- Generic alendronate may be the first choice for a patient whose primary concern is keeping cost to a minimum, but risedronate or ibandronate may be best for a patient who prefers the convenience of monthly oral dosing.
Educate patients to improve compliance
Many patients who are prescribed medication do not start taking it, take it incorrectly, or stop taking it before obtaining benefit.
Some patients may not understand the goal of therapy (reduction of fracture risk) or the serious consequences of fractures. The lay press and medical journals contain much information on potential adverse effects of drug therapy (eg, osteonecrosis of the jaw, atrial fibrillation, mid-shaft femur fractures, esophageal cancer with bisphosphonates), often presented without consideration of the benefit-risk ratio. Patients may fear real or perceived adverse effects, and physicians may not be sufficiently knowledgeable to allay those fears.
For drugs that are complex to administer, such as oral bisphosphonates, patients may not fully understand the requirements or their rationale and importance.
For these reasons, a patient who is prescribed a drug must also be educated about the importance of filling the prescription, taking it regularly and correctly (particularly important for oral bisphosphonates), and taking it long enough to benefit.
Keep in touch with the patient
The causes of poor compliance and persistence are many, and effective interventions to help are few.50 One approach that has been shown to be helpful is regular contact with a health care provider.51
Patients often stop treatment when they develop an adverse effect (real or perceived), or when a news release of a new medical report raises the fear of an adverse effect. Without contact with a health care provider, these issues cannot be recognized and addressed.
Monitor for effectiveness
Monitoring for effectiveness of therapy, usually with DXA 1 to 2 years after starting therapy, provides useful clinical information.
Stability or an increase in bone mineral density is considered a good response that is associated with a reduction in fracture risk.7 A significant loss of bone mineral density is cause for concern and consideration of evaluation for secondary causes.52,53
Allowable intervals for insurance coverage of DXA may vary according to Medicare jurisdiction and health plan.
SHOULD BISPHOSPHONATES BE STOPPED AFTER LONG-TERM USE?
The concept of a “drug holiday” has arisen because bisphosphonates have a prolonged but waning antiresorptive effect with discontinuation after long-term use. A drug holiday, for a period of 1 year or perhaps longer, may be considered for patients on alendronate who are no longer or never were at high risk of fracture. On the other hand, given the evidence of increased risk of clinical vertebral fracture54 and hip fracture55 after bisphosphonates are discontinued, a drug holiday is probably not a reasonable choice in patients at high risk of fracture.
For bisphosphonates with very long dosing intervals, such as zoledronic acid given intravenously every 12 months, there may be opportunities for extending the dosing interval, but the lack of data means that no recommendations can be made at this time.
WHEN TO REFER
Most patients with osteoporosis can be effectively managed by the primary care provider. Referral to an osteoporosis specialist should be considered when the evaluation or treatment is beyond the comfort zone or level of expertise of the provider. Examples of situations in which referral may be appropriate are young patients with fragility fractures, patients with normal bone mineral density and fragility fractures, unusual laboratory findings on the evaluation for secondary osteoporosis, poor response to therapy (declining bone mineral density, failure of bone turnover markers to change as expected, fractures), and inability to tolerate therapy.
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004.
- National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995; 17( suppl 5):505S–511S.
- Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993; 137:1001–1005.
- Kanis JA; on behalf of the World Health Organization Scientific Group 2007. Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK, 2008.
- Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 2008; 11:75–91.
- Curtis JR, Carbone L, Cheng H, et al. Longitudinal patterns in bone mass measurement among U.S. Medicare beneficiaries [abstract]. J Bone Miner Res 2007; 22( suppl 1):S193.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285:785–795.
- van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 2002; 13:624–629.
- Cummings SR, Stone KL, Lui LL, et al. Are traumatic fractures osteoporotic? [abstract] J Bone Miner Res 2002; 17(suppl 1):S175.
- Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 2005; 20:557–563.
- Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int 2000; 11:577–582.
- Cooper C, O’Neill T, Silman A. The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 1993; 14( suppl 1):S89–S97.
- Foley KA, Foster SA, Meadows ES, Baser O, Long SR. Assessment of the clinical management of fragility fractures and implications for the new HEDIS osteoporosis measure. Med Care 2007; 45:902–906.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000; 109:326–328.
- Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002; 162:2217–2222.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15:721–739.
- Quintos-Macasa AM, Quinet R, Spady M, et al. Implementation of a mandatory rheumatology osteoporosis consultation in patients with low-impact hip fracture. J Clin Rheumatol 2007; 13:70–72.
- Streeten EA, Mohamed A, Gandhi A, et al. The inpatient consultation approach to osteoporosis treatment in patients with a fracture. Is automatic consultation needed? J Bone Joint Surg Am 2006; 88:1968–1974.
- World Health Organization. FRAX WHO Fracture Risk Assessment Tool. World Health Organization 2008. www.shef.ac.uk/FRAX/. Accessed 6/28/2008.
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000; 85:231–236.
- Lewiecki EM, Borges JL. Bone density testing in clinical practice. Arq Bras Endocrinol Metabol 2006; 50:586–595.
- Lewiecki EM, Richmond B, Miller PD. Uses and misuses of quantitative ultrasonography in managing osteoporosis. Cleve Clin J Med 2006; 73:742–752.
- Lewiecki EM, Binkley N, Petak S. Impact of DXA quality on patient care: clinician and technologist perceptions [abstract]. J Bone Miner Res 2006; 21( suppl 1):S355.
- Lewiecki EM, Binkley N, Petak SM. DXA quality matters. J Clin Densitom 2006; 9:388–392.
- Lewiecki EM, Baim S, Siris ES. Osteoporosis care at risk in the United States. Osteoporos Int 2008; 19:1505–1509.
- The Lewin Group. Assessing the costs of performing DXA services in the office-based setting. Final Report. www.aace.com/advocacy/leg/pdfs/DXAExecutiveSummary.pdf. Accessed 6/28.2009.
- Lewiecki EM. Crisis in osteoporosis care. The Female Patient 2009; 34:1–2.
- Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008; 90(suppl 4):188–194.
- Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood GC. Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int 2003; 14:146–151.
- Ayoub WT, Newman ED, Blosky MA, Stewart WF, Wood GC. Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int 2009; 20:37–42.
- Harrington JT, Barash HL, Day S, Lease J. Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum 2005; 53:198–204.
- McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 2003; 14:1028–1034.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312:1254–1259.
- Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001; 12:417–427.
- Lewiecki EM. Review of guidelines for bone mineral density testing and treatment of osteoporosis. Curr Osteoporos Rep 2005; 3:75–83.
- Tosteson AN, Melton LJ, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 2008; 19:437–447.
- Dawson-Hughes B, Tosteson AN, Melton LJ, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 2008; 19:449–458.
- Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005; 90:2787–2793.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004; 164:1108–1112.
- Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 1999; 14:1061–1066.
- van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc 2002; 77:453–468.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Luckey MM, Tannenbaum C. Authors' response: Recommended testing in patients with low bone density. J Clin Endocrinol Metab 2003; 88:1405.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007; 18:1023–1031.
- Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2004; 89:1117–1123.
- Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int 2008; 19:1363–1368.
- Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom 2003; 6:307–314.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004.
- National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 1995; 17( suppl 5):505S–511S.
- Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993; 137:1001–1005.
- Kanis JA; on behalf of the World Health Organization Scientific Group 2007. Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK, 2008.
- Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 2008; 11:75–91.
- Curtis JR, Carbone L, Cheng H, et al. Longitudinal patterns in bone mass measurement among U.S. Medicare beneficiaries [abstract]. J Bone Miner Res 2007; 22( suppl 1):S193.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285:785–795.
- van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 2002; 13:624–629.
- Cummings SR, Stone KL, Lui LL, et al. Are traumatic fractures osteoporotic? [abstract] J Bone Miner Res 2002; 17(suppl 1):S175.
- Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 2005; 20:557–563.
- Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int 2000; 11:577–582.
- Cooper C, O’Neill T, Silman A. The epidemiology of vertebral fractures. European Vertebral Osteoporosis Study Group. Bone 1993; 14( suppl 1):S89–S97.
- Foley KA, Foster SA, Meadows ES, Baser O, Long SR. Assessment of the clinical management of fragility fractures and implications for the new HEDIS osteoporosis measure. Med Care 2007; 45:902–906.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000; 109:326–328.
- Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002; 162:2217–2222.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15:721–739.
- Quintos-Macasa AM, Quinet R, Spady M, et al. Implementation of a mandatory rheumatology osteoporosis consultation in patients with low-impact hip fracture. J Clin Rheumatol 2007; 13:70–72.
- Streeten EA, Mohamed A, Gandhi A, et al. The inpatient consultation approach to osteoporosis treatment in patients with a fracture. Is automatic consultation needed? J Bone Joint Surg Am 2006; 88:1968–1974.
- World Health Organization. FRAX WHO Fracture Risk Assessment Tool. World Health Organization 2008. www.shef.ac.uk/FRAX/. Accessed 6/28/2008.
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000; 85:231–236.
- Lewiecki EM, Borges JL. Bone density testing in clinical practice. Arq Bras Endocrinol Metabol 2006; 50:586–595.
- Lewiecki EM, Richmond B, Miller PD. Uses and misuses of quantitative ultrasonography in managing osteoporosis. Cleve Clin J Med 2006; 73:742–752.
- Lewiecki EM, Binkley N, Petak S. Impact of DXA quality on patient care: clinician and technologist perceptions [abstract]. J Bone Miner Res 2006; 21( suppl 1):S355.
- Lewiecki EM, Binkley N, Petak SM. DXA quality matters. J Clin Densitom 2006; 9:388–392.
- Lewiecki EM, Baim S, Siris ES. Osteoporosis care at risk in the United States. Osteoporos Int 2008; 19:1505–1509.
- The Lewin Group. Assessing the costs of performing DXA services in the office-based setting. Final Report. www.aace.com/advocacy/leg/pdfs/DXAExecutiveSummary.pdf. Accessed 6/28.2009.
- Lewiecki EM. Crisis in osteoporosis care. The Female Patient 2009; 34:1–2.
- Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008; 90(suppl 4):188–194.
- Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood GC. Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int 2003; 14:146–151.
- Ayoub WT, Newman ED, Blosky MA, Stewart WF, Wood GC. Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int 2009; 20:37–42.
- Harrington JT, Barash HL, Day S, Lease J. Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum 2005; 53:198–204.
- McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 2003; 14:1028–1034.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312:1254–1259.
- Kanis JA, Oden A, Johnell O, Jonsson B, De Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001; 12:417–427.
- Lewiecki EM. Review of guidelines for bone mineral density testing and treatment of osteoporosis. Curr Osteoporos Rep 2005; 3:75–83.
- Tosteson AN, Melton LJ, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 2008; 19:437–447.
- Dawson-Hughes B, Tosteson AN, Melton LJ, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 2008; 19:449–458.
- Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005; 90:2787–2793.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004; 164:1108–1112.
- Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 1999; 14:1061–1066.
- van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc 2002; 77:453–468.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Luckey MM, Tannenbaum C. Authors' response: Recommended testing in patients with low bone density. J Clin Endocrinol Metab 2003; 88:1405.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007; 18:1023–1031.
- Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2004; 89:1117–1123.
- Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int 2008; 19:1363–1368.
- Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom 2003; 6:307–314.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
KEY POINTS
- Osteoporosis is underdiagnosed. Patients discharged from the hospital after hip fractures are commonly not diagnosed with or treated for osteoporosis although the risk of future fractures is very high.
- The Fracture Risk Assessment Tool (FRAX) estimates the 10-year probability of fracture on the basis of clinical risk factors for fracture and the bone mineral density of the femoral neck. The combination of bone mineral density and clinical risk factors predicts fracture risk better than either alone.
- A drug holiday, for 1 year or perhaps longer, may be considered for patients on alendronate (Fosamax) who are no longer at high risk of fracture. On the other hand, given the evidence of increased risk of clinical vertebral fracture and hip fracture after bisphosphonates are discontinued, a drug holiday is probably not a reasonable choice in patients at high risk of fracture.
- Patient education and regular contact with a health care provider may improve compliance and persistence with therapy.