User login
Gender Disparity in Breast Cancer Among US Veterans
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
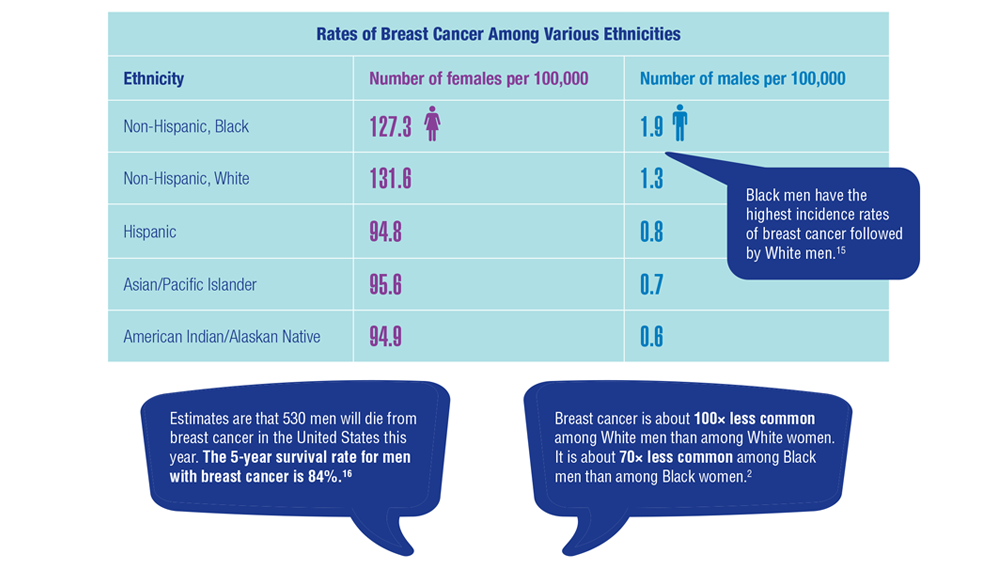
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
Screening High-Risk Women Veterans for Breast Cancer
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
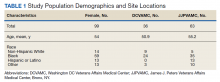
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
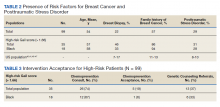
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
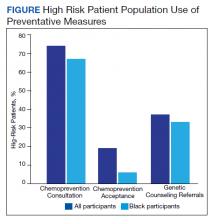
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
Breast Cancer Tumor Board
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
Case Presentation
This case represents a composite of many different patients and is not meant to represent an individual. Any resemblance to an actual patient is coincidental.
A 32-year-old African American woman presented with a self-palpated left breast mass (axillary tail at 9 o’clock position). The patient was a nonsmoker, was otherwise healthy, and had no family history of breast or any other cancer. She had never used oral contraceptives or hormones, was never pregnant, her menarche was at age 12 years, and she had regular menstrual periods. On physical examination she had a 1-cm left breast mass and a palpable left axillary lymph node. A complete diagnostic workup revealed a 2-cm left breast mass. An ultrasound-guided biopsy of the axillary lymph node was positive for invasive ductal carcinoma (IDC). The final diagnosis was left breast cancer, stage IIB IDC, T1N1M0, ER+, PR+, HER2 2+ by immunohistochemistry, fluorescence in situ hybridization (FISH) was 2.4, confirming a HER2+ tumor.
Anita Aggarwal, DO, PhD. What is the role of genetic counseling and testing in this young patient who does not have a family history of breast cancer?
Vickie L. Venne, MS. This patient absolutely would be a candidate for counseling and testing. From a genetic counseling perspective, one of the first points has to do with what “no family history of cancer” means. Typically, in a fast-paced clinic, a patient will be asked “Does anybody else in your family have cancer?” And it’s not uncommon to get the answer “no.” Genetic counselors collect specific information on at least the first- and second-degree relatives, so we end up with 3 generations. This includes both the maternal and the paternal histories. We find that people who initially report no family history of cancer are often just thinking of breast cancer, even if the provider’s question is broad. When we start digging, we often find other cancers because cancer is common.
The other issue is that she was diagnosed at a young age. Clearly, 32 years is much younger than we typically see in breast cancer, and we know that individuals with hereditary cancers often have an earlier age of onset. With no other information, her a priori risk of having a BRCA1/2 mutation would be < 2.5%.
Regardless, based on current National Comprehensive Cancer Network (NCCN) guidelines, she would be a testing candidate. We would also recommend testing for more than just BRCA1/2. In the last few decades, there have been many genes identified that are associated with an increased susceptibility to cancer. Many of these genes are part of syndromes, so if you had a mutation, that also would increase the risk for a cancer in another organ. If this woman’s mother and father lived into their 70s or 80s and she had a number of aunts on both sides who never developed breast cancer, it would be less likely to be BRCA1/2. However, P53 also can present in young women and as a de novo mutation. Therefore, we would offer her a panel of actionable genes. Genes that if, in fact, we identified a mutation in one of them, would mean we could do something different for this young lady.
JoAnn Manning, MD. Let’s say she does have testing, and she comes back BRCA+. Then what would be the recommendations or guidance?
Ms. Venne. Women (and men as well!) with mutations have an increased risk for a second primary breast cancer as well as cancer in other organs. Focusing first on the breast story and all the media around BRCA1/2 mutations and surgery, this is a woman who may consider a more aggressive surgery, including prophylactic contralateral mastectomy, if she is concerned. She is young, so we also would explore her fertility plans. While her next few months will be filled with breast cancer treatment choices, women with BRCA mutations also are at an increased risk to develop ovarian cancer, so that might be a decision she makes as well. Her health care team may also eventually discuss chemotherapeutic options available specifically to women with mutations.
However, we often see young women who are extremely nervous because there is a sense that if you’re younger, your cancer must be inherited. Part of the pretest counseling is to explore psychosocial issues and help these young ladies understand that, especially if she does not have a family history of cancer and the only indication is her age, then it’s highly likely that we’re not going to find an identifiable mutation. And in that circumstance, she probably could consider a more conservative surgical decision.
Dr. Aggarwal. How common is a BRCA1 or BRCA2 mutation in African American females?
Ms. Venne. I have not paid attention to the prevalence of mutations based on ethnicity, so I don’t know. While many of the initial mutations were discovered in women of European ancestry, there are large cohorts of women with African ancestry whose specimens are now available for identifying genetic markers that will improve breast cancer risk assessment in them.1 However, because those mutations are still being characterized, it is more common to find a variant of uncertain significance (VUS) in African American women. A VUS is an alteration—a change in the gene—that we simply don’t know what it means yet. Clinicians don’t have enough information to know if that alteration is pathogenic or benign. The problem is that people try to make sense out of everything in their lives, so they also will try to make a VUS mean something. We try hard to help people understand that a VUS is really no more significant than if we had not tested in the first place, and they should not act on that information. They should use their family history, their age, their other psychosocial concerns about their experiences with cancer as they make their treatment decisions. But they also should check back periodically with their genetic counselor because VUSs can be reclassified. And if that happens, the information might be more useful for not only them, but their family members.
Dr. Manning. Would you consider this patient for any neoadjuvant chemotherapy?
Dr. Aggarwal. The patient is a young female with a small tumor that is HER2+. The indication for neoadjuvant chemotherapy is typically a big tumor or inoperable disease. Neoadjuvant chemotherapy is considered the standard of care for patients with inflammatory breast cancer and may confer a survival benefit in these patients. Of all the breast cancer subtypes, triple negative and HER2+ are considered the most chemosensitive and may benefit from neoadjuvant therapy. This patient has a small tumor, and I don’t think she’s a candidate for neoadjuvant chemotherapy unless the patient wants to see if her tumor is chemosensitive or not.
Dr. Manning, What’s the role and benefit of lumpectomy vs mastectomy?
Dr. Manning. Historically, mastectomy would have been considered the standard of care, but luckily, in the 1970s and the 1980s, we had a significant number of randomized controlled trials that demonstrated that certain women with particular characteristics would get the same overall survival if they chose mastectomy vs lumpectomy, the removal of the tumor with negative margin and whole-breast radiation. The key thing to understand is that breast-conserving surgery is now very well established with more than 20 years of data to support it. And that breast irradiation after breast-conserving surgery is essential to maximizing the local control and the overall survival (OS).
There have been a lot of major studies, but the one with the greatest follow-up now is the National Surgical Adjuvant Breast and Bowel Project (NSABP) B06 protocol, which was the only trial to compare mastectomy to lumpectomy and radiation or lumpectomy alone. It required negative margins. With 20 years of follow-up, the data still support that mastectomy or lumpectomy with radiation offers equivalent OS and local control. It’s really about patient preference if they are candidates.
Who is a candidate? Clearly, there are contraindications. We tend to look primarily at the size of the tumor. However, removing an average-sized tumor (< 2 cm) with a margin may not have a good cosmetic result for a patient with very small breasts. That patient may opt to go forward with a mastectomy instead. Young patients who are candidates must have to have negative margins. If they have persistently positive resection margins after excision or reexcision, then they need to go forward with mastectomy.
A patient who has imaging evidence of multicentric disease with 2 or more primary tumors in separate quadrants would not be a candidate for breast-conserving therapy. Diffuse malignant-appearing microcalcifications on a mammogram also would suggest multicentric disease. And a patient with a prior history of radiation therapy to the breast or chest wall cannot go through breast-conserving therapy.
In the case we are discussing, we also should make sure this young lady is not pregnant. If the patient is adamant about breast-conserving surgery and pregnant, especially in the third trimester, radiation could be deferred until after delivery. Another relative contraindication is for patients who have connective tissue disorders. Sometimes if they are given whole-breast radiation, the cosmetic result is poor. So if you’re doing this procedure to save the breast, then having a good cosmetic result is an important consideration for many patients.
When you look at the size of the tumor for this patient, she seems to be a good candidate for breast-conserving surgery. I would recommend that she go forward with lumpectomy followed by whole-breast radiation.
Ms. Venne. Although the numbers aren’t nearly as large as they were in the original trials looking at the lumpectomy vs mastectomy, there are now survival data for women with BRCA1/2 mutations. With all of the caveats that Dr. Manning mentioned, even if you have an identifiable mutation, you may not necessarily need that more aggressive surgery.2 Clearly, individuals with identifiable mutations would have a higher chance of a contralateral breast cancer, a second primary, so some individuals consider a prophylactic bilateral mastectomy. But from a survival perspective, there are a fair amount of data now available that say that lumpectomy vs mastectomy should really be the conversation based on all of the information that Dr. Manning outlines rather than using primarily the mutation status
Dr. Manning. I agree.
Dr. Aggarwal. This patient had a lumpectomy and axillary lymph node dissection. Pathology reported 1.5-cm mass, grade 3 IDC; the margins were negative. There was no skin involvement, 27 lymph nodes removed were all negative. Dr. Manning, can you please discuss the role of radiation in early stage breast cancer in patients like this case?
Dr. Manning. One of the questions that is always controversial for radiation in these early stage breast cancer cases is what do you do with the nodal irradiation? Previously, radiation oncologists based treatment plans on retrospective data, but in 2015, there were 2 major studies, 1 from Canada, and 1 from the European Organisation for Research and Treatment of Cancer (EORTC).3,4 Both studies tried to determine whether there was an advantage to doing regional nodal irradiation in early breast cancer cases. That encompassed axillary, supraclavicular, and internal mammary nodes. The studies showed that there was no survival advantage, but there was a statistically significant improvement in disease free survival and in local regional recurrence and distant mets.
Unfortunately, there are still a lot of unanswered questions, like what group potentially would benefit the most? In the MA.20 Study, some observers questioned that maybe the ER-/PR- women had the most benefit, but then, in the other study the benefit wasn’t clear.4,5 One question is which lymph node group is having the most impact? Was the benefit from radiating the supraclavicular nodes or was it from radiating the internal mammary nodes? Determining the answer is important from a technical point of view because when you radiate the internal mammary nodes, you have the potential to expose more heart and lung to radiation. You have to put all these together and make a recommendation.
Clearly, for a patient with negative nodes there is no question: You would not treat the regional nodes. However, for a patient with positive nodes you really have to individualize the approach and consider age, anatomy, tumor location, and burden of axillary disease.
I would sit down and have a discussion with this young woman to weigh the risks and the benefits. There is a slight increased risk of lymphedema in these patients, and radiation pneumonitis increases, but not significantly. A key concern is to minimize the total dose of radiation to the heart. There have been great developments in radiation oncology technology and capabilities, so the cardiac dose is now less. But when you think about a 32-year-old patient and weigh the benefit of a 2% to 3% decrease in the incidence of distant metastases and no OS advantage, then you really need to have a conversation about how to safely treat her. At a minimum, I would treat the high axilla and the supraclavicular nodes because she had a pretty extensive lymph node dissection with more than 20 nodes, and then with her getting systemic therapy, that should be more than adequate.
Dr. Aggarwal. Is there any cutoff for age or size of the tumor where you would not do any radiation to the breast?
Dr. Manning. In this particular patient absolutely not because of the lymph node. She had breast-conserving therapy, and she’s only 32-years-old. The PRIME 2 study offered lumpectomy alone vs lumpectomy and radiation for women aged ≥ 65 years with tumors ≤ 3 cm, low grade.6 The study participants had to have negative lymph nodes, be ER+, and low grade. It was a very select group. The lumpectomy patients had a recurrence rate around 4%, and the other was closer to 1.3%.
You have to look at the whole picture. Is this a healthy 70-year-old woman? Is it an inconvenience for her to get treatment? Is she going to get hormone therapy and will she be adherent? There’s a very small group of women who underwent breast-conserving surgery that I would feel safe about not offering radiation.
Dr. Aggarwal. About 15% to 20% of all breast cancers are HER2 over expressors, which used to be a poor prognostic characteristic. However, the development of anti-HER2 therapies has changed the picture of HER2 prognosis. After the initial discovery of activity, the pivotal study by Slamon et al showed benefit in terms of progression-free survival (PFS) and OS with chemotherapy and trastuzumab. The NCCN guideline recommends anti-HER2 antibody trastuzumab in combination with chemotherapy.7
Patients with tumor < 0.5 cm who are HER2+ and ER+ may not benefit from trastuzumab, but those who are ER- and HER2+ will still benefit from trastuzumab. The combination is adriamycin/cyclophosphamide followed by a taxane with trastuzumab and to complete 1 year of trastuzumab or trastuzumab in combination with carboplatin and taxanes.
Pertuzumab, in combination with trastuzumab and docetaxel (PHT) has been FDA-approved in neoadjuvant and metastatic HER2+ disease, but is not FDA approved yet in the adjuvant setting. However, these are expensive drugs, and we don’t know how long these drugs should be given.
Mr. Crawford, What are the adverse effects (AEs) of an anti-HER2 or trastuzumab treatment, and what is the cost of trastuzumab?
Russell Crawford, BPharm. The anti-HER2 antibodies have certainly changed treatment plans and outcomes for patients with breast cancer who test HER2+. There are actually 3 of these anti-HER2 drugs on the U.S. market, and they can be used in a variety of settings. Trastuzumab and pertuzumab are indicated in women or patients who have HER2+ disease, and they work by binding to the extracellular domain of the HER2 proteins and mediate antibody-dependent cellular toxicity by inhibiting proliferation of the cells that overexpress HER2.
In this patient, we would be looking at using adjuvant trastuzumab to complete a 1-year course of therapy while she’s getting her dose-dense doxorubicin and cyclophosphamide (AC) on a weekly basis for the first 12 weeks. Trastuzumab is dosed with an initial loading dose of 4 mg/kg as the first dose, and then it’s 2 mg/kg/wk until adjuvant chemotherapy is completed. We usually extend the dosing out to 6 mg/kg every 3 weeks to complete the year of treatment.
These drugs are fairly well tolerated. They are monoclonal proteins, so a lot of the AEs that patients experience are the things that we’re used to seeing with other monoclonal proteins like the infusion-related reactions and some flulike symptoms. The biggest concern with these patients is that being on the drug for a year, there is a risk of decreasing the left ventricular ejection fraction (LVEF) of the heart. That risk is increased when these drugs are combined with anthracyclines that we know are cardiotoxic. As a single agent, the impact on left ventricular function is not significant, but when it is combined with chemotherapy, it does become a problem. Usually, we recommend routine and periodic monitoring of the LVEF with a multiple-gated acquisition or an echocardiogram to make sure that we’re not causing harm related to this treatment.
The cost of these drugs depends on the frequency, is it every week, every 2 weeks, or every 3 weeks? There are different ways to give trastuzumab, but for most patients, we prefer the every 3-week dose. And it’s estimated that for a 70-kg patient, a dose of trastuzumab at 6 mg/kg at the rate of every 3 weeks costs about $2,500 per dose. The VA pays about $6 a milligram, but it’s certainly money well spent because it has changed the playing field and the outcomes for these patients.
The cost of pertuzumab is dosed a little bit differently. It’s a flat dose not a weight-based dose. Patients get an initial loading dose of 840 mg and a continuation dose of 420 mg every 3 weeks. The cost of the 420-mg dose of pertuzumab is just under $3,000, so that first-time loading dose would be a $6,000 dose, and the continuing doses are about $3,000 per dose every 3 weeks. The AE profile is no different from what you would expect with trastuzumab. There is a similar toxicity profile for these 2 drugs. It does not appear that there is any additional cardiotoxicity if you are using the combination in the neoadjuvant setting.
The third targeted agent that goes after the HER2 is ado-trastuzumab, but it is only used in the metastatic setting, so we’ll reserve that for down the road for this patient should we ever need it.
Dr. Aggarwal. The patient received adriamycin/cyclophosphamide followed by paclitaxel weekly for 12 weeks with trastuzumab. After the 12 weekly doses, she went on trastuzumab every 3 weeks. Because she was ER+, she was a candidate for additional endocrine ablation therapy. She was started on tamoxifen and leuprolide acetate for complete hormonal ablation.
Tamoxifen was the first targeted therapy for breast cancer. In women with ER+ breast cancer, with tamoxifen given for 5 years as adjuvant treatment, the odds of recurrences decreased by 39%, and death decreased by 30% in both pre- and postmenopausal women.8 Then the ATLAS data came, which randomly allocated patients to continue another 5 years of tamoxifen vs placebo, for a total of 10 years of treatment with tamoxifen. With a mean of 7.6 years of further follow-up after entry at year 5 in this trial showed that recurrence and breast cancer mortality during the second decade after diagnosis are reduced more effectively by 10 years of adjuvant tamoxifen than by 5 years.9 The current recommendation for pre- and postmenopausal is 10 years of tamoxifen.
In addition we have 3 aromatase inhibitors (AIs), anastrozole, letrozole, and exemestane, which block the production of estrogen in postmenopausal females. Anastrozole and letrozole are nonsteroidal, and exemestane is steroidal. There are countless big randomized trials using all of these drug in different combinations. In most of these trials, AIs are shown to be equal to tamoxifen when they are compared with each other, but their AE profile is different.
The recommendation by the American Society of Clinical Oncology and NCCN guidelines is to use only AIs for 5 years. There are different combinations: You can give tamoxifen for 2 to 3 years, followed by 5 years of an AI, or 5 years of tamoxifen and 5 years of an AI. Some patients wants to stop because of AEs, but others want to continue. Patients can develop osteoporosis and arthritis from an AI and hot flashes from tamoxifen.
Mr. Crawford, How would you manage of these AEs from these treatments?
Mr. Crawford. Because this woman is young, age 32, and premenopausal, tamoxifen would be the recommended endocrine therapy for her being ER+/PR+. But the role of the leuprolide acetate is to induce a chemical oophorectomy. We are putting her into ovarian ablation by using the leuprolide acetate.
The tamoxifen is relatively well tolerated, but as an ER blocker, it has a different AE profile than does an estrogen production decreaser. With tamoxifen patients tend to complain about hot flashes, edema, fluid retention, altered menses, spotting vaginal discharge, vaginal bleeding, and dryness. These medications also increase the risk of venous thromboembolism (VTE), and there is some concern about increased risk of developing endometrial cancers with these medications. We can give it either once or twice daily. There’s nothing that really says 10 mg twice daily vs 20 mg once daily is any different. So we may play with dosing to see if patients tolerate it better one way or the other.
There are medications that we can offer to help manage the hot flashes. These medications don’t necessarily make the hot flashes go away, but they can decrease the hot flash intensity or and/or frequency. Many medications have been evaluated for hot flashes. The best data are for venlafaxine, which is usually given once a day at bedtime (dosage 37.5-75.0 mg). There has been success with gabapentin titrated up to a dose of about 300 mg 3 times daily. They are fairly similar for decreasing hot flash scores and intensities, but the patient preferences were more favorable toward the venlafaxine than for the gabapentin.
The AIs, on the other hand, have a different AE profile. With tamoxifen we see vaginal discharges, bleeding, endometrial cancer risk, and VTE risk, but these are not significant problems with any of the AIs. The AE profiles for AIs include hot flashes, but more often it is complaints of bone pain, arthralgias, and myalgias. Probably the top reason why most patients discontinue taking AIs is arthralgia and myalgia.
Because we have shut off estrogen production with the AIs, and estrogen is an important component of maintaining good bone health and bone homeostasis, patients are at an increased risked of losing or declining bone mineral density (BMD). It is recommended that these patients get placed on routine calcium and vitamin D supplementation with routine dual-energy X-ray absorptiometry scans, so we know whether we will need to initiate osteoporosis treatment, whether with oral bisphosphonates, intravenous bisphosphonates, or subcutaneous rank ligand inhibitors.
With bisphosphonates there may be a slight increase in fracture rates. But we have to balance that with the BMD concerns. If the patient progresses into the metastatic setting and we know that there’s a fair chance that there’s going to be some skeletal involvement, those people are also at an increased risk of fracture. While there is a slight concern about the increased risk of fractures with bisphosphonates, I tend to believe that the benefits outweigh the risks.
Go to www.fedprac.com/AVAHO for a discussion of the next steps in the treatment for the patient after she returned 2 years later with nausea, vomiting, acute onset headache, and 2 brain lesions that were about 2 cm.
Click here to read the digital edition.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
1. Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of african ancestry. Cancer Epidemiol Biomarkers. 2017;26(7):1016-1026.
2. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180.
3. Poortmans PM, Collette S, Kirkove C, et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327.
4. Whelan TJ, Olivotto IA, Parulekar WR, et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316.
5. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
6. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273.
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
9. Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816.
Why Am I Being Treated Like a Female Breast Cancer Patient? (FULL)
Patient Perspective
Breast cancer has been one of my life’s greatest blessings. Its highs and lows, prospects, and disappointments have only strengthened my faith and turned me more to God.
In March 2012, I had a bad cold, and while I was coughing and grabbing my chest, I discovered a small knot in my left breast, and for whatever reason, I suspected it was cancer. I immediately woke my wife. She, groggy and in usual humor exclaimed, “Oh great! You have breast cancer! Well guess what? I have prostate cancer…now go back to sleep!” I laughed at the prospect of her having prostate cancer. It certainly would’ve changed a few dynamics in our relationship.
Two weeks later my fears were confirmed. I was told that I needed to have a mastectomy of my left breast. I wanted nothing but to have this poison removed. Yesterday would not have been too soon.
My surgery was scheduled a month later; it was a long wait. And it soon became clear that as I recovered from the impending mastectomy, I also would be in line for open-heart surgery.
The mastectomy was a textbook procedure with no complications. My surgeon apprehensively warned me that follow-up visits would be at the Women’s Health Center. I must admit, it was awkward every time I went. Realistically though, I cared more about my health than about others’ perceptions.
While I prepared for my cardiac surgery, the blood test revealed triglyceride levels that were through the roof. In fact, the cardiac surgeon described them as “industrial strength.” After an exhaustive review, it was determined that my adjuvant therapy with tamoxifen was the culprit! I immediately stopped taking it, and within days my levels returned to normal. I was now left to fight any future bouts of cancer with just my body’s own defenses.
It probably seems strange, but if I had not found the breast lump, the problems with my heart would have gone undetected. I most likely would’ve died. Had the cancer not been a part of my life, I wouldn’t have been able to keep on living.
In the middle of March 2016, during preliminary testing for surgery to remove a skin tag, my chest X-ray displayed abnormalities. The workup showed that my breast cancer had returned. Worse yet, it had metastasized to my lungs. It had gone into my lymph nodes and lower spine.
The fight was on. A treatment plan was outlined; 12 weeks of chemotherapy infusions was a reasonable plan of attack. A second opinion was not necessarily an opportunity to find a differing plan, but as in my case, it was comforting affirmation of a good plan. I remember wondering if the rest of my life was going to be a mix of hospital visits, blood transfusions, chemotherapies, and injections.
While fear of the unknown works on one’s psyche, I made a decision to focus on my faith and God. My cancer experiences are probably no worse or different from the experiences of most other patients. I do believe that my perception of how cancer affected me psychologically is a different story. I know and trust that I am in the capable and knowledgeable hands of my doctor.
While the experience of good health care is remarkable, living with cancer does not end with medical care. I am blessed to have a partner who loves me infinitely. I cannot imagine my life without her.
I am grateful my cancer has allowed me to remain alive. The prospect of death does not shake me. I plan on living my life to the fullest.
Oncologist Perspective
Yes, men do get breast cancer! Unlike female breast cancer (FBC), male breast cancer (MBC) makes up about 1% of all cases in the U.S. The lifetime risk of a man developing breast cancer is about 1 in 1,000 vs 1 in 8 women.1 Little is known about MBC because its rarity renders prospective randomized trials problematic. As a result, the management of breast cancer in males from diagnosis to treatment is based on research on FBC. Patients with MBC have higher mortality, and the incidence is rising 1.1% per year; by comparison both trends are decreasing for females with breast cancer.2,3
Males are usually older and present with an advanced stage of the disease at the time of the diagnosis. Most MBC is ER+/PR+ and HER2−.4 Comparison data of 1,123 male veterans with 5,320 females revealed that the mean age at diagnosis was 70 years for MBC and 57 years for FBC, respectively (P < .01); 95% of patients with MBC and 72% of patients with FBC were aged > 50 years (P < .01). Patients with MBC were more likely to present with stage III or IV disease (40% vs 24%, respectively). Eighty percent of patients with MBC had ER+/PR+ tumors. Mortality was 31.6% in males vs 14.9% in females.
Given the high prevalence of ER/PR positivity, MBC usually is considered to have a better prognosis, but that does not explain the high mortality. Unlike FBC, delay in diagnosis due to lack of MBC awareness and no screening guidelines for MBC, older age at diagnosis, and comorbidities have been considered the etiology of higher mortality in MBC, but there has to be more than that. I believe that the differences in MBC biology and pathology also have to be contributing factors to MBC mortality.
As a VA oncologist, I have treated a number of patients with MBC. Surprisingly, my experience treating these patients has been different from treating FBC. In 2011, when I first met Mr. Lewis, he laughed and questioned his diagnosis—how could he have breast cancer if males don’t have breasts, and none of his family member had any type of cancer. Prior to his cancer diagnoses, he had gone through multiple cardiac stents and had a history of hypertriglyceridemia. His cancer workup and treatment plan were the same as that of females with breast cancer, and he questioned me again, “Why am I being treated like a female breast cancer patient?”
Unlike females with breast cancer, he had to have a complete mastectomy given the small breast tissue. His final diagnosis was stage IIA invasive ductal carcinoma of the left breast.
Because of Mr. Lewis’ cardiac history and recent stent placement, I was hesitant to give him first-line adjuvant anthracycline. The Oncotype DX test is highly recommended and easily done for FBC, but I had to go through great difficulty to order this test for him. The Oncotype Dx RS score for him was 17 (a so-called low score) with distant recurrence risk of 11%. I interpreted the test the same way as I would for a patient with FBC. We were happy that he did not have to be exposed to toxic chemotherapies.
Because of the lack of data for aromatase inhibitors (AIs) use in males, adjuvant tamoxifen was given but had to be stopped after a month because of hypertriglyceridemia > 8,000 mg/dL and cholesterol > 700 mg/dL. Tamoxifen as well as an AI was deemed not to be the right adjuvant treatment for him. There were no data on adjuvant fulvestrant; not even for females in 2012. Mr. Lewis was among the unlucky 11% and presented with stage IV disease in his lungs and bones 4 years after the initial diagnosis. He has not had a great response to taxanes and now is being treated with fulvestrant. He remains positive and hopeful, he told me only God—not medical science—has the power to take back the gift of life.
My experience with Mr. Lewis and others has underscored that MBC is not the same disease as FBC. I am hopeful we will see more clinical trials to further identify MBC biology and genomics.
Click here to read the digital edition.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232-239.
3. Howlander N, Noone AM, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2009: fast stats. http://seer.cancer.gov/csr/1975_2009_pops09. Updated April 2012. Accessed January 20, 2018.
4. Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918-1926.
Patient Perspective
Breast cancer has been one of my life’s greatest blessings. Its highs and lows, prospects, and disappointments have only strengthened my faith and turned me more to God.
In March 2012, I had a bad cold, and while I was coughing and grabbing my chest, I discovered a small knot in my left breast, and for whatever reason, I suspected it was cancer. I immediately woke my wife. She, groggy and in usual humor exclaimed, “Oh great! You have breast cancer! Well guess what? I have prostate cancer…now go back to sleep!” I laughed at the prospect of her having prostate cancer. It certainly would’ve changed a few dynamics in our relationship.
Two weeks later my fears were confirmed. I was told that I needed to have a mastectomy of my left breast. I wanted nothing but to have this poison removed. Yesterday would not have been too soon.
My surgery was scheduled a month later; it was a long wait. And it soon became clear that as I recovered from the impending mastectomy, I also would be in line for open-heart surgery.
The mastectomy was a textbook procedure with no complications. My surgeon apprehensively warned me that follow-up visits would be at the Women’s Health Center. I must admit, it was awkward every time I went. Realistically though, I cared more about my health than about others’ perceptions.
While I prepared for my cardiac surgery, the blood test revealed triglyceride levels that were through the roof. In fact, the cardiac surgeon described them as “industrial strength.” After an exhaustive review, it was determined that my adjuvant therapy with tamoxifen was the culprit! I immediately stopped taking it, and within days my levels returned to normal. I was now left to fight any future bouts of cancer with just my body’s own defenses.
It probably seems strange, but if I had not found the breast lump, the problems with my heart would have gone undetected. I most likely would’ve died. Had the cancer not been a part of my life, I wouldn’t have been able to keep on living.
In the middle of March 2016, during preliminary testing for surgery to remove a skin tag, my chest X-ray displayed abnormalities. The workup showed that my breast cancer had returned. Worse yet, it had metastasized to my lungs. It had gone into my lymph nodes and lower spine.
The fight was on. A treatment plan was outlined; 12 weeks of chemotherapy infusions was a reasonable plan of attack. A second opinion was not necessarily an opportunity to find a differing plan, but as in my case, it was comforting affirmation of a good plan. I remember wondering if the rest of my life was going to be a mix of hospital visits, blood transfusions, chemotherapies, and injections.
While fear of the unknown works on one’s psyche, I made a decision to focus on my faith and God. My cancer experiences are probably no worse or different from the experiences of most other patients. I do believe that my perception of how cancer affected me psychologically is a different story. I know and trust that I am in the capable and knowledgeable hands of my doctor.
While the experience of good health care is remarkable, living with cancer does not end with medical care. I am blessed to have a partner who loves me infinitely. I cannot imagine my life without her.
I am grateful my cancer has allowed me to remain alive. The prospect of death does not shake me. I plan on living my life to the fullest.
Oncologist Perspective
Yes, men do get breast cancer! Unlike female breast cancer (FBC), male breast cancer (MBC) makes up about 1% of all cases in the U.S. The lifetime risk of a man developing breast cancer is about 1 in 1,000 vs 1 in 8 women.1 Little is known about MBC because its rarity renders prospective randomized trials problematic. As a result, the management of breast cancer in males from diagnosis to treatment is based on research on FBC. Patients with MBC have higher mortality, and the incidence is rising 1.1% per year; by comparison both trends are decreasing for females with breast cancer.2,3
Males are usually older and present with an advanced stage of the disease at the time of the diagnosis. Most MBC is ER+/PR+ and HER2−.4 Comparison data of 1,123 male veterans with 5,320 females revealed that the mean age at diagnosis was 70 years for MBC and 57 years for FBC, respectively (P < .01); 95% of patients with MBC and 72% of patients with FBC were aged > 50 years (P < .01). Patients with MBC were more likely to present with stage III or IV disease (40% vs 24%, respectively). Eighty percent of patients with MBC had ER+/PR+ tumors. Mortality was 31.6% in males vs 14.9% in females.
Given the high prevalence of ER/PR positivity, MBC usually is considered to have a better prognosis, but that does not explain the high mortality. Unlike FBC, delay in diagnosis due to lack of MBC awareness and no screening guidelines for MBC, older age at diagnosis, and comorbidities have been considered the etiology of higher mortality in MBC, but there has to be more than that. I believe that the differences in MBC biology and pathology also have to be contributing factors to MBC mortality.
As a VA oncologist, I have treated a number of patients with MBC. Surprisingly, my experience treating these patients has been different from treating FBC. In 2011, when I first met Mr. Lewis, he laughed and questioned his diagnosis—how could he have breast cancer if males don’t have breasts, and none of his family member had any type of cancer. Prior to his cancer diagnoses, he had gone through multiple cardiac stents and had a history of hypertriglyceridemia. His cancer workup and treatment plan were the same as that of females with breast cancer, and he questioned me again, “Why am I being treated like a female breast cancer patient?”
Unlike females with breast cancer, he had to have a complete mastectomy given the small breast tissue. His final diagnosis was stage IIA invasive ductal carcinoma of the left breast.
Because of Mr. Lewis’ cardiac history and recent stent placement, I was hesitant to give him first-line adjuvant anthracycline. The Oncotype DX test is highly recommended and easily done for FBC, but I had to go through great difficulty to order this test for him. The Oncotype Dx RS score for him was 17 (a so-called low score) with distant recurrence risk of 11%. I interpreted the test the same way as I would for a patient with FBC. We were happy that he did not have to be exposed to toxic chemotherapies.
Because of the lack of data for aromatase inhibitors (AIs) use in males, adjuvant tamoxifen was given but had to be stopped after a month because of hypertriglyceridemia > 8,000 mg/dL and cholesterol > 700 mg/dL. Tamoxifen as well as an AI was deemed not to be the right adjuvant treatment for him. There were no data on adjuvant fulvestrant; not even for females in 2012. Mr. Lewis was among the unlucky 11% and presented with stage IV disease in his lungs and bones 4 years after the initial diagnosis. He has not had a great response to taxanes and now is being treated with fulvestrant. He remains positive and hopeful, he told me only God—not medical science—has the power to take back the gift of life.
My experience with Mr. Lewis and others has underscored that MBC is not the same disease as FBC. I am hopeful we will see more clinical trials to further identify MBC biology and genomics.
Click here to read the digital edition.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Patient Perspective
Breast cancer has been one of my life’s greatest blessings. Its highs and lows, prospects, and disappointments have only strengthened my faith and turned me more to God.
In March 2012, I had a bad cold, and while I was coughing and grabbing my chest, I discovered a small knot in my left breast, and for whatever reason, I suspected it was cancer. I immediately woke my wife. She, groggy and in usual humor exclaimed, “Oh great! You have breast cancer! Well guess what? I have prostate cancer…now go back to sleep!” I laughed at the prospect of her having prostate cancer. It certainly would’ve changed a few dynamics in our relationship.
Two weeks later my fears were confirmed. I was told that I needed to have a mastectomy of my left breast. I wanted nothing but to have this poison removed. Yesterday would not have been too soon.
My surgery was scheduled a month later; it was a long wait. And it soon became clear that as I recovered from the impending mastectomy, I also would be in line for open-heart surgery.
The mastectomy was a textbook procedure with no complications. My surgeon apprehensively warned me that follow-up visits would be at the Women’s Health Center. I must admit, it was awkward every time I went. Realistically though, I cared more about my health than about others’ perceptions.
While I prepared for my cardiac surgery, the blood test revealed triglyceride levels that were through the roof. In fact, the cardiac surgeon described them as “industrial strength.” After an exhaustive review, it was determined that my adjuvant therapy with tamoxifen was the culprit! I immediately stopped taking it, and within days my levels returned to normal. I was now left to fight any future bouts of cancer with just my body’s own defenses.
It probably seems strange, but if I had not found the breast lump, the problems with my heart would have gone undetected. I most likely would’ve died. Had the cancer not been a part of my life, I wouldn’t have been able to keep on living.
In the middle of March 2016, during preliminary testing for surgery to remove a skin tag, my chest X-ray displayed abnormalities. The workup showed that my breast cancer had returned. Worse yet, it had metastasized to my lungs. It had gone into my lymph nodes and lower spine.
The fight was on. A treatment plan was outlined; 12 weeks of chemotherapy infusions was a reasonable plan of attack. A second opinion was not necessarily an opportunity to find a differing plan, but as in my case, it was comforting affirmation of a good plan. I remember wondering if the rest of my life was going to be a mix of hospital visits, blood transfusions, chemotherapies, and injections.
While fear of the unknown works on one’s psyche, I made a decision to focus on my faith and God. My cancer experiences are probably no worse or different from the experiences of most other patients. I do believe that my perception of how cancer affected me psychologically is a different story. I know and trust that I am in the capable and knowledgeable hands of my doctor.
While the experience of good health care is remarkable, living with cancer does not end with medical care. I am blessed to have a partner who loves me infinitely. I cannot imagine my life without her.
I am grateful my cancer has allowed me to remain alive. The prospect of death does not shake me. I plan on living my life to the fullest.
Oncologist Perspective
Yes, men do get breast cancer! Unlike female breast cancer (FBC), male breast cancer (MBC) makes up about 1% of all cases in the U.S. The lifetime risk of a man developing breast cancer is about 1 in 1,000 vs 1 in 8 women.1 Little is known about MBC because its rarity renders prospective randomized trials problematic. As a result, the management of breast cancer in males from diagnosis to treatment is based on research on FBC. Patients with MBC have higher mortality, and the incidence is rising 1.1% per year; by comparison both trends are decreasing for females with breast cancer.2,3
Males are usually older and present with an advanced stage of the disease at the time of the diagnosis. Most MBC is ER+/PR+ and HER2−.4 Comparison data of 1,123 male veterans with 5,320 females revealed that the mean age at diagnosis was 70 years for MBC and 57 years for FBC, respectively (P < .01); 95% of patients with MBC and 72% of patients with FBC were aged > 50 years (P < .01). Patients with MBC were more likely to present with stage III or IV disease (40% vs 24%, respectively). Eighty percent of patients with MBC had ER+/PR+ tumors. Mortality was 31.6% in males vs 14.9% in females.
Given the high prevalence of ER/PR positivity, MBC usually is considered to have a better prognosis, but that does not explain the high mortality. Unlike FBC, delay in diagnosis due to lack of MBC awareness and no screening guidelines for MBC, older age at diagnosis, and comorbidities have been considered the etiology of higher mortality in MBC, but there has to be more than that. I believe that the differences in MBC biology and pathology also have to be contributing factors to MBC mortality.
As a VA oncologist, I have treated a number of patients with MBC. Surprisingly, my experience treating these patients has been different from treating FBC. In 2011, when I first met Mr. Lewis, he laughed and questioned his diagnosis—how could he have breast cancer if males don’t have breasts, and none of his family member had any type of cancer. Prior to his cancer diagnoses, he had gone through multiple cardiac stents and had a history of hypertriglyceridemia. His cancer workup and treatment plan were the same as that of females with breast cancer, and he questioned me again, “Why am I being treated like a female breast cancer patient?”
Unlike females with breast cancer, he had to have a complete mastectomy given the small breast tissue. His final diagnosis was stage IIA invasive ductal carcinoma of the left breast.
Because of Mr. Lewis’ cardiac history and recent stent placement, I was hesitant to give him first-line adjuvant anthracycline. The Oncotype DX test is highly recommended and easily done for FBC, but I had to go through great difficulty to order this test for him. The Oncotype Dx RS score for him was 17 (a so-called low score) with distant recurrence risk of 11%. I interpreted the test the same way as I would for a patient with FBC. We were happy that he did not have to be exposed to toxic chemotherapies.
Because of the lack of data for aromatase inhibitors (AIs) use in males, adjuvant tamoxifen was given but had to be stopped after a month because of hypertriglyceridemia > 8,000 mg/dL and cholesterol > 700 mg/dL. Tamoxifen as well as an AI was deemed not to be the right adjuvant treatment for him. There were no data on adjuvant fulvestrant; not even for females in 2012. Mr. Lewis was among the unlucky 11% and presented with stage IV disease in his lungs and bones 4 years after the initial diagnosis. He has not had a great response to taxanes and now is being treated with fulvestrant. He remains positive and hopeful, he told me only God—not medical science—has the power to take back the gift of life.
My experience with Mr. Lewis and others has underscored that MBC is not the same disease as FBC. I am hopeful we will see more clinical trials to further identify MBC biology and genomics.
Click here to read the digital edition.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232-239.
3. Howlander N, Noone AM, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2009: fast stats. http://seer.cancer.gov/csr/1975_2009_pops09. Updated April 2012. Accessed January 20, 2018.
4. Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918-1926.
1. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232-239.
3. Howlander N, Noone AM, Krapcho M, et al. eds. SEER cancer statistics review, 1975-2009: fast stats. http://seer.cancer.gov/csr/1975_2009_pops09. Updated April 2012. Accessed January 20, 2018.
4. Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918-1926.
Thirteen Years and Still Growing: An AVAHO History (FULL)
The Association of VA Hematology/Oncology (AVAHO) is now 13 years old, and we have much to celebrate! Like an adolescent, the organization has grown significantly over the past 13 years, and there is still a lot to learn and plenty of opportunities to grow. To understand where we are going, it is helpful to reflect on our past before looking ahead to a bright future.
It is true that in the 1980s the VHA hosted an annual cancer symposium. This forum was focused on veterans with cancer and provided a unique opportunity for VA health care professionals (HCPs) to meet. Lack of funding, strict rules, concerns over conflicts of interest eventually meant that the symposia could not continue. There was a dire need to fill the void, and VA HCPs were nostalgic.
Because of the VA’s population of patients and structure, VA HCPs face unique challengesand opportunities. The founders of AVAHO saw the benefits and positive influences that providing a platform for education, networking, and research opportunities would bring to VA care. The seed for AVAHO was planted in the summer of 2005 by a group of passionate hematology and oncology professionals working at the VHA, including Abdul-Rahman Jazieh, MD, MPH, who was professor of medicine in the division of hematology/medical oncology at the University of Cincinnati in Ohio. This group sought to implement a forum for interaction among VA hematology/oncology professionals across the nation with the simple goal of providing the best care possible to our nation’s veterans facing cancer. Dr. Jazieh, developed a partnership that joined VA HCPs; the pharmaceutical industry (including Celgene’s Jackie Rychel), which provided financial support; and workforce Strategies (Tammy Pritchard); which provided logistic and legal support. Sue Lentz was employed as administrator.
The first official AVAHO inaugural meeting was held in Cincinnati, Ohio, on September 17, 2005, and the first executive committee consisted of Dr. Jazieh (president); Malek Safa, MD (vice president); Rami Komrokji, MD (secretary); and Zeina Nahleh, MD (treasurer).
A Maturing Organization
The society has grown over the past 13 years from fewer than 100 to a robust 630-member organization that provides a high-quality conference annually, including continuing medical education (CME) and non-CME sessions. Attendance at the annual conferences grew from 35 members in 2005 to nearly 400 in 2016. The first conference program lasted about 6 hours and included a business meeting and education and breakout sessions. Since 2005, the AVAHO meeting has expanded to 2 days to include CME and non-CME concurrent education sessions, special interest group breakout discussions, scientific posters, an exhibitor showcase, and networking opportunities. In addition, pharmaceutical company-sponsored satellite symposia have become very attractive to our members in the past 2 years and help support the organization’s goals. Now it has become difficult to fit the growing agenda into 2 days.
From its inception, AVAHO has been interdisciplinary so that professionals from across the cancer care team could connect, share their expertise and experience, and develop new strategies for cancer care delivery and research. From Nashville to Portland, Atlanta to Omaha, Washington, DC, to Dallas, the annual conference location varies to facilitate attendance from all areas of the country.
The society also has been at the vanguard of promoting cutting-edge science, precision medicine, and fostering innovation in cancer care as can be seen in the pages that follow. In addition, AVAHO is committed to providing essential CME for all hematology and oncology professionals and opportunities for HCPs to network and collaborate.
Beginning in 2012, AVAHO forged a strategic relationship with Federal Practitioner, and that relationship has grown to include the publication of AVAHO abstracts and a series of special issues focused on hematology and oncology—including this one.
A Unique Organization
AVAHO is unique. We are a 501(c)(3) nonprofit organization that is volunteer-led and managed with the support of a single paid staff member. This is the only professional association where all disciplines of hematology oncology professionals meet with only one focus—care of veterans with cancer.
The AVAHO mission is to provide leadership in delivering quality comprehensive care to veterans with cancer; education for members to improve the quality of cancer care for veterans; a mechanism for networking among members to gain knowledge and best practices from the experiences of colleagues; and a venue to explore and facilitate new multidisciplinary research. One priority has been to continually increase the benefits of being an AVAHO member. Members have access to a variety of resources to keep current with trends in cancer care as well as to low-cost continuing education credits that are essential for licensure across disciplines.
The education AVAHO provides is focused on the unique nature of providing “the best care, anywhere” inside the VHA system. In addition, AVAHO continues to help minimize the costs associated with travel to the conference site, cost of stay, some of the associated meals provided in part by AVAHO. As members, HCPs also have the opportunity to raise issues and concerns and share successes and almost-successes, and support career development of professional peers.
Last year, AVAHO announced its first scholarship, a $10,000 research scholarship to a young investigator. Anyone who completed education and/or training within the past 10 years and has a minimum 5/8ths appointment at a VA facility can apply for these funds.
The teen years are not always easy but offer great promise. Over the past 13 years, AVAHO has grown significantly as an organization and continues to offer more opportunities for education, research, and networking, all with the hope of improving the quality of care for veterans with cancer and increasing support for their caregivers.
Click here to read the digital edition.
The Association of VA Hematology/Oncology (AVAHO) is now 13 years old, and we have much to celebrate! Like an adolescent, the organization has grown significantly over the past 13 years, and there is still a lot to learn and plenty of opportunities to grow. To understand where we are going, it is helpful to reflect on our past before looking ahead to a bright future.
It is true that in the 1980s the VHA hosted an annual cancer symposium. This forum was focused on veterans with cancer and provided a unique opportunity for VA health care professionals (HCPs) to meet. Lack of funding, strict rules, concerns over conflicts of interest eventually meant that the symposia could not continue. There was a dire need to fill the void, and VA HCPs were nostalgic.
Because of the VA’s population of patients and structure, VA HCPs face unique challengesand opportunities. The founders of AVAHO saw the benefits and positive influences that providing a platform for education, networking, and research opportunities would bring to VA care. The seed for AVAHO was planted in the summer of 2005 by a group of passionate hematology and oncology professionals working at the VHA, including Abdul-Rahman Jazieh, MD, MPH, who was professor of medicine in the division of hematology/medical oncology at the University of Cincinnati in Ohio. This group sought to implement a forum for interaction among VA hematology/oncology professionals across the nation with the simple goal of providing the best care possible to our nation’s veterans facing cancer. Dr. Jazieh, developed a partnership that joined VA HCPs; the pharmaceutical industry (including Celgene’s Jackie Rychel), which provided financial support; and workforce Strategies (Tammy Pritchard); which provided logistic and legal support. Sue Lentz was employed as administrator.
The first official AVAHO inaugural meeting was held in Cincinnati, Ohio, on September 17, 2005, and the first executive committee consisted of Dr. Jazieh (president); Malek Safa, MD (vice president); Rami Komrokji, MD (secretary); and Zeina Nahleh, MD (treasurer).
A Maturing Organization
The society has grown over the past 13 years from fewer than 100 to a robust 630-member organization that provides a high-quality conference annually, including continuing medical education (CME) and non-CME sessions. Attendance at the annual conferences grew from 35 members in 2005 to nearly 400 in 2016. The first conference program lasted about 6 hours and included a business meeting and education and breakout sessions. Since 2005, the AVAHO meeting has expanded to 2 days to include CME and non-CME concurrent education sessions, special interest group breakout discussions, scientific posters, an exhibitor showcase, and networking opportunities. In addition, pharmaceutical company-sponsored satellite symposia have become very attractive to our members in the past 2 years and help support the organization’s goals. Now it has become difficult to fit the growing agenda into 2 days.
From its inception, AVAHO has been interdisciplinary so that professionals from across the cancer care team could connect, share their expertise and experience, and develop new strategies for cancer care delivery and research. From Nashville to Portland, Atlanta to Omaha, Washington, DC, to Dallas, the annual conference location varies to facilitate attendance from all areas of the country.
The society also has been at the vanguard of promoting cutting-edge science, precision medicine, and fostering innovation in cancer care as can be seen in the pages that follow. In addition, AVAHO is committed to providing essential CME for all hematology and oncology professionals and opportunities for HCPs to network and collaborate.
Beginning in 2012, AVAHO forged a strategic relationship with Federal Practitioner, and that relationship has grown to include the publication of AVAHO abstracts and a series of special issues focused on hematology and oncology—including this one.
A Unique Organization
AVAHO is unique. We are a 501(c)(3) nonprofit organization that is volunteer-led and managed with the support of a single paid staff member. This is the only professional association where all disciplines of hematology oncology professionals meet with only one focus—care of veterans with cancer.
The AVAHO mission is to provide leadership in delivering quality comprehensive care to veterans with cancer; education for members to improve the quality of cancer care for veterans; a mechanism for networking among members to gain knowledge and best practices from the experiences of colleagues; and a venue to explore and facilitate new multidisciplinary research. One priority has been to continually increase the benefits of being an AVAHO member. Members have access to a variety of resources to keep current with trends in cancer care as well as to low-cost continuing education credits that are essential for licensure across disciplines.
The education AVAHO provides is focused on the unique nature of providing “the best care, anywhere” inside the VHA system. In addition, AVAHO continues to help minimize the costs associated with travel to the conference site, cost of stay, some of the associated meals provided in part by AVAHO. As members, HCPs also have the opportunity to raise issues and concerns and share successes and almost-successes, and support career development of professional peers.
Last year, AVAHO announced its first scholarship, a $10,000 research scholarship to a young investigator. Anyone who completed education and/or training within the past 10 years and has a minimum 5/8ths appointment at a VA facility can apply for these funds.
The teen years are not always easy but offer great promise. Over the past 13 years, AVAHO has grown significantly as an organization and continues to offer more opportunities for education, research, and networking, all with the hope of improving the quality of care for veterans with cancer and increasing support for their caregivers.
Click here to read the digital edition.
The Association of VA Hematology/Oncology (AVAHO) is now 13 years old, and we have much to celebrate! Like an adolescent, the organization has grown significantly over the past 13 years, and there is still a lot to learn and plenty of opportunities to grow. To understand where we are going, it is helpful to reflect on our past before looking ahead to a bright future.
It is true that in the 1980s the VHA hosted an annual cancer symposium. This forum was focused on veterans with cancer and provided a unique opportunity for VA health care professionals (HCPs) to meet. Lack of funding, strict rules, concerns over conflicts of interest eventually meant that the symposia could not continue. There was a dire need to fill the void, and VA HCPs were nostalgic.
Because of the VA’s population of patients and structure, VA HCPs face unique challengesand opportunities. The founders of AVAHO saw the benefits and positive influences that providing a platform for education, networking, and research opportunities would bring to VA care. The seed for AVAHO was planted in the summer of 2005 by a group of passionate hematology and oncology professionals working at the VHA, including Abdul-Rahman Jazieh, MD, MPH, who was professor of medicine in the division of hematology/medical oncology at the University of Cincinnati in Ohio. This group sought to implement a forum for interaction among VA hematology/oncology professionals across the nation with the simple goal of providing the best care possible to our nation’s veterans facing cancer. Dr. Jazieh, developed a partnership that joined VA HCPs; the pharmaceutical industry (including Celgene’s Jackie Rychel), which provided financial support; and workforce Strategies (Tammy Pritchard); which provided logistic and legal support. Sue Lentz was employed as administrator.
The first official AVAHO inaugural meeting was held in Cincinnati, Ohio, on September 17, 2005, and the first executive committee consisted of Dr. Jazieh (president); Malek Safa, MD (vice president); Rami Komrokji, MD (secretary); and Zeina Nahleh, MD (treasurer).
A Maturing Organization
The society has grown over the past 13 years from fewer than 100 to a robust 630-member organization that provides a high-quality conference annually, including continuing medical education (CME) and non-CME sessions. Attendance at the annual conferences grew from 35 members in 2005 to nearly 400 in 2016. The first conference program lasted about 6 hours and included a business meeting and education and breakout sessions. Since 2005, the AVAHO meeting has expanded to 2 days to include CME and non-CME concurrent education sessions, special interest group breakout discussions, scientific posters, an exhibitor showcase, and networking opportunities. In addition, pharmaceutical company-sponsored satellite symposia have become very attractive to our members in the past 2 years and help support the organization’s goals. Now it has become difficult to fit the growing agenda into 2 days.
From its inception, AVAHO has been interdisciplinary so that professionals from across the cancer care team could connect, share their expertise and experience, and develop new strategies for cancer care delivery and research. From Nashville to Portland, Atlanta to Omaha, Washington, DC, to Dallas, the annual conference location varies to facilitate attendance from all areas of the country.
The society also has been at the vanguard of promoting cutting-edge science, precision medicine, and fostering innovation in cancer care as can be seen in the pages that follow. In addition, AVAHO is committed to providing essential CME for all hematology and oncology professionals and opportunities for HCPs to network and collaborate.
Beginning in 2012, AVAHO forged a strategic relationship with Federal Practitioner, and that relationship has grown to include the publication of AVAHO abstracts and a series of special issues focused on hematology and oncology—including this one.
A Unique Organization
AVAHO is unique. We are a 501(c)(3) nonprofit organization that is volunteer-led and managed with the support of a single paid staff member. This is the only professional association where all disciplines of hematology oncology professionals meet with only one focus—care of veterans with cancer.
The AVAHO mission is to provide leadership in delivering quality comprehensive care to veterans with cancer; education for members to improve the quality of cancer care for veterans; a mechanism for networking among members to gain knowledge and best practices from the experiences of colleagues; and a venue to explore and facilitate new multidisciplinary research. One priority has been to continually increase the benefits of being an AVAHO member. Members have access to a variety of resources to keep current with trends in cancer care as well as to low-cost continuing education credits that are essential for licensure across disciplines.
The education AVAHO provides is focused on the unique nature of providing “the best care, anywhere” inside the VHA system. In addition, AVAHO continues to help minimize the costs associated with travel to the conference site, cost of stay, some of the associated meals provided in part by AVAHO. As members, HCPs also have the opportunity to raise issues and concerns and share successes and almost-successes, and support career development of professional peers.
Last year, AVAHO announced its first scholarship, a $10,000 research scholarship to a young investigator. Anyone who completed education and/or training within the past 10 years and has a minimum 5/8ths appointment at a VA facility can apply for these funds.
The teen years are not always easy but offer great promise. Over the past 13 years, AVAHO has grown significantly as an organization and continues to offer more opportunities for education, research, and networking, all with the hope of improving the quality of care for veterans with cancer and increasing support for their caregivers.
Click here to read the digital edition.
Advances in Targeted Therapy for Breast Cancer
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
It is estimated that there were more than 3.1 million women living in the U.S. with a history of invasive breast cancer as of January 1, 2014, and an additional 231,840 women will be newly diagnosed with invasive breast cancer in 2015.1,2 The median age at the time of breast cancer diagnosis is 61 years. About 20% of breast cancers occur among women aged < 50 years, and 43% occur in women aged > 65 years.
The treatment and prognosis for breast cancer depend on the stage at diagnosis, the biologic characteristics of the tumor, and the age and health of the patient. The overall 5-year relative survival rate for female patients with breast cancer has improved from 75% to 90% from 1975 to 1977 and from 2003 to 2009, respectively, largely due to improvements in treatment (ie, chemotherapy, hormone therapy, and targeted drugs) and because of earlier diagnosis resulting from the widespread use of mammography and other screening tools.2
Estrogen Receptor-Positive Therapies
Women with breast cancer who test positive for hormone receptors are candidates for treatment with hormone therapy to reduce the likelihood of recurrence or as a core component of treatment for advanced disease. Currently available endocrine strategies for the treatment of estrogen receptor- (ER) positive breast cancer include targeting the ER with the antiestrogen drug tamoxifen. Another option is suppressing the amount of available ligand (estrogen) for the receptor either with gonadal suppression in premenopausal oophorectomy, or luteinizing hormonereleasing hormone agonists, or with the aromatase inhibitors (AIs) anastrozole, exemestane, and letrozole in postmenopausal women and by downregulating the receptor with fulvestrant. Given their proven efficacy and generally favorable adverse effect (AE) profile, these endocrine therapies are widely used in the treatment of both early-stage and recurrent and/or metastatic breast cancer.
Recent studies have offered new treatments for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Innovative hormonal and targeted therapies for advanced disease as well as new data on adjuvant hormonal therapy for young high-risk patients are changing the available therapeutic options.
Advanced Metastatic Treatments
Treatment for metastatic hormone receptor-positive breast cancer has shifted from traditional cytotoxic chemotherapies to targeted therapeutic options. Most treatment guidelines, including the National Comprehensive Cancer Network guidelines, recommend targeted therapy with AIs or selective ER modulators rather than chemotherapy, except in the case of visceral crisis.3
Until recently, there had been relatively little guidance to inform which hormonal therapy was most appropriate. Aromatase inhibitors were generally reserved for postmenopausal women, whereas tamoxifen was preferred in premenopausal women.
Fulvestrant
The FDA initially approved fulvestrant, a hormone receptor downregulator, in 2002 at a 250-mg dose, following progression on an anti-estrogen therapy, such as tamoxifen in postmenopausal women with stage IV breast cancer. The FDA approval was based on similar response rates for the already approved agent anastrozole.4 However, pharmacokinetic findings from the phase 3 EFECT trial in 2008 prompted researchers to explore a 500-mg dose of fulvestrant.5
The recently published FIRST study is a phase 2, randomized, open-label study comparing fulvestrant 500 mg with anastrozole 1 mg as first-line hormonal therapy for postmenopausal women with hormone receptorpositive advanced breast cancer. Fulvestrant was given 500 mg once monthly with an extra dose given on day 14 of month 1. The trial enrolled 233 patients. The median time to progression was 23.4 months for fulvestrant and 13.1 months for anastrozole. These results translate into a 34% reduction in the risk of progression.6
These outcomes suggest that fulvestrant is as viable and perhaps even preferred first-line therapy for postmenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer. The impressive results from this trial are likely, because the study used the 500-mg dose of fulvestrant, which is twice the dose used in the original trials. However, the 500-mg dose has previously been studied, and long-term outcome data suggest both safety and efficiency. The large randomized, double-blinded phase 3 CONFIRM trial, published in 2013, compared the 250-mg dose with the 500-mg dose and found that the higher dose was associated with a 19% reduction in the risk of death and a 4.1 month increase in median overall survival (OS) without any new safety concerns.5
Palbociclib
The FDA recently granted accelerated approval to palbociclib in combination with letrozole for the first-line therapy of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women. Palbociclib is an oral small-molecular inhibitor of cyclindependent kinases 4 and 6. Preclinical data suggested synergy with anti-estrogen therapies and inhibition of breast cancer cell growth.7
A phase 2, open-label randomized trial (PALOMA-1/TRIO-18) enrolled 165 patients. Progression-free survival (PFS) was 20.2 months for the palbociclib plus letrozole arm and 10.2 months for the letrozole alone arm. Significant toxicities were noted in the palbociclib arm, including 54% of people experiencing grade 3 to 4 neutropenia (vs 1% in the letrozole arm), leukopenia in 19% (vs 0%) and fatigue in 4% (vs 1%). A phase 3 trial is currently enrolling patients.7 While we await the results of the phase 3 trial and long-term follow-up data, palbociclib plus letrozole is a new, viable option for metastatic hormone receptor-positive advanced breast cancer.
Although many practitioners will continue to reasonably use any AI or selective ER modulator when treating metastatic breast cancer, both fulvestrant and palbociclib in combination with letrozole are new evidence-based, first-line options worth considering.
Early-Stage Treatment Options
There are many acceptable therapeutic options for treating early stage breast cancer. Tamoxifen has traditionally been used in the adjuvant setting for premenopausal women, whereas AIs are often used in postmenopausal women. There has also been a long-standing debate about the role of ovarian suppression in premenopausal women.
The recently published phase 3 TEXT and SOFT trials attempted to provide answers to these long-standing therapeutic dilemmas. The SOFT trial randomly assigned 3,066 premenopausal women to 5 years of tamoxifen, 5 years of tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The TEXT trial randomly assigned 2,672 women to receive either exemestane plus ovarian suppression or tamoxifen plus ovarian suppression. The studies showed that subjecting all women receiving tamoxifen to ovarian suppression did not provide any significant benefit.8,9
However, the subgroup of women with high-risk disease who required adjuvant chemotherapy and remained premenopausal experienced improved outcomes from ovarian suppression. This high-risk subgroup when given tamoxifen plus ovarian suppression had a 4.5% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone. When this high-risk subgroup was given exemestane plus ovarian suppression, the women had a 7.7% absolute reduction in breast cancer recurrence at 5 years compared with the group that received tamoxifen alone.8
Ovarian suppression resulted in significant additional AEs, including depression and menopausal symptoms. The authors of the study also pointed out the additional risk of hypertension, musculoskeletal AEs, and decreased bone density. Furthermore, the OS data from these studies are premature, because the patients had fewer AEs than initially anticipated; this resulted in an only 5% mortality at publication.
The study design also raised several interesting questions. The primary endpoint was disease-free survival. The authors defined this as the time from randomization to the first appearance of invasive recurrence of breast cancer (local, regional, or distant), invasive contralateral breast cancer, second (non-breast) invasive cancer, or death without breast cancer recurrence or second invasive cancer. When studying adjuvant therapy for diseases, such as breast cancer, which carry long-term survival, studies often use PFS with various modified definitions as a surrogate marker for OS. Clinicians are then left to decide whether this surrogate marker is an accurate predictor of OS or other important clinical outcomes.
In the combined analysis of the TEXT and SOFT trials, only 60% of the first recurrences, second invasive cancers, or deaths involved recurrence of breast cancer
at a distant site.9 Because locally recurrent breast cancer is highly treatable and often curable, clinicians must ask whether the increased toxicities of ovarian suppression are worth the large number of women who experienced local recurrence given the still relatively small absolute reduction in recurrence risk.
Last, the study authors retrospectively reviewed data from the International Breast Cancer Study Group and U.S. Intergroup trials and concluded that women aged < 35 years were most likely to be at high-risk for AEs.10,11 A subgroup analysis of women aged < 35 years in the SOFT trial noted that breast cancer recurred within 5 years in one-third of women receiving tamoxifen alone, whereas only in one-sixth of women receiving exemestane plus ovarian suppression.8 This is the basis for the conclusion that premenopausal women, particularly those aged < 35 years, with high-risk disease who receive chemotherapy and remain premenopausal after chemotherapy, benefit from ovarian suppression in combination with tamoxifen, and even more impressively from ovarian suppression combined with exemestane.
The problem is that the study did not risk-stratify patients based on those aged < 35 years, and the conclusion is based on a subgroup analysis using a primary endpoint that may not accurately predict OS. Nonetheless, although not definitive, the data from the TEXT and SOFT trials raise interesting therapeutic questions that require further study and certainly provide tempting therapeutic options in patients who are clinically at high risk for recurrence.
HER2-Positive Breast Cancer
Up to 20% of invasive breast cancers are a result of HER2 gene amplification or overexpression of the HER2 protein, a tyrosine kinase transmembrane receptor, resulting in a more aggressive phenotype and a poor prognosis. Anti-HER2 drugs have changed the landscape of the disease previously known as aggressive breast cancer with a poor survival rate.
Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, compared with chemotherapy alone, significantly improves PFS and OS among patients with HER2-positive metastatic as well as early breast cancer. However, in most patients with HER2-positive metastatic breast cancer, the disease progresses, highlighting the need for new, targeted therapies for advanced disease.
New Standard of Care
The original studies of trastuzumab showed improved OS in late-stage (metastatic) breast cancer from 20.3 to 25.1 months, and in early-stage breast cancer, it reduced the risk of cancer returning after surgery by an absolute risk of 9.5% and the risk of death by an absolute risk of 3%.
New therapies directed at HER2 are being developed, among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain 2) than that at which trastuzumab binds. Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity. Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these 2 agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than does either agent alone in HER2-positive tumor models.12 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer and in patients with early breast cancer.13
In the phase 3 CLEOPATRA study, the combination of pertuzumab plus trastuzumab plus docetaxel, used as first-line treatment for HER2-positive metastatic breast cancer compared with placebo plus trastuzumab plus docetaxel, significantly prolonged PFS (18.5 months vs 12.4 months), with no increase in cardiac toxic effects.12 In a recent updated follow-up of the CLEOPATRA study, the addition of pertuzumab to trastuzumab and docetaxel showed a significantly better median OS (56.5 months vs 40.8 months; hazard ratio, 0.68; P < .001).14 From these results, this combination regimen is now considered a first-line therapy for patients with HER2-positive metastatic breast cancer.
However, the cost of cancer treatment has become a mounting concern during the past decade, as new therapies come down the pipeline with ever-increasing price tags. Trastuzumab costs about $4,500 a month, and the newer pertuzumab runs about 30% higher, at $6,000 a month. For a full course of treatment, the cost of the pertuzumab and trastuzumab combination could go as high as $195,000, depending on the duration of therapy and the choice of taxanes.
Conclusions
The landscape of therapeutic options in high-risk, young patients with early-stage breast cancer as well as patients with advanced or metastatic disease is changing rapidly.
Clinicians now have 2 new first-line options for the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. A phase 3 trial demonstrated that fulvestrant monotherapy offers improved PFS and some improvement in OS compared with anastrazole in postmenopausal women. A phase 2 trial showed that palbociclib plus letrozole offers improved PFS in postmenopausal women. Based on the SOFT and TEXT trials, clinicians treating high-risk premenopausal women now have some data to inform the debate about whether ovarian suppression should be added to hormone therapy.
Based on the CLEOPATRA trial, clinicians can now consider combination pertuzumab and trastuzumab and docetaxel as first-line therapy for patients with HER2-positive metastatic breast cancer.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.
1. American Cancer Society. Cancer facts & figures, 2015. Atlanta, GA: American Cancer Society; 2015.
2. American Cancer Society. Cancer treatment & survivorship facts & figures, 2014-2015. Atlanta, GA: American Cancer Society; 2014.
3. National Comprehensive Cancer Network. NCCN clinical Practice guidelines in oncology: breast Cancer. Version 1. 2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:BINV-19.
4. Howell A, Robertson JF, Quaresma Albano J. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396-3403.
5. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):djt337.
6. Robertson JF, Lindemann JB, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503-511.
7. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
8. Francis PA, Regan MM, Fleming GF, et al; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436-446.
9. Pagani O. Regan MM, Walley BA, et al. TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107-118.
10. Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869-1874.
11. Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001;(30):44-51
12. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
13. Baselga J, Cortés J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119.
14. Swain SM, Baselga J, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734.