User login
Routine Chest Radiographs after Uncomplicated Thoracentesis
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care, but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Bedside thoracentesis can cause serious complications, such as pneumothorax, re-expansion pulmonary edema, or hemorrhage. These rare complications have led many hospitalists to routinely order chest radiographs (CXRs) following thoracentesis. However, post-thoracentesis CXRs are usually not indicated and can lead to unnecessary radiation exposure and expense. Rather than obtaining routine CXRs, hospitalists should use postprocedural signs and symptoms to identify the occasional patients who require imaging. A risk-stratified approach is a safe and cost-effective way to avoid unnecessary radiographs.
CASE REPORT
A 52-year-old man with decompensated liver disease and hepatic hydrothorax is hospitalized for increasing dyspnea caused by a recurrent pleural effusion. Diuretics do not improve his dyspnea, and his hospitalist recommends a therapeutic thoracentesis for symptom relief. The patient does not have any significant procedural risk factors: He does not have preexisting pulmonary or pleural disease, his platelet count is 105,000 × 103/µl, and his international normalized ratio is 1.3. Bedside sonography demonstrates a large, free-flowing, right-sided pleural effusion. The hospitalist performs an uncomplicated ultrasound-guided removal of 1.5 L of straw-colored fluid with a catheter-over-needle kit. The patient does not have any pain or increased shortness of breath during or after the procedure. The hospitalist reflexively orders a routine chest radiograph to assess for pneumothorax.
Why You Might Think a Chest Radiograph is Helpful after Thoracentesis
Pleural effusions are newly diagnosed in more than 1.5 million Americans annually,1 and hospitalists frequently care for patients requiring thoracentesis. Internal medicine residents traditionally learn to perform this procedure during residency, and thoracentesis remains a common task for both residents and hospitalists.2 Patients typically tolerate thoracentesis well, but they can develop serious complications such as pneumothorax, re-expansion pulmonary edema, or hemothorax. Before the advent of bedside ultrasound, these complications occurred relatively commonly; a 2010 systematic review, for example, found that the rate of pneumothorax from thoracentesis performed without ultrasound was 9.3%.3 Other studies have identified even higher rates of complications, including two case series in which investigators found a 14% rate of major complications4 and a pneumothorax rate of nearly 30%.5 Postprocedure radiographs became common practice because of the high rate of complications, and this practice has persisted for many practitioners despite the substantial safety improvements introduced by bedside ultrasonography.6
Hospitalists might think routine CXRs are helpful after ultrasound-guided thoracentesis for additional reasons. First, modern guidelines reflecting the low risk of complications after ultrasound-guided procedures have not been released by United States pulmonary medicine societies, and some clinicians may continue to follow practices acquired during the era of unguided thoracentesis. Second, performing postprocedure imaging has become ingrained as a standard part of some institutional procedure checklists6 and some prominent textbooks continue to recommend the practice.7 For some hospitalists, this testing reflex may be reinforced by other common procedures, such as placing a nasogastric tube or a central venous catheter, for which a postprocedure CXR is standard practice. Thus, ordering postprocedure imaging can become internalized as the safe, checklist-based final step of a procedure. Third, hospitalists may order a postprocedure CXR for reasons other than detecting procedural complications. The pleural effusion might be thought to obscure a parenchymal or endobronchial lesion for which a postprocedure CXR may reveal an important finding. Finally, a CXR also may also satisfy the clinician’s curiosity regarding the completeness of drainage.
Why a Routine Postprocedure Chest Radiograph is Not Helpful after Thoracentesis
A routine post-thoracentesis CXR is not necessary for three reasons. First, the use of ultrasound marking or guidance has substantially improved site selection and reduced the rate of complications for experienced operators. For example, a 2010 systematic review found an overall rate of pneumothorax of 4% for ultrasound-guided procedures performed between 1986 and 2006,3 whereas more recently published data suggest the current rate of pneumothorax is closer to 1% when ultrasound marking or guidance is used.8,9 One study of 462 consecutive patients with malignant pleural effusions, for example, showed that the rate of pneumothorax with ultrasound-guided needle-over-catheter thoracentesis was 0.97% (3/310 patients), compared with a rate of 8.89% (12/135 patients) when the procedure was performed without ultrasound.9 Another prospective, randomized study of 160 patients with various causes of pleural effusion showed that the rate of pneumothorax with ultrasound-marked thoracentesis was 1.25% (1/80 patients), compared with 12.5% (10/80 patients) for procedures performed without ultrasound.8 Hospitalists who competently use ultrasound guidance should act on modern estimates of complications and may also choose to incorporate postprocedure ultrasound into their practice. Indeed, the Society of Hospital Medicine recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized on postprocedure ultrasound.10
Second, procedural factors and postprocedural symptoms (new chest pain, dyspnea, or persistent cough) reliably identify patients with high risk of clinically meaningful complications. On one hand, only 1% to 2% of asymptomatic patients have a postprocedure pneumothorax, and clinical monitoring does not lead to chest tube placement in almost all of these cases.11 On the other hand, 67% to 72% of symptomatic patients are found to have complications.12 Doyle et al13 showed that the use of symptoms and procedure-specific factors (such as the aspiration of air, difficult procedure, multiple needle passes, or high operator suspicion of pneumothorax) could obviate the need for routine CXRs in approximately 60% of their procedures without any serious consequences.
Third, postprocedural CXRs very rarely reveal new or unexpected findings. For example, in one series,12 only 3.8% of postdrainage radiographs uncovered new findings, none of which clarified the underlying diagnosis or changed management. To assess the utility of an initial thoracentesis and decide about repeat procedures, begin by asking the patient about symptoms and perform a physical exam.
Why PostProcedural CHEST RADIOGRAPHS Might be Helpful in Certain Circumstances
CXRs might be helpful in certain scenarios, even when a complication is not suspected. For example, a postprocedure CXR to detect nonexpandable lung or evaluate the rate of recurrence may guide definitive management of patients with recurrent or malignant pleural effusion. Determining completeness of drainage may also assist with planning for palliative measures such as pleurodesis or indwelling pleural catheter placement. A postprocedure CXR is also helpful in patients with a technically difficult procedure or in those with symptoms during or immediately after the procedure. This recommendation is consistent with the 2010 British Thoracic Society guidelines, which recommend CXRs for procedures where air was withdrawn, the procedure was difficult, multiple needle passes were required, or the patient became symptomatic.14 The Society of Hospital Medicine’s recent Position Statement concurs with these guidelines and recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized by postprocedure ultrasound.10
What You Should Do Instead
Hospitalists should not rountinely obtain post-thoracentesis CXRs in asymptomatic patients. Clinical monitoring with subsequent symptom-guided evaluation lowers costs, avoids unnecessary radiation exposure, and has been shown to be successful in a large case series of more than 9,300 patients.15 Some coughing should be expected with all large-volume thoracenteses as a normal response to re-expansion of atelectatic lung. The coughing should not persist past the immediate postprocedure period. If symptoms arise or if a complication is expected, the test of choice is either CXR or, if the hospitalist is a competent sonographer, bedside sonography. Bedside sonography is a low-cost, noninvasive method and has been well studied in the diagnosis of post-thoracentesis pneumothorax.16 CXRs may still be needed to confirm findings by sonography, to investigate postprocedural symptoms in those with pleural adhesions or other lung/pleural diseases (because ultrasonography is less reliable in these patients), or if reexpansion pulmonary edema or other complications are suspected. A robust quality improvement strategy to reduce unnecessary post-thoracentesis CXRs could result in cost savings and spare patients from radiation exposure, because a recent study of almost 1,000 thoracenteses performed at an academic medical center demonstrated that internal medicine residents, pulmonologists, and interventional radiologists order a CXR following 95% of thoracenteses.17 For a hypothetical hospital that orders 100 unnecessary post-thoracentesis CXRs annually, hospitalists could avoid approximately $7,000 in wasted expense per year.18
RECOMMENDATIONS:
- Do not routinely order post-thoracentesis CXRs.
- Order a post-thoracentesis CXR if (1) the patient had new chest pain, dyspnea, or persistent cough during or after the procedure; (2) procedural features suggest increased risk of a complication (multiple needle passes, aspiration of air, difficulty obtaining fluid); or (3) a definitive palliative procedure will be arranged based on lung expansion.
- If qualified, use bedside sonography as a first step in the diagnosis of pneumothorax, reserving CXRs for those patients in whom accurate sonography is not possible, an alternative diagnosis is suspected, or when sonography findings are equivocal.
CONCLUSION
Following the uncomplicated thoracentesis, the hospitalist reconsidered the initial decision to order a CXR and rapidly assessed the patient’s risk of complications. Because the procedure required only one needle pass, air was not aspirated, and the patient did not experience prolonged coughing or pain, the CXR order was canceled. The patient recovered uneventfully and was spared the cost and radiation associated with the proposed CXR.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.cknowledgments
Acknowledgements
The authors would like to thank Patricia Kritek and Somnath Mookherjee for their comments on an early version of this manuscript.
Disclosures
The au
1. Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055-1070. doi: 10.1016/j.mcna.2011.08.005. PubMed
2. ABIM Policies and Procedures for Certification. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed 10th February 2018.
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. doi: 10.1001/archinternmed.2009.548. PubMed
4. Seneff MG, Corwin RW, Gold LH, Irwin RS. Complications associated with thoracocentesis. Chest. 1986;90:97-100. doi: 10.1378/chest.90.1.97 PubMed
5. Grogan DR, Irwin RS, Channick R, Raptopoulos V, Curley FJ, Bartter T. Complications associated with thoracentesis a prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150:873-877. doi: 10.1001/archinte.150.4.873 PubMed
6. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis preliminary results. Am J Med Qual. 2013;28:220-226. doi: 10.1177/1062860612459881. PubMed
7. Morris CA, Wolf A. Video 482e-1 clinical procedure tutorial: thoracentesis. Harrison’s Principles of Internal Medicine, 19th edition. http://accessmedicine.mhmedical.com/MultimediaPlayer.aspx?MultimediaID=12986897. Accessed 28th September 2017.
8. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis?* , ** A ultrassonografia pode reduzir o risco de pneumotórax após toracocentese? J Bras Pneumol. 2013;40:6-12. doi: 10.1590/S1806-37132014000100002 PubMed
9. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. doi: 10.1186/1477-7819-12-139. PubMed
10. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13:126-135. doi: 10.12788/jhm.2940. PubMed
11. Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107:340-343. doi: 10.1016/S0002-9343(99)00238-7 PubMed
12. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117:1038-1042. doi: 10.1378/chest.117.4.1038 PubMed
13. Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med. 1996;124: 816-820. doi: 10.7326/0003-4819-124-9-199605010-00005 PubMed
14. BTS- British Thoracic Society. BTS Pleural Disease Guideline 2010. Thorax 2010;65:1-76. doi: 10.1136/thx.2010.137026.
15. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-132. 10.1136/thoraxjnl-2014-206114. PubMed
16. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside ultrasonography in detection of post procedure pneumothorax. J Ultrasound Med. 2013;32:1003-1009. doi: 10.7863/ultra.32.6.1003 PubMed
17. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes. Acad Med. 2017; doi: 10.1097/ACM.0000000000001965 PubMed
18. Healthcare Bluebook. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?cftId=137&g=Chest+X-Ray. Accessed 10th February 2018.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care, but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Bedside thoracentesis can cause serious complications, such as pneumothorax, re-expansion pulmonary edema, or hemorrhage. These rare complications have led many hospitalists to routinely order chest radiographs (CXRs) following thoracentesis. However, post-thoracentesis CXRs are usually not indicated and can lead to unnecessary radiation exposure and expense. Rather than obtaining routine CXRs, hospitalists should use postprocedural signs and symptoms to identify the occasional patients who require imaging. A risk-stratified approach is a safe and cost-effective way to avoid unnecessary radiographs.
CASE REPORT
A 52-year-old man with decompensated liver disease and hepatic hydrothorax is hospitalized for increasing dyspnea caused by a recurrent pleural effusion. Diuretics do not improve his dyspnea, and his hospitalist recommends a therapeutic thoracentesis for symptom relief. The patient does not have any significant procedural risk factors: He does not have preexisting pulmonary or pleural disease, his platelet count is 105,000 × 103/µl, and his international normalized ratio is 1.3. Bedside sonography demonstrates a large, free-flowing, right-sided pleural effusion. The hospitalist performs an uncomplicated ultrasound-guided removal of 1.5 L of straw-colored fluid with a catheter-over-needle kit. The patient does not have any pain or increased shortness of breath during or after the procedure. The hospitalist reflexively orders a routine chest radiograph to assess for pneumothorax.
Why You Might Think a Chest Radiograph is Helpful after Thoracentesis
Pleural effusions are newly diagnosed in more than 1.5 million Americans annually,1 and hospitalists frequently care for patients requiring thoracentesis. Internal medicine residents traditionally learn to perform this procedure during residency, and thoracentesis remains a common task for both residents and hospitalists.2 Patients typically tolerate thoracentesis well, but they can develop serious complications such as pneumothorax, re-expansion pulmonary edema, or hemothorax. Before the advent of bedside ultrasound, these complications occurred relatively commonly; a 2010 systematic review, for example, found that the rate of pneumothorax from thoracentesis performed without ultrasound was 9.3%.3 Other studies have identified even higher rates of complications, including two case series in which investigators found a 14% rate of major complications4 and a pneumothorax rate of nearly 30%.5 Postprocedure radiographs became common practice because of the high rate of complications, and this practice has persisted for many practitioners despite the substantial safety improvements introduced by bedside ultrasonography.6
Hospitalists might think routine CXRs are helpful after ultrasound-guided thoracentesis for additional reasons. First, modern guidelines reflecting the low risk of complications after ultrasound-guided procedures have not been released by United States pulmonary medicine societies, and some clinicians may continue to follow practices acquired during the era of unguided thoracentesis. Second, performing postprocedure imaging has become ingrained as a standard part of some institutional procedure checklists6 and some prominent textbooks continue to recommend the practice.7 For some hospitalists, this testing reflex may be reinforced by other common procedures, such as placing a nasogastric tube or a central venous catheter, for which a postprocedure CXR is standard practice. Thus, ordering postprocedure imaging can become internalized as the safe, checklist-based final step of a procedure. Third, hospitalists may order a postprocedure CXR for reasons other than detecting procedural complications. The pleural effusion might be thought to obscure a parenchymal or endobronchial lesion for which a postprocedure CXR may reveal an important finding. Finally, a CXR also may also satisfy the clinician’s curiosity regarding the completeness of drainage.
Why a Routine Postprocedure Chest Radiograph is Not Helpful after Thoracentesis
A routine post-thoracentesis CXR is not necessary for three reasons. First, the use of ultrasound marking or guidance has substantially improved site selection and reduced the rate of complications for experienced operators. For example, a 2010 systematic review found an overall rate of pneumothorax of 4% for ultrasound-guided procedures performed between 1986 and 2006,3 whereas more recently published data suggest the current rate of pneumothorax is closer to 1% when ultrasound marking or guidance is used.8,9 One study of 462 consecutive patients with malignant pleural effusions, for example, showed that the rate of pneumothorax with ultrasound-guided needle-over-catheter thoracentesis was 0.97% (3/310 patients), compared with a rate of 8.89% (12/135 patients) when the procedure was performed without ultrasound.9 Another prospective, randomized study of 160 patients with various causes of pleural effusion showed that the rate of pneumothorax with ultrasound-marked thoracentesis was 1.25% (1/80 patients), compared with 12.5% (10/80 patients) for procedures performed without ultrasound.8 Hospitalists who competently use ultrasound guidance should act on modern estimates of complications and may also choose to incorporate postprocedure ultrasound into their practice. Indeed, the Society of Hospital Medicine recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized on postprocedure ultrasound.10
Second, procedural factors and postprocedural symptoms (new chest pain, dyspnea, or persistent cough) reliably identify patients with high risk of clinically meaningful complications. On one hand, only 1% to 2% of asymptomatic patients have a postprocedure pneumothorax, and clinical monitoring does not lead to chest tube placement in almost all of these cases.11 On the other hand, 67% to 72% of symptomatic patients are found to have complications.12 Doyle et al13 showed that the use of symptoms and procedure-specific factors (such as the aspiration of air, difficult procedure, multiple needle passes, or high operator suspicion of pneumothorax) could obviate the need for routine CXRs in approximately 60% of their procedures without any serious consequences.
Third, postprocedural CXRs very rarely reveal new or unexpected findings. For example, in one series,12 only 3.8% of postdrainage radiographs uncovered new findings, none of which clarified the underlying diagnosis or changed management. To assess the utility of an initial thoracentesis and decide about repeat procedures, begin by asking the patient about symptoms and perform a physical exam.
Why PostProcedural CHEST RADIOGRAPHS Might be Helpful in Certain Circumstances
CXRs might be helpful in certain scenarios, even when a complication is not suspected. For example, a postprocedure CXR to detect nonexpandable lung or evaluate the rate of recurrence may guide definitive management of patients with recurrent or malignant pleural effusion. Determining completeness of drainage may also assist with planning for palliative measures such as pleurodesis or indwelling pleural catheter placement. A postprocedure CXR is also helpful in patients with a technically difficult procedure or in those with symptoms during or immediately after the procedure. This recommendation is consistent with the 2010 British Thoracic Society guidelines, which recommend CXRs for procedures where air was withdrawn, the procedure was difficult, multiple needle passes were required, or the patient became symptomatic.14 The Society of Hospital Medicine’s recent Position Statement concurs with these guidelines and recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized by postprocedure ultrasound.10
What You Should Do Instead
Hospitalists should not rountinely obtain post-thoracentesis CXRs in asymptomatic patients. Clinical monitoring with subsequent symptom-guided evaluation lowers costs, avoids unnecessary radiation exposure, and has been shown to be successful in a large case series of more than 9,300 patients.15 Some coughing should be expected with all large-volume thoracenteses as a normal response to re-expansion of atelectatic lung. The coughing should not persist past the immediate postprocedure period. If symptoms arise or if a complication is expected, the test of choice is either CXR or, if the hospitalist is a competent sonographer, bedside sonography. Bedside sonography is a low-cost, noninvasive method and has been well studied in the diagnosis of post-thoracentesis pneumothorax.16 CXRs may still be needed to confirm findings by sonography, to investigate postprocedural symptoms in those with pleural adhesions or other lung/pleural diseases (because ultrasonography is less reliable in these patients), or if reexpansion pulmonary edema or other complications are suspected. A robust quality improvement strategy to reduce unnecessary post-thoracentesis CXRs could result in cost savings and spare patients from radiation exposure, because a recent study of almost 1,000 thoracenteses performed at an academic medical center demonstrated that internal medicine residents, pulmonologists, and interventional radiologists order a CXR following 95% of thoracenteses.17 For a hypothetical hospital that orders 100 unnecessary post-thoracentesis CXRs annually, hospitalists could avoid approximately $7,000 in wasted expense per year.18
RECOMMENDATIONS:
- Do not routinely order post-thoracentesis CXRs.
- Order a post-thoracentesis CXR if (1) the patient had new chest pain, dyspnea, or persistent cough during or after the procedure; (2) procedural features suggest increased risk of a complication (multiple needle passes, aspiration of air, difficulty obtaining fluid); or (3) a definitive palliative procedure will be arranged based on lung expansion.
- If qualified, use bedside sonography as a first step in the diagnosis of pneumothorax, reserving CXRs for those patients in whom accurate sonography is not possible, an alternative diagnosis is suspected, or when sonography findings are equivocal.
CONCLUSION
Following the uncomplicated thoracentesis, the hospitalist reconsidered the initial decision to order a CXR and rapidly assessed the patient’s risk of complications. Because the procedure required only one needle pass, air was not aspirated, and the patient did not experience prolonged coughing or pain, the CXR order was canceled. The patient recovered uneventfully and was spared the cost and radiation associated with the proposed CXR.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.cknowledgments
Acknowledgements
The authors would like to thank Patricia Kritek and Somnath Mookherjee for their comments on an early version of this manuscript.
Disclosures
The au
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care, but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Bedside thoracentesis can cause serious complications, such as pneumothorax, re-expansion pulmonary edema, or hemorrhage. These rare complications have led many hospitalists to routinely order chest radiographs (CXRs) following thoracentesis. However, post-thoracentesis CXRs are usually not indicated and can lead to unnecessary radiation exposure and expense. Rather than obtaining routine CXRs, hospitalists should use postprocedural signs and symptoms to identify the occasional patients who require imaging. A risk-stratified approach is a safe and cost-effective way to avoid unnecessary radiographs.
CASE REPORT
A 52-year-old man with decompensated liver disease and hepatic hydrothorax is hospitalized for increasing dyspnea caused by a recurrent pleural effusion. Diuretics do not improve his dyspnea, and his hospitalist recommends a therapeutic thoracentesis for symptom relief. The patient does not have any significant procedural risk factors: He does not have preexisting pulmonary or pleural disease, his platelet count is 105,000 × 103/µl, and his international normalized ratio is 1.3. Bedside sonography demonstrates a large, free-flowing, right-sided pleural effusion. The hospitalist performs an uncomplicated ultrasound-guided removal of 1.5 L of straw-colored fluid with a catheter-over-needle kit. The patient does not have any pain or increased shortness of breath during or after the procedure. The hospitalist reflexively orders a routine chest radiograph to assess for pneumothorax.
Why You Might Think a Chest Radiograph is Helpful after Thoracentesis
Pleural effusions are newly diagnosed in more than 1.5 million Americans annually,1 and hospitalists frequently care for patients requiring thoracentesis. Internal medicine residents traditionally learn to perform this procedure during residency, and thoracentesis remains a common task for both residents and hospitalists.2 Patients typically tolerate thoracentesis well, but they can develop serious complications such as pneumothorax, re-expansion pulmonary edema, or hemothorax. Before the advent of bedside ultrasound, these complications occurred relatively commonly; a 2010 systematic review, for example, found that the rate of pneumothorax from thoracentesis performed without ultrasound was 9.3%.3 Other studies have identified even higher rates of complications, including two case series in which investigators found a 14% rate of major complications4 and a pneumothorax rate of nearly 30%.5 Postprocedure radiographs became common practice because of the high rate of complications, and this practice has persisted for many practitioners despite the substantial safety improvements introduced by bedside ultrasonography.6
Hospitalists might think routine CXRs are helpful after ultrasound-guided thoracentesis for additional reasons. First, modern guidelines reflecting the low risk of complications after ultrasound-guided procedures have not been released by United States pulmonary medicine societies, and some clinicians may continue to follow practices acquired during the era of unguided thoracentesis. Second, performing postprocedure imaging has become ingrained as a standard part of some institutional procedure checklists6 and some prominent textbooks continue to recommend the practice.7 For some hospitalists, this testing reflex may be reinforced by other common procedures, such as placing a nasogastric tube or a central venous catheter, for which a postprocedure CXR is standard practice. Thus, ordering postprocedure imaging can become internalized as the safe, checklist-based final step of a procedure. Third, hospitalists may order a postprocedure CXR for reasons other than detecting procedural complications. The pleural effusion might be thought to obscure a parenchymal or endobronchial lesion for which a postprocedure CXR may reveal an important finding. Finally, a CXR also may also satisfy the clinician’s curiosity regarding the completeness of drainage.
Why a Routine Postprocedure Chest Radiograph is Not Helpful after Thoracentesis
A routine post-thoracentesis CXR is not necessary for three reasons. First, the use of ultrasound marking or guidance has substantially improved site selection and reduced the rate of complications for experienced operators. For example, a 2010 systematic review found an overall rate of pneumothorax of 4% for ultrasound-guided procedures performed between 1986 and 2006,3 whereas more recently published data suggest the current rate of pneumothorax is closer to 1% when ultrasound marking or guidance is used.8,9 One study of 462 consecutive patients with malignant pleural effusions, for example, showed that the rate of pneumothorax with ultrasound-guided needle-over-catheter thoracentesis was 0.97% (3/310 patients), compared with a rate of 8.89% (12/135 patients) when the procedure was performed without ultrasound.9 Another prospective, randomized study of 160 patients with various causes of pleural effusion showed that the rate of pneumothorax with ultrasound-marked thoracentesis was 1.25% (1/80 patients), compared with 12.5% (10/80 patients) for procedures performed without ultrasound.8 Hospitalists who competently use ultrasound guidance should act on modern estimates of complications and may also choose to incorporate postprocedure ultrasound into their practice. Indeed, the Society of Hospital Medicine recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized on postprocedure ultrasound.10
Second, procedural factors and postprocedural symptoms (new chest pain, dyspnea, or persistent cough) reliably identify patients with high risk of clinically meaningful complications. On one hand, only 1% to 2% of asymptomatic patients have a postprocedure pneumothorax, and clinical monitoring does not lead to chest tube placement in almost all of these cases.11 On the other hand, 67% to 72% of symptomatic patients are found to have complications.12 Doyle et al13 showed that the use of symptoms and procedure-specific factors (such as the aspiration of air, difficult procedure, multiple needle passes, or high operator suspicion of pneumothorax) could obviate the need for routine CXRs in approximately 60% of their procedures without any serious consequences.
Third, postprocedural CXRs very rarely reveal new or unexpected findings. For example, in one series,12 only 3.8% of postdrainage radiographs uncovered new findings, none of which clarified the underlying diagnosis or changed management. To assess the utility of an initial thoracentesis and decide about repeat procedures, begin by asking the patient about symptoms and perform a physical exam.
Why PostProcedural CHEST RADIOGRAPHS Might be Helpful in Certain Circumstances
CXRs might be helpful in certain scenarios, even when a complication is not suspected. For example, a postprocedure CXR to detect nonexpandable lung or evaluate the rate of recurrence may guide definitive management of patients with recurrent or malignant pleural effusion. Determining completeness of drainage may also assist with planning for palliative measures such as pleurodesis or indwelling pleural catheter placement. A postprocedure CXR is also helpful in patients with a technically difficult procedure or in those with symptoms during or immediately after the procedure. This recommendation is consistent with the 2010 British Thoracic Society guidelines, which recommend CXRs for procedures where air was withdrawn, the procedure was difficult, multiple needle passes were required, or the patient became symptomatic.14 The Society of Hospital Medicine’s recent Position Statement concurs with these guidelines and recommends against routine chest radiography in asymptomatic patients when sliding lung is visualized by postprocedure ultrasound.10
What You Should Do Instead
Hospitalists should not rountinely obtain post-thoracentesis CXRs in asymptomatic patients. Clinical monitoring with subsequent symptom-guided evaluation lowers costs, avoids unnecessary radiation exposure, and has been shown to be successful in a large case series of more than 9,300 patients.15 Some coughing should be expected with all large-volume thoracenteses as a normal response to re-expansion of atelectatic lung. The coughing should not persist past the immediate postprocedure period. If symptoms arise or if a complication is expected, the test of choice is either CXR or, if the hospitalist is a competent sonographer, bedside sonography. Bedside sonography is a low-cost, noninvasive method and has been well studied in the diagnosis of post-thoracentesis pneumothorax.16 CXRs may still be needed to confirm findings by sonography, to investigate postprocedural symptoms in those with pleural adhesions or other lung/pleural diseases (because ultrasonography is less reliable in these patients), or if reexpansion pulmonary edema or other complications are suspected. A robust quality improvement strategy to reduce unnecessary post-thoracentesis CXRs could result in cost savings and spare patients from radiation exposure, because a recent study of almost 1,000 thoracenteses performed at an academic medical center demonstrated that internal medicine residents, pulmonologists, and interventional radiologists order a CXR following 95% of thoracenteses.17 For a hypothetical hospital that orders 100 unnecessary post-thoracentesis CXRs annually, hospitalists could avoid approximately $7,000 in wasted expense per year.18
RECOMMENDATIONS:
- Do not routinely order post-thoracentesis CXRs.
- Order a post-thoracentesis CXR if (1) the patient had new chest pain, dyspnea, or persistent cough during or after the procedure; (2) procedural features suggest increased risk of a complication (multiple needle passes, aspiration of air, difficulty obtaining fluid); or (3) a definitive palliative procedure will be arranged based on lung expansion.
- If qualified, use bedside sonography as a first step in the diagnosis of pneumothorax, reserving CXRs for those patients in whom accurate sonography is not possible, an alternative diagnosis is suspected, or when sonography findings are equivocal.
CONCLUSION
Following the uncomplicated thoracentesis, the hospitalist reconsidered the initial decision to order a CXR and rapidly assessed the patient’s risk of complications. Because the procedure required only one needle pass, air was not aspirated, and the patient did not experience prolonged coughing or pain, the CXR order was canceled. The patient recovered uneventfully and was spared the cost and radiation associated with the proposed CXR.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.cknowledgments
Acknowledgements
The authors would like to thank Patricia Kritek and Somnath Mookherjee for their comments on an early version of this manuscript.
Disclosures
The au
1. Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055-1070. doi: 10.1016/j.mcna.2011.08.005. PubMed
2. ABIM Policies and Procedures for Certification. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed 10th February 2018.
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. doi: 10.1001/archinternmed.2009.548. PubMed
4. Seneff MG, Corwin RW, Gold LH, Irwin RS. Complications associated with thoracocentesis. Chest. 1986;90:97-100. doi: 10.1378/chest.90.1.97 PubMed
5. Grogan DR, Irwin RS, Channick R, Raptopoulos V, Curley FJ, Bartter T. Complications associated with thoracentesis a prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150:873-877. doi: 10.1001/archinte.150.4.873 PubMed
6. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis preliminary results. Am J Med Qual. 2013;28:220-226. doi: 10.1177/1062860612459881. PubMed
7. Morris CA, Wolf A. Video 482e-1 clinical procedure tutorial: thoracentesis. Harrison’s Principles of Internal Medicine, 19th edition. http://accessmedicine.mhmedical.com/MultimediaPlayer.aspx?MultimediaID=12986897. Accessed 28th September 2017.
8. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis?* , ** A ultrassonografia pode reduzir o risco de pneumotórax após toracocentese? J Bras Pneumol. 2013;40:6-12. doi: 10.1590/S1806-37132014000100002 PubMed
9. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. doi: 10.1186/1477-7819-12-139. PubMed
10. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13:126-135. doi: 10.12788/jhm.2940. PubMed
11. Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107:340-343. doi: 10.1016/S0002-9343(99)00238-7 PubMed
12. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117:1038-1042. doi: 10.1378/chest.117.4.1038 PubMed
13. Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med. 1996;124: 816-820. doi: 10.7326/0003-4819-124-9-199605010-00005 PubMed
14. BTS- British Thoracic Society. BTS Pleural Disease Guideline 2010. Thorax 2010;65:1-76. doi: 10.1136/thx.2010.137026.
15. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-132. 10.1136/thoraxjnl-2014-206114. PubMed
16. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside ultrasonography in detection of post procedure pneumothorax. J Ultrasound Med. 2013;32:1003-1009. doi: 10.7863/ultra.32.6.1003 PubMed
17. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes. Acad Med. 2017; doi: 10.1097/ACM.0000000000001965 PubMed
18. Healthcare Bluebook. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?cftId=137&g=Chest+X-Ray. Accessed 10th February 2018.
1. Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055-1070. doi: 10.1016/j.mcna.2011.08.005. PubMed
2. ABIM Policies and Procedures for Certification. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed 10th February 2018.
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. doi: 10.1001/archinternmed.2009.548. PubMed
4. Seneff MG, Corwin RW, Gold LH, Irwin RS. Complications associated with thoracocentesis. Chest. 1986;90:97-100. doi: 10.1378/chest.90.1.97 PubMed
5. Grogan DR, Irwin RS, Channick R, Raptopoulos V, Curley FJ, Bartter T. Complications associated with thoracentesis a prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150:873-877. doi: 10.1001/archinte.150.4.873 PubMed
6. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis preliminary results. Am J Med Qual. 2013;28:220-226. doi: 10.1177/1062860612459881. PubMed
7. Morris CA, Wolf A. Video 482e-1 clinical procedure tutorial: thoracentesis. Harrison’s Principles of Internal Medicine, 19th edition. http://accessmedicine.mhmedical.com/MultimediaPlayer.aspx?MultimediaID=12986897. Accessed 28th September 2017.
8. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis?* , ** A ultrassonografia pode reduzir o risco de pneumotórax após toracocentese? J Bras Pneumol. 2013;40:6-12. doi: 10.1590/S1806-37132014000100002 PubMed
9. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. doi: 10.1186/1477-7819-12-139. PubMed
10. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13:126-135. doi: 10.12788/jhm.2940. PubMed
11. Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107:340-343. doi: 10.1016/S0002-9343(99)00238-7 PubMed
12. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117:1038-1042. doi: 10.1378/chest.117.4.1038 PubMed
13. Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med. 1996;124: 816-820. doi: 10.7326/0003-4819-124-9-199605010-00005 PubMed
14. BTS- British Thoracic Society. BTS Pleural Disease Guideline 2010. Thorax 2010;65:1-76. doi: 10.1136/thx.2010.137026.
15. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-132. 10.1136/thoraxjnl-2014-206114. PubMed
16. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside ultrasonography in detection of post procedure pneumothorax. J Ultrasound Med. 2013;32:1003-1009. doi: 10.7863/ultra.32.6.1003 PubMed
17. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes. Acad Med. 2017; doi: 10.1097/ACM.0000000000001965 PubMed
18. Healthcare Bluebook. https://www.healthcarebluebook.com/page_ProcedureDetails.aspx?cftId=137&g=Chest+X-Ray. Accessed 10th February 2018.
© 2018 Society of Hospital Medicine
Scratching Beneath the Surface
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
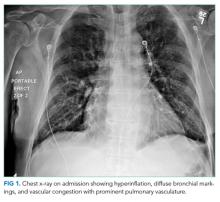
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
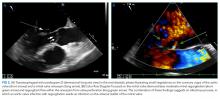
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
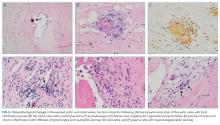
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
© 2018 Society of Hospital Medicine
Physicians' Posture at Patients' Bedside
Sitting while interacting with patients is standard in the outpatient setting and encouraged in the inpatient setting as a best practice.[1, 2] Michael W. Kahn defined etiquette‐based medicine as a set of easily taught behaviors that demonstrate respect for the patient; sitting at the bedside is included.[1] A prominent healthcare consulting group also recommends that physicians and nurses sit at the bedside, claiming that the patient will estimate you were in the room 3 times longer.[3] Previous studies suggest patients may perceive physicians who sit at the bedside as more compassionate and as spending more time with them, and may perceive the overall interaction as more positive when the physician sits.[4, 5, 6] Two small studies found that patients perceived the physician as having spent more time with them if he or she sat rather than stood.[5, 6] A study in the emergency department found no effect of posture on patient perception of physician communication skills, and a study of a single attending neurosurgeon found that patients reported a better understanding of their condition when the physician sat.[5, 6] The effect of physician posture on hospitalist physician‐patient communication has not been previously studied. Despite evidence that sitting in the inpatient setting may improve physician‐patient communication, studies suggest that physicians rarely sit at the bedside of inpatients.[7, 8]
We conducted a cluster‐randomized trial of the impact of hospitalist physician posture during morning rounds. We hypothesized that patients whose physician sat rather than stood would perceive that their physician spent more time with them and would rate the physician's communication skills more highly. We also hypothesized that sitting would not prolong the length of the patient‐physician encounter.
PATIENTS AND METHODS
We conducted a cluster‐randomized clinical trial with a crossover component randomizing physicians on the order of sit/stand within a consecutive 7‐day workweek. We enrolled patients being cared for by attending hospitalists on a resident‐uncovered general internal medicine service in an academic tertiary care hospital. We also enrolled the hospitalists and collected demographics and practice information. Wall‐mounted folding chairs (Figure 1) were installed in all rooms on two 28‐bed units for use by physicians. Eligible patients were newly admitted or transferred from the intensive care unit between June 2014 and June 2015, English speaking, and adults who consented to their own medical care. Physicians were randomly assigned to sit or stand during morning rounds for the first 3 days of their workweek. The last 4 days they provided care using the other posture. Blocks of 4 weeks were used to randomize the sit/stand order.

We measured the length of the physician‐patient interaction, asked both the physician and the patient to estimate the length of the interaction, and administered a written survey to the patient with questions about the physician's communication skills. Research assistants timed the interaction from outside the room and entered the room to consent patients and administer the survey after the physician departed. Survey questions were modeled on the physician communication questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. We aggregated all answers other than the most positive answer because HCAHPS questions are analyzed according to a top box methodology. Adherence to the intervention was measured by asking the physician whether he or she actually sat or stood for each interaction. We administered a survey to physicians to collect demographics and feedback.
We estimated descriptive statistics for physician and patient participants using cross‐tabs and means. To estimate associations, we used logistic and linear regression that employed cluster‐adjusted t statistics and clustered patients within providers. This method optimizes estimation of standard errors (and corresponding confidence intervals and P values) when the number of clusters is small (16 physicians).[9] For our primary analysis, we analyzed as randomized using an intent‐to‐treat approach. In other words, those assigned to the standing group were analyzed in the standing group even if they actually sat (and vice versa). In a sensitivity analysis we used the same methods to analyze the data according actual provider posture as reported by the physician and not as randomized. We calculated the mean and range of the number of patients seen by physicians. We compared estimates of time spent between patients and providers and patients' satisfaction according to provider posture. We complied with the Consolidated Standards of Reporting Trials 2010 guidelines.[10] Our institutional review board approved this project. All participants provided written consent.
RESULTS
All 17 hospitalists attending on the service consented to participate; 1 did not see any patients involved in the study and was removed from the analysis. Sixty‐nine percent were female and 81% had been in practice for 3 years or less at the time of study enrollment; 94% reported standing when assigned to stand and 83% reported sitting when assigned to sit. We found 31% of physicians reported they routinely sat before participating in the study, and 81% said they would sit more after the study; this result approached statistical significance (exact McNemar P = 0.06). Of the 11 physicians who reported not routinely sitting before the study all, 7 cited not having a place to sit as a reason for not sitting. Other rationale provided included being too short to see the patient if seated, believing rounds would take more time if seated, and concerns about contact precautions. Comments in the postintervention survey regarding why providers planned to sit more centered around themes of having chairs available, thinking that sitting improves communication, and thinking that patients prefer providers to sit.
Two hundred eleven patients were assessed for eligibility. Fifty‐two were excluded (27 did not meet inclusion criteria and 25 declined to participate), leaving 159 participating patients. Seven patient‐physician pairs were inadvertently assigned the wrong intervention but were analyzed as randomized. There were no demographic differences between patient groups (Table 1). Physicians participating in the study saw an average of 13 study patients (range, 118) during the study. Mean time spent in the patient's room during rounds was 12:00 minutes for seated physicians and 12:10 for standing physicians (P = 0.84). Regardless of provider posture, patients overestimated the amount of time their physician spent in the room (mean difference 4:10 minutes, P = 0.01). Patients' estimates of the time the physician spent did not vary by posture (16:00 minutes for seated, 16:19 for standing, P = 0.86).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patient age, y | |||||
| 1839 | 16 | 25.4 | 25 | 27.5 | 0.59 |
| 4059 | 17 | 27.0 | 30 | 33.0 | |
| 60+ | 30 | 47.6 | 36 | 39.6 | |
| Gender | |||||
| Male | 32 | 49.2 | 43 | 46.2 | 0.71 |
| Female | 33 | 50.8 | 50 | 53.8 | |
| Ethnicity | |||||
| Caucasian | 54 | 84.4 | 67 | 73.6 | 0.24 |
| Asian or Pacific Islander | 3 | 4.7 | 5 | 5.5 | |
| Other | 7 | 10.9 | 19 | 20.9 | |
Patients whose physician sat on rounds were statistically significantly more likely to choose the answer always to the questions regarding their physician listening carefully to them (P = 0.02) and explaining things in a way that was easy to understand (P = 0.05, Table 2). There was no difference in the patients' response to questions about the physician interrupting the patient when talking or treating them with courtesy and respect. Nearly all patients chose just right when asked to rate the amount of time their physician had spent with them on rounds (Table 2). The results of our sensitivity analysis that classified physicians according to their actual posture yielded different results; none of the findings in that analysis including questions regarding the physician listening carefully or explaining things in a way that was easy to understand were statistically significant (see Supporting Information, Appendix 1, in the online version of this article).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Patient perception of physician communication on that day's rounds | |||||
| Today on rounds, how often did this physician. | |||||
| Explain things in a way that was easy to understand? | |||||
| Never, sometimes, or usually | 7 | 10.9 | 22 | 23.9 | 0.05 |
| Always | 57 | 89.1 | 71 | 76.1 | |
| Listen carefully to you? | |||||
| Never, sometimes, or usually | 4 | 6.1 | 19 | 20.4 | 0.02 |
| Always | 62 | 93.4 | 74 | 79.6 | |
| Interrupt you when you were talking? | |||||
| Always, sometimes, or usually | 4 | 6.5 | 9 | 10 | 0.46 |
| Never | 58 | 93.6 | 81 | 90 | |
| Treat you with courtesy and respect? | |||||
| Never, sometimes, or usually | 0 | 0 | 7 | 7.6 | Not estimable |
| Always | 63 | 100 | 85 | 92.4 | |
| Please rate the amount of time this physician spent with you today during morning rounds. | |||||
| Too little | 1 | 1.6 | 3 | 3.5 | 0.41 |
| Just right | 63 | 98.4 | 84 | 96.5 | |
| Did you have any important questions or concerns about your care that you did not bring up with this doctor today?* | |||||
| Yes | 4 | 6.6 | 9 | 10.3 | 0.26 |
| No | 57 | 94.4 | 78 | 89.7 | |
DISCUSSION
In our study involving general medicine inpatients cared for by academic hospitalists, physicians did not spend more time in the room when seated, and were willing to adopt this practice. Patients perceived that seated compared to standing physicians listened more carefully and explained things in a way that was easy to understand when analyzed using an intent‐to‐treat approach. Patients did not perceive that seated physicians spent more time with them than standing physicians. To our knowledge, this is the first study showing the effects of hospitalist rounding posture on patient experience.
Our finding that patients rated seated physicians more highly on listening carefully and explaining things well indicates that training hospitalists to sit at the bedside may ultimately improve patient satisfaction. Our findings suggest seated interaction may improve satisfaction with communication without increasing time burden on physicians. However, given that these findings were not statistically significant when we analyzed our data according to actual behavior, larger studies should verify the impact of physician posture on patient experience.
Previous studies found that a minority of physicians sit in the inpatient setting, but did not study barriers to sitting while on rounds.[7, 8] A majority of physicians in our study sat when instructed to do so and when chairs were provided, and over 80% of physicians in our study said they planned to continue sitting while on rounds after the study was complete. A lack of chairs may be a major barrier to physicians adopting this facet of etiquette‐based medicine, and institutions wishing to promote this practice should consider providing chairs. Written comments from physician participants suggest physicians who are introduced to this practice enjoy sitting and think it improves physician‐patient communication. Further studies are needed to test our assumption that physicians continue sitting when chairs are provided.
Our work differs from previous studies. Johnson et al. studied interactions in the emergency room with a mean length of 8.6 minutes,[5] and Swayden et al. studied postoperative visits by a single neurosurgeon with a mean length of about 1 minute.[6] One explanation for the lack of a difference in time spent by posture might be that an average visit time of 12 minutes passes a threshold where patients make more accurate estimates of visit length or where factors other than posture more strongly influence perceptions of duration.
Limitations of our study include the relatively small sample size, single location, and limitation to English‐speaking patients able to consent themselves. Reasons for the limited sample size include that chairs were only installed in 2 units, and not all patients on the unit were under the care of participating physicians. Physician subjects were not blinded to their interactions being timed or to the fact that patients were surveyed about their communication skills. It is possible that factors that may have affected patients' responses such as severity of illness, number of consultants involved in their care, or prior experiences in the healthcare system were not equally distributed between our 2 groups. Additionally, our use of questions similar to those used in the HCAHPS instrument is not compliant with Centers for Medicare and Medicaid Services (CMS) policy. We caution others against using questions that might invalidate their hospital's participation in CMS payment programs.
Our study was limited to rounds involving 1 physician; our practice is that in a larger team the presenting member is encouraged to sit and others sit if there are additional chairs. Best practices on a teaching service are unclear and could be the subject of further study. The longer‐term sustainability of the practice of sitting on rounds is unclear. However, our physician subjects reported that they plan to continue to sit after the study, and we have shared the results with physicians in order to provide them with evidence supporting this practice. Not having a place to sit and thinking that sitting increases the amount of time spent on rounds were concerns provided in our preintervention survey, and we believe our study addresses these concerns.
Our study demonstrates the effects of a simple intervention on patient satisfaction without increasing burden on providers. Sitting at the bedside does not impact the amount of time spent with the patient, but may improve the patient's perception of the physician's communication skills and thus impact the patient experience. This simple intervention could improve patient satisfaction at little cost.
Acknowledgements
The authors acknowledge Tom Staiger, MD, UWMC Medical Director, for his assistance with obtaining chairs for this study.
Disclosure: Nothing to report.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , . Enhancing patient satisfaction in dermatology. Am J Clin Dermatol. 2015;16:1–4.
- The Studer Group. Q21:501–505.
- , , , . To sit or not to sit? Ann Emerg Med. 2008;51:188–193.
- , , , , , . Effect of sitting vs. standing on perception of provider time at bedside: a pilot study. Patient Educ Couns. 2012;86(2):166–171.
- , , , , . Appraising the Practice of Etiquette‐Based Medicine in the Inpatient Setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013; 8:631–634.
- , . Practical and effective approaches to dealing with clustered data [unpublished manuscript]. Department of Political Science, Rice University, Houston, TX. Available at: http://jee3.web.rice.edu/cluster‐paper.pdf. Accessed February 29, 2016.
- , , . Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732.
Sitting while interacting with patients is standard in the outpatient setting and encouraged in the inpatient setting as a best practice.[1, 2] Michael W. Kahn defined etiquette‐based medicine as a set of easily taught behaviors that demonstrate respect for the patient; sitting at the bedside is included.[1] A prominent healthcare consulting group also recommends that physicians and nurses sit at the bedside, claiming that the patient will estimate you were in the room 3 times longer.[3] Previous studies suggest patients may perceive physicians who sit at the bedside as more compassionate and as spending more time with them, and may perceive the overall interaction as more positive when the physician sits.[4, 5, 6] Two small studies found that patients perceived the physician as having spent more time with them if he or she sat rather than stood.[5, 6] A study in the emergency department found no effect of posture on patient perception of physician communication skills, and a study of a single attending neurosurgeon found that patients reported a better understanding of their condition when the physician sat.[5, 6] The effect of physician posture on hospitalist physician‐patient communication has not been previously studied. Despite evidence that sitting in the inpatient setting may improve physician‐patient communication, studies suggest that physicians rarely sit at the bedside of inpatients.[7, 8]
We conducted a cluster‐randomized trial of the impact of hospitalist physician posture during morning rounds. We hypothesized that patients whose physician sat rather than stood would perceive that their physician spent more time with them and would rate the physician's communication skills more highly. We also hypothesized that sitting would not prolong the length of the patient‐physician encounter.
PATIENTS AND METHODS
We conducted a cluster‐randomized clinical trial with a crossover component randomizing physicians on the order of sit/stand within a consecutive 7‐day workweek. We enrolled patients being cared for by attending hospitalists on a resident‐uncovered general internal medicine service in an academic tertiary care hospital. We also enrolled the hospitalists and collected demographics and practice information. Wall‐mounted folding chairs (Figure 1) were installed in all rooms on two 28‐bed units for use by physicians. Eligible patients were newly admitted or transferred from the intensive care unit between June 2014 and June 2015, English speaking, and adults who consented to their own medical care. Physicians were randomly assigned to sit or stand during morning rounds for the first 3 days of their workweek. The last 4 days they provided care using the other posture. Blocks of 4 weeks were used to randomize the sit/stand order.

We measured the length of the physician‐patient interaction, asked both the physician and the patient to estimate the length of the interaction, and administered a written survey to the patient with questions about the physician's communication skills. Research assistants timed the interaction from outside the room and entered the room to consent patients and administer the survey after the physician departed. Survey questions were modeled on the physician communication questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. We aggregated all answers other than the most positive answer because HCAHPS questions are analyzed according to a top box methodology. Adherence to the intervention was measured by asking the physician whether he or she actually sat or stood for each interaction. We administered a survey to physicians to collect demographics and feedback.
We estimated descriptive statistics for physician and patient participants using cross‐tabs and means. To estimate associations, we used logistic and linear regression that employed cluster‐adjusted t statistics and clustered patients within providers. This method optimizes estimation of standard errors (and corresponding confidence intervals and P values) when the number of clusters is small (16 physicians).[9] For our primary analysis, we analyzed as randomized using an intent‐to‐treat approach. In other words, those assigned to the standing group were analyzed in the standing group even if they actually sat (and vice versa). In a sensitivity analysis we used the same methods to analyze the data according actual provider posture as reported by the physician and not as randomized. We calculated the mean and range of the number of patients seen by physicians. We compared estimates of time spent between patients and providers and patients' satisfaction according to provider posture. We complied with the Consolidated Standards of Reporting Trials 2010 guidelines.[10] Our institutional review board approved this project. All participants provided written consent.
RESULTS
All 17 hospitalists attending on the service consented to participate; 1 did not see any patients involved in the study and was removed from the analysis. Sixty‐nine percent were female and 81% had been in practice for 3 years or less at the time of study enrollment; 94% reported standing when assigned to stand and 83% reported sitting when assigned to sit. We found 31% of physicians reported they routinely sat before participating in the study, and 81% said they would sit more after the study; this result approached statistical significance (exact McNemar P = 0.06). Of the 11 physicians who reported not routinely sitting before the study all, 7 cited not having a place to sit as a reason for not sitting. Other rationale provided included being too short to see the patient if seated, believing rounds would take more time if seated, and concerns about contact precautions. Comments in the postintervention survey regarding why providers planned to sit more centered around themes of having chairs available, thinking that sitting improves communication, and thinking that patients prefer providers to sit.
Two hundred eleven patients were assessed for eligibility. Fifty‐two were excluded (27 did not meet inclusion criteria and 25 declined to participate), leaving 159 participating patients. Seven patient‐physician pairs were inadvertently assigned the wrong intervention but were analyzed as randomized. There were no demographic differences between patient groups (Table 1). Physicians participating in the study saw an average of 13 study patients (range, 118) during the study. Mean time spent in the patient's room during rounds was 12:00 minutes for seated physicians and 12:10 for standing physicians (P = 0.84). Regardless of provider posture, patients overestimated the amount of time their physician spent in the room (mean difference 4:10 minutes, P = 0.01). Patients' estimates of the time the physician spent did not vary by posture (16:00 minutes for seated, 16:19 for standing, P = 0.86).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patient age, y | |||||
| 1839 | 16 | 25.4 | 25 | 27.5 | 0.59 |
| 4059 | 17 | 27.0 | 30 | 33.0 | |
| 60+ | 30 | 47.6 | 36 | 39.6 | |
| Gender | |||||
| Male | 32 | 49.2 | 43 | 46.2 | 0.71 |
| Female | 33 | 50.8 | 50 | 53.8 | |
| Ethnicity | |||||
| Caucasian | 54 | 84.4 | 67 | 73.6 | 0.24 |
| Asian or Pacific Islander | 3 | 4.7 | 5 | 5.5 | |
| Other | 7 | 10.9 | 19 | 20.9 | |
Patients whose physician sat on rounds were statistically significantly more likely to choose the answer always to the questions regarding their physician listening carefully to them (P = 0.02) and explaining things in a way that was easy to understand (P = 0.05, Table 2). There was no difference in the patients' response to questions about the physician interrupting the patient when talking or treating them with courtesy and respect. Nearly all patients chose just right when asked to rate the amount of time their physician had spent with them on rounds (Table 2). The results of our sensitivity analysis that classified physicians according to their actual posture yielded different results; none of the findings in that analysis including questions regarding the physician listening carefully or explaining things in a way that was easy to understand were statistically significant (see Supporting Information, Appendix 1, in the online version of this article).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Patient perception of physician communication on that day's rounds | |||||
| Today on rounds, how often did this physician. | |||||
| Explain things in a way that was easy to understand? | |||||
| Never, sometimes, or usually | 7 | 10.9 | 22 | 23.9 | 0.05 |
| Always | 57 | 89.1 | 71 | 76.1 | |
| Listen carefully to you? | |||||
| Never, sometimes, or usually | 4 | 6.1 | 19 | 20.4 | 0.02 |
| Always | 62 | 93.4 | 74 | 79.6 | |
| Interrupt you when you were talking? | |||||
| Always, sometimes, or usually | 4 | 6.5 | 9 | 10 | 0.46 |
| Never | 58 | 93.6 | 81 | 90 | |
| Treat you with courtesy and respect? | |||||
| Never, sometimes, or usually | 0 | 0 | 7 | 7.6 | Not estimable |
| Always | 63 | 100 | 85 | 92.4 | |
| Please rate the amount of time this physician spent with you today during morning rounds. | |||||
| Too little | 1 | 1.6 | 3 | 3.5 | 0.41 |
| Just right | 63 | 98.4 | 84 | 96.5 | |
| Did you have any important questions or concerns about your care that you did not bring up with this doctor today?* | |||||
| Yes | 4 | 6.6 | 9 | 10.3 | 0.26 |
| No | 57 | 94.4 | 78 | 89.7 | |
DISCUSSION
In our study involving general medicine inpatients cared for by academic hospitalists, physicians did not spend more time in the room when seated, and were willing to adopt this practice. Patients perceived that seated compared to standing physicians listened more carefully and explained things in a way that was easy to understand when analyzed using an intent‐to‐treat approach. Patients did not perceive that seated physicians spent more time with them than standing physicians. To our knowledge, this is the first study showing the effects of hospitalist rounding posture on patient experience.
Our finding that patients rated seated physicians more highly on listening carefully and explaining things well indicates that training hospitalists to sit at the bedside may ultimately improve patient satisfaction. Our findings suggest seated interaction may improve satisfaction with communication without increasing time burden on physicians. However, given that these findings were not statistically significant when we analyzed our data according to actual behavior, larger studies should verify the impact of physician posture on patient experience.
Previous studies found that a minority of physicians sit in the inpatient setting, but did not study barriers to sitting while on rounds.[7, 8] A majority of physicians in our study sat when instructed to do so and when chairs were provided, and over 80% of physicians in our study said they planned to continue sitting while on rounds after the study was complete. A lack of chairs may be a major barrier to physicians adopting this facet of etiquette‐based medicine, and institutions wishing to promote this practice should consider providing chairs. Written comments from physician participants suggest physicians who are introduced to this practice enjoy sitting and think it improves physician‐patient communication. Further studies are needed to test our assumption that physicians continue sitting when chairs are provided.
Our work differs from previous studies. Johnson et al. studied interactions in the emergency room with a mean length of 8.6 minutes,[5] and Swayden et al. studied postoperative visits by a single neurosurgeon with a mean length of about 1 minute.[6] One explanation for the lack of a difference in time spent by posture might be that an average visit time of 12 minutes passes a threshold where patients make more accurate estimates of visit length or where factors other than posture more strongly influence perceptions of duration.
Limitations of our study include the relatively small sample size, single location, and limitation to English‐speaking patients able to consent themselves. Reasons for the limited sample size include that chairs were only installed in 2 units, and not all patients on the unit were under the care of participating physicians. Physician subjects were not blinded to their interactions being timed or to the fact that patients were surveyed about their communication skills. It is possible that factors that may have affected patients' responses such as severity of illness, number of consultants involved in their care, or prior experiences in the healthcare system were not equally distributed between our 2 groups. Additionally, our use of questions similar to those used in the HCAHPS instrument is not compliant with Centers for Medicare and Medicaid Services (CMS) policy. We caution others against using questions that might invalidate their hospital's participation in CMS payment programs.
Our study was limited to rounds involving 1 physician; our practice is that in a larger team the presenting member is encouraged to sit and others sit if there are additional chairs. Best practices on a teaching service are unclear and could be the subject of further study. The longer‐term sustainability of the practice of sitting on rounds is unclear. However, our physician subjects reported that they plan to continue to sit after the study, and we have shared the results with physicians in order to provide them with evidence supporting this practice. Not having a place to sit and thinking that sitting increases the amount of time spent on rounds were concerns provided in our preintervention survey, and we believe our study addresses these concerns.
Our study demonstrates the effects of a simple intervention on patient satisfaction without increasing burden on providers. Sitting at the bedside does not impact the amount of time spent with the patient, but may improve the patient's perception of the physician's communication skills and thus impact the patient experience. This simple intervention could improve patient satisfaction at little cost.
Acknowledgements
The authors acknowledge Tom Staiger, MD, UWMC Medical Director, for his assistance with obtaining chairs for this study.
Disclosure: Nothing to report.
Sitting while interacting with patients is standard in the outpatient setting and encouraged in the inpatient setting as a best practice.[1, 2] Michael W. Kahn defined etiquette‐based medicine as a set of easily taught behaviors that demonstrate respect for the patient; sitting at the bedside is included.[1] A prominent healthcare consulting group also recommends that physicians and nurses sit at the bedside, claiming that the patient will estimate you were in the room 3 times longer.[3] Previous studies suggest patients may perceive physicians who sit at the bedside as more compassionate and as spending more time with them, and may perceive the overall interaction as more positive when the physician sits.[4, 5, 6] Two small studies found that patients perceived the physician as having spent more time with them if he or she sat rather than stood.[5, 6] A study in the emergency department found no effect of posture on patient perception of physician communication skills, and a study of a single attending neurosurgeon found that patients reported a better understanding of their condition when the physician sat.[5, 6] The effect of physician posture on hospitalist physician‐patient communication has not been previously studied. Despite evidence that sitting in the inpatient setting may improve physician‐patient communication, studies suggest that physicians rarely sit at the bedside of inpatients.[7, 8]
We conducted a cluster‐randomized trial of the impact of hospitalist physician posture during morning rounds. We hypothesized that patients whose physician sat rather than stood would perceive that their physician spent more time with them and would rate the physician's communication skills more highly. We also hypothesized that sitting would not prolong the length of the patient‐physician encounter.
PATIENTS AND METHODS
We conducted a cluster‐randomized clinical trial with a crossover component randomizing physicians on the order of sit/stand within a consecutive 7‐day workweek. We enrolled patients being cared for by attending hospitalists on a resident‐uncovered general internal medicine service in an academic tertiary care hospital. We also enrolled the hospitalists and collected demographics and practice information. Wall‐mounted folding chairs (Figure 1) were installed in all rooms on two 28‐bed units for use by physicians. Eligible patients were newly admitted or transferred from the intensive care unit between June 2014 and June 2015, English speaking, and adults who consented to their own medical care. Physicians were randomly assigned to sit or stand during morning rounds for the first 3 days of their workweek. The last 4 days they provided care using the other posture. Blocks of 4 weeks were used to randomize the sit/stand order.

We measured the length of the physician‐patient interaction, asked both the physician and the patient to estimate the length of the interaction, and administered a written survey to the patient with questions about the physician's communication skills. Research assistants timed the interaction from outside the room and entered the room to consent patients and administer the survey after the physician departed. Survey questions were modeled on the physician communication questions from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. We aggregated all answers other than the most positive answer because HCAHPS questions are analyzed according to a top box methodology. Adherence to the intervention was measured by asking the physician whether he or she actually sat or stood for each interaction. We administered a survey to physicians to collect demographics and feedback.
We estimated descriptive statistics for physician and patient participants using cross‐tabs and means. To estimate associations, we used logistic and linear regression that employed cluster‐adjusted t statistics and clustered patients within providers. This method optimizes estimation of standard errors (and corresponding confidence intervals and P values) when the number of clusters is small (16 physicians).[9] For our primary analysis, we analyzed as randomized using an intent‐to‐treat approach. In other words, those assigned to the standing group were analyzed in the standing group even if they actually sat (and vice versa). In a sensitivity analysis we used the same methods to analyze the data according actual provider posture as reported by the physician and not as randomized. We calculated the mean and range of the number of patients seen by physicians. We compared estimates of time spent between patients and providers and patients' satisfaction according to provider posture. We complied with the Consolidated Standards of Reporting Trials 2010 guidelines.[10] Our institutional review board approved this project. All participants provided written consent.
RESULTS
All 17 hospitalists attending on the service consented to participate; 1 did not see any patients involved in the study and was removed from the analysis. Sixty‐nine percent were female and 81% had been in practice for 3 years or less at the time of study enrollment; 94% reported standing when assigned to stand and 83% reported sitting when assigned to sit. We found 31% of physicians reported they routinely sat before participating in the study, and 81% said they would sit more after the study; this result approached statistical significance (exact McNemar P = 0.06). Of the 11 physicians who reported not routinely sitting before the study all, 7 cited not having a place to sit as a reason for not sitting. Other rationale provided included being too short to see the patient if seated, believing rounds would take more time if seated, and concerns about contact precautions. Comments in the postintervention survey regarding why providers planned to sit more centered around themes of having chairs available, thinking that sitting improves communication, and thinking that patients prefer providers to sit.
Two hundred eleven patients were assessed for eligibility. Fifty‐two were excluded (27 did not meet inclusion criteria and 25 declined to participate), leaving 159 participating patients. Seven patient‐physician pairs were inadvertently assigned the wrong intervention but were analyzed as randomized. There were no demographic differences between patient groups (Table 1). Physicians participating in the study saw an average of 13 study patients (range, 118) during the study. Mean time spent in the patient's room during rounds was 12:00 minutes for seated physicians and 12:10 for standing physicians (P = 0.84). Regardless of provider posture, patients overestimated the amount of time their physician spent in the room (mean difference 4:10 minutes, P = 0.01). Patients' estimates of the time the physician spent did not vary by posture (16:00 minutes for seated, 16:19 for standing, P = 0.86).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patient age, y | |||||
| 1839 | 16 | 25.4 | 25 | 27.5 | 0.59 |
| 4059 | 17 | 27.0 | 30 | 33.0 | |
| 60+ | 30 | 47.6 | 36 | 39.6 | |
| Gender | |||||
| Male | 32 | 49.2 | 43 | 46.2 | 0.71 |
| Female | 33 | 50.8 | 50 | 53.8 | |
| Ethnicity | |||||
| Caucasian | 54 | 84.4 | 67 | 73.6 | 0.24 |
| Asian or Pacific Islander | 3 | 4.7 | 5 | 5.5 | |
| Other | 7 | 10.9 | 19 | 20.9 | |
Patients whose physician sat on rounds were statistically significantly more likely to choose the answer always to the questions regarding their physician listening carefully to them (P = 0.02) and explaining things in a way that was easy to understand (P = 0.05, Table 2). There was no difference in the patients' response to questions about the physician interrupting the patient when talking or treating them with courtesy and respect. Nearly all patients chose just right when asked to rate the amount of time their physician had spent with them on rounds (Table 2). The results of our sensitivity analysis that classified physicians according to their actual posture yielded different results; none of the findings in that analysis including questions regarding the physician listening carefully or explaining things in a way that was easy to understand were statistically significant (see Supporting Information, Appendix 1, in the online version of this article).
| Patients Seen by Seated Physician, N = 66 | Patients Seen by Standing Physician, N = 93 | P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Patient perception of physician communication on that day's rounds | |||||
| Today on rounds, how often did this physician. | |||||
| Explain things in a way that was easy to understand? | |||||
| Never, sometimes, or usually | 7 | 10.9 | 22 | 23.9 | 0.05 |
| Always | 57 | 89.1 | 71 | 76.1 | |
| Listen carefully to you? | |||||
| Never, sometimes, or usually | 4 | 6.1 | 19 | 20.4 | 0.02 |
| Always | 62 | 93.4 | 74 | 79.6 | |
| Interrupt you when you were talking? | |||||
| Always, sometimes, or usually | 4 | 6.5 | 9 | 10 | 0.46 |
| Never | 58 | 93.6 | 81 | 90 | |
| Treat you with courtesy and respect? | |||||
| Never, sometimes, or usually | 0 | 0 | 7 | 7.6 | Not estimable |
| Always | 63 | 100 | 85 | 92.4 | |
| Please rate the amount of time this physician spent with you today during morning rounds. | |||||
| Too little | 1 | 1.6 | 3 | 3.5 | 0.41 |
| Just right | 63 | 98.4 | 84 | 96.5 | |
| Did you have any important questions or concerns about your care that you did not bring up with this doctor today?* | |||||
| Yes | 4 | 6.6 | 9 | 10.3 | 0.26 |
| No | 57 | 94.4 | 78 | 89.7 | |
DISCUSSION
In our study involving general medicine inpatients cared for by academic hospitalists, physicians did not spend more time in the room when seated, and were willing to adopt this practice. Patients perceived that seated compared to standing physicians listened more carefully and explained things in a way that was easy to understand when analyzed using an intent‐to‐treat approach. Patients did not perceive that seated physicians spent more time with them than standing physicians. To our knowledge, this is the first study showing the effects of hospitalist rounding posture on patient experience.
Our finding that patients rated seated physicians more highly on listening carefully and explaining things well indicates that training hospitalists to sit at the bedside may ultimately improve patient satisfaction. Our findings suggest seated interaction may improve satisfaction with communication without increasing time burden on physicians. However, given that these findings were not statistically significant when we analyzed our data according to actual behavior, larger studies should verify the impact of physician posture on patient experience.
Previous studies found that a minority of physicians sit in the inpatient setting, but did not study barriers to sitting while on rounds.[7, 8] A majority of physicians in our study sat when instructed to do so and when chairs were provided, and over 80% of physicians in our study said they planned to continue sitting while on rounds after the study was complete. A lack of chairs may be a major barrier to physicians adopting this facet of etiquette‐based medicine, and institutions wishing to promote this practice should consider providing chairs. Written comments from physician participants suggest physicians who are introduced to this practice enjoy sitting and think it improves physician‐patient communication. Further studies are needed to test our assumption that physicians continue sitting when chairs are provided.
Our work differs from previous studies. Johnson et al. studied interactions in the emergency room with a mean length of 8.6 minutes,[5] and Swayden et al. studied postoperative visits by a single neurosurgeon with a mean length of about 1 minute.[6] One explanation for the lack of a difference in time spent by posture might be that an average visit time of 12 minutes passes a threshold where patients make more accurate estimates of visit length or where factors other than posture more strongly influence perceptions of duration.
Limitations of our study include the relatively small sample size, single location, and limitation to English‐speaking patients able to consent themselves. Reasons for the limited sample size include that chairs were only installed in 2 units, and not all patients on the unit were under the care of participating physicians. Physician subjects were not blinded to their interactions being timed or to the fact that patients were surveyed about their communication skills. It is possible that factors that may have affected patients' responses such as severity of illness, number of consultants involved in their care, or prior experiences in the healthcare system were not equally distributed between our 2 groups. Additionally, our use of questions similar to those used in the HCAHPS instrument is not compliant with Centers for Medicare and Medicaid Services (CMS) policy. We caution others against using questions that might invalidate their hospital's participation in CMS payment programs.
Our study was limited to rounds involving 1 physician; our practice is that in a larger team the presenting member is encouraged to sit and others sit if there are additional chairs. Best practices on a teaching service are unclear and could be the subject of further study. The longer‐term sustainability of the practice of sitting on rounds is unclear. However, our physician subjects reported that they plan to continue to sit after the study, and we have shared the results with physicians in order to provide them with evidence supporting this practice. Not having a place to sit and thinking that sitting increases the amount of time spent on rounds were concerns provided in our preintervention survey, and we believe our study addresses these concerns.
Our study demonstrates the effects of a simple intervention on patient satisfaction without increasing burden on providers. Sitting at the bedside does not impact the amount of time spent with the patient, but may improve the patient's perception of the physician's communication skills and thus impact the patient experience. This simple intervention could improve patient satisfaction at little cost.
Acknowledgements
The authors acknowledge Tom Staiger, MD, UWMC Medical Director, for his assistance with obtaining chairs for this study.
Disclosure: Nothing to report.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , . Enhancing patient satisfaction in dermatology. Am J Clin Dermatol. 2015;16:1–4.
- The Studer Group. Q21:501–505.
- , , , . To sit or not to sit? Ann Emerg Med. 2008;51:188–193.
- , , , , , . Effect of sitting vs. standing on perception of provider time at bedside: a pilot study. Patient Educ Couns. 2012;86(2):166–171.
- , , , , . Appraising the Practice of Etiquette‐Based Medicine in the Inpatient Setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013; 8:631–634.
- , . Practical and effective approaches to dealing with clustered data [unpublished manuscript]. Department of Political Science, Rice University, Houston, TX. Available at: http://jee3.web.rice.edu/cluster‐paper.pdf. Accessed February 29, 2016.
- , , . Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , . Enhancing patient satisfaction in dermatology. Am J Clin Dermatol. 2015;16:1–4.
- The Studer Group. Q21:501–505.
- , , , . To sit or not to sit? Ann Emerg Med. 2008;51:188–193.
- , , , , , . Effect of sitting vs. standing on perception of provider time at bedside: a pilot study. Patient Educ Couns. 2012;86(2):166–171.
- , , , , . Appraising the Practice of Etiquette‐Based Medicine in the Inpatient Setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013; 8:631–634.
- , . Practical and effective approaches to dealing with clustered data [unpublished manuscript]. Department of Political Science, Rice University, Houston, TX. Available at: http://jee3.web.rice.edu/cluster‐paper.pdf. Accessed February 29, 2016.
- , , . Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732.
Interhospital Transfer Patients
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
Interhospital transfers (IHTs) to academic medical centers (AMCs) or their affiliated hospitals may benefit patients who require unique specialty and procedural services. However, IHTs also introduce a potentially risky transition of care for patients suffering from complex or unstable medical problems.[1] Components of this risk include the dangers associated with transportation and the disrupted continuity of care that may lead to delays or errors in care.[2, 3] Furthermore, referring and accepting providers may face barriers to optimal handoffs including a lack of shared communication standards and difficulty accessing external medical records.[3, 4, 5] Although some authors have recommended the creation of formal guidelines for interhospital transfer processes for all patients to mitigate the risks of transfer, the available guidelines governing the IHT triage and communication process are limited to critically ill patients.[6]
A recent study of a diverse patient and hospital dataset demonstrated that interhospital transfer patients have a higher risk of mortality, increased length of stay (LOS), and increased risk of adverse events as compared with non‐transfer patients.[7] However, it is unknown if these findings persist in the population of patients transferred specifically to AMCs or their affiliated hospitals (the combination is hereafter referred to as academic health systems [AHSs]). AMCs provide a disproportionate share of IHT care for complex patients and have a vested interest in improving the outcomes of these transitions.[8] Prior single‐center studies of acute care adult medical patients accepted to AMCs have shown that IHT is associated with a longer LOS, increased in‐hospital mortality, and higher resource use.[9, 10] However, it is difficult to generalize from single‐center studies due to the variation in referral practices, geography, and network characteristics. Additionally, AMC referral systems, patient mix, and utilization of hospitalists have likely changed substantially in the nearly 2 decades since those reports were published.
Hospitalists and general internists often manage the transfer acceptance processes for internal medicine services at receiving hospitals, helping to triage and coordinate care for IHT patients. As a result, it is important for hospitalists to understand the characteristics and outcomes of the IHT population. In addition to informing the decision making around transfer for a given patient, such an understanding is the foundation for helping providers and institutions begin to systematically identify and mitigate peritransfer risks.
We conducted this large multicenter study to describe the characteristics and outcomes of a current, nationally representative IHT patient population discharged by hospitalists and general internists at AHSs. To identify unique features of the IHT population, we compared patients transferred from another hospital to an AHS to those admitted to the AHS directly from the AHS's emergency department (ED). Based on our anecdotal experiences and the prior single‐center study findings in adult medical populations,[9, 10] we hypothesized that the IHT population would be sicker, stay in the hospital and intensive care unit (ICU) longer, and have higher costs and in‐hospital mortality than ED patients. Although there may be fundamental differences between the 2 groups related to disease and patient condition, we hypothesized that outcome differences would persist even after adjusting for patient factors such as demographics, disease‐specific risk of mortality, and ICU utilization.
PATIENTS AND METHODS
We conducted a retrospective cohort study using data from the University HealthSystem Consortium (UHC) Clinical Database and Resource Manager (CDB/RM). UHC is an alliance of 120 academic medical centers and 300 of their affiliated hospitals for the purposes of collaboration on performance improvement. Each year, a subset of participating hospitals submits data on all of their inpatient discharges to the CDB/RM, which totals approximately 5 million records. The CDB/RM includes information from billing forms including demographics, diagnoses, and procedures as captured by International Classification of Diseases, Ninth Revision (ICD‐9) codes, discharge disposition, and line item charge detail for the type of bed (eg, floor, ICU). Most hospitals also provide detailed charge information including pharmacy, imaging, blood products, lab tests, and supplies. Some hospitals do not provide any charge data. The Beth Israel Deaconess Medical Center and University of Washington institutional review boards reviewed and approved the conduct of this study.
We included all inpatients discharged by hospitalists or general internal medicine physicians from UHC hospitals between April 1, 2011 and March 31, 2012. We excluded minors, pregnant patients, and prisoners. One hundred fifty‐eight adult academic medical centers and affiliated hospitals submitted data throughout this time period. Our primary independent variable, IHT status, was defined by patients whose admission source was another acute care institution. ED admissions were defined as patients admitted from the AHS ED whose source of origination was not another hospital or ambulatory surgery site.
Admission Characteristics
Admission characteristics of interest included age, gender, insurance status, the most common diagnoses in each cohort based on Medicare Severity Diagnosis‐Related Group (MS‐DRG), the most common Agency for Healthcare Research and Quality (AHRQ) comorbitidies,[11] the most common procedures, and the admission 3M All‐Patient Refined Diagnosis‐Related Group (APR‐DRG) risk of mortality (ROM) scores. 3M APR‐DRG ROM scores are proprietary categorical measures specific to the base APR‐DRG to which a patient is assigned, which are calculated using data available at the time of admission, including comorbid condition diagnosis codes, age, procedure codes, and principal diagnosis codes. A patient can fall into 1 of 4 categories with this score: minor, moderate, major, or extreme.[12]
Outcomes
Our primary outcome of interest was in‐hospital mortality. Secondary outcomes included LOS, the cost of care, ICU utilization, and discharge destination. The cost of care is a standardized estimate of the direct costs based on an adjustment of the charges submitted by CDB/RM participants. If an IHT is triaged through a receiving hospital's ED, the cost of care reflects those charges as well as the inpatient charges.
Statistical Analysis
We used descriptive statistics to characterize the IHT and ED patient populations. For bivariate comparisons of continuous variables, 2‐sample t tests with unequal variance were used. For categorical variables, 2 analysis was performed. We assessed the impact of IHT status on in‐hospital mortality using logistic regression to estimate unadjusted and adjusted relative risks, 95% confidence intervals (CIs), and P values. We included age, gender, insurance status, race, timing of ICU utilization, and 3M APR‐DRG ROM scores as independent variables. Prior studies have used this type of risk‐adjustment methodology with 3M APR‐DRG ROM scores,[13, 14, 15] including with interhospital transfer patients.[16] For all comparisons, a P value of <0.05 was considered statistically significant. Our sample size was determined by the data available for the 1‐year period.
Subgroup Analyses
We performed a stratified analysis based on the timing of ICU transfer to allow for additional comparisons of mortality within more homogeneous patient groups, and to control for the possibility that delays in ICU transfer could explain the association between IHT and in‐hospital mortality. We determined whether and when a patient spent time in the ICU based on daily accommodation charges. If a patient was charged for an ICU bed on the day of admission, we coded them as a direct ICU admission, and if the first ICU bed charge was on a subsequent day, they were coded as a delayed ICU admission. Approximately 20% of patients did not have the data necessary to determine the timing of ICU utilization, because the hospitals where they received care did not submit detailed charge data to the UHC.
Data analysis was performed by the UHC. Analysis was performed using Stata version 10 (StataCorp, College Station, TX). For all comparisons, a P value of <0.05 was considered significant.
RESULTS
Patient Characteristics
We identified 885,392 patients who met study criteria: 75,524 patients admitted as an IHT and 809,868 patients admitted from the ED. The proportion of each hospital's admissions that were IHTs that met our study criteria varied widely (median 9%, 25th percentile 3%, 75th percentile 14%). The average age and gender of the IHT and ED populations were similar and reflective of a nationally representative adult inpatient sample (Table 1). Racial compositions of the populations were notable for a higher portion of black patients in the ED admission group than the IHT group (25.4% vs 13.2%, P < 0.001). A slightly higher portion of the IHT population was covered by commercial insurance compared with the ED admissions (22.7% vs 19.1%, P < 0.001).
| Demographic/Clinical Variables | ED | IHT | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | Rank | ||
| ||||||
| No. of patients | 809,868 | 91.5 | 75,524 | 8.5 | ||
| Age, y | 62.2 19.1 | 60.2 18.2 | ||||
| Male | 381,563 | 47.1 | 38,850 | 51.4 | ||
| Female | 428,303 | 52.9 | 36,672 | 48.6 | ||
| Race | ||||||
| White | 492,894 | 60.9 | 54,780 | 72.5 | ||
| Black | 205,309 | 25.4 | 9,968 | 13.2 | ||
| Other | 66,709 | 8.1 | 7,777 | 10.3 | ||
| Hispanic | 44,956 | 5.6 | 2,999 | 4.0 | ||
| Primary payer | ||||||
| Commercial | 154,826 | 19.1 | 17,130 | 22.7 | ||
| Medicaid | 193,585 | 23.9 | 15,924 | 21.1 | ||
| Medicare | 445,227 | 55.0 | 39,301 | 52.0 | ||
| Other | 16,230 | 2.0 | 3,169 | 4.2 | ||
| Most common MS‐DRGs (top 5 for each group) | ||||||
| Esophagitis, gastroenteritis, and miscellaneous digest disorders without MCC | 34,116 | 4.2 | 1st | 1,517 | 2.1 | 2nd |
| Septicemia or severe sepsis without MV 96+ hours with MCC | 25,710 | 3.2 | 2nd | 2,625 | 3.7 | 1st |
| Cellulitis without MCC | 21,686 | 2.7 | 3rd | 871 | 1.2 | 8th |
| Kidney and urinary tract infections without MCC | 19,937 | 2.5 | 4th | 631 | 0.9 | 21st |
| Chest pain | 18,056 | 2.2 | 5th | 495 | 0.7 | 34th |
| Renal failure with CC | 15,478 | 1.9 | 9th | 1,018 | 1.4 | 5th |
| GI hemorrhage with CC | 12,855 | 1.6 | 12th | 1,234 | 1.7 | 3rd |
| Respiratory system diagnosis w ventilator support | 4,773 | 0.6 | 47th | 1,118 | 1.6 | 4th |
| AHRQ comorbidities (top 5 for each group) | ||||||
| Hypertension | 468,026 | 17.8 | 1st | 39,340 | 16.4 | 1st |
| Fluid and electrolyte disorders | 251,339 | 9.5 | 2nd | 19,825 | 8.3 | 2nd |
| Deficiency anemia | 208,722 | 7.9 | 3rd | 19,663 | 8.2 | 3rd |
| Diabetes without CCs | 190,140 | 7.2 | 4th | 17,131 | 7.1 | 4th |
| Chronic pulmonary disease | 178,164 | 6.8 | 5th | 16,319 | 6.8 | 5th |
| Most common procedures (top 5 for each group) | ||||||
| Packed cell transfusion | 72,590 | 7.0 | 1st | 9,756 | 5.0 | 2nd |
| (Central) venous catheter insertion | 68,687 | 6.7 | 2nd | 13,755 | 7.0 | 1st |
| Hemodialysis | 41,557 | 4.0 | 3rd | 5,351 | 2.7 | 4th |
| Heart ultrasound (echocardiogram) | 37,762 | 3.7 | 4th | 5,441 | 2.8 | 3rd |
| Insert endotracheal tube | 25,360 | 2.5 | 5th | 4,705 | 2.4 | 6th |
| Continuous invasive mechanical ventilation | 19,221 | 1.9 | 9th | 5,280 | 2.7 | 5th |
| 3M APR‐DRG admission ROM score | ||||||
| Minor | 271,702 | 33.6 | 18,620 | 26.1 | ||
| Moderate | 286,427 | 35.4 | 21,775 | 30.5 | ||
| Major | 193,652 | 23.9 | 20,531 | 28.7 | ||
| Extreme | 58,081 | 7.2 | 10,527 | 14.7 | ||
Primary discharge diagnoses (MS‐DRGs) varied widely, with no single diagnosis accounting for more than 4.2% of admissions in either group. The most common primary diagnoses among IHTs included severe sepsis (3.7%), esophagitis and gastroenteritis (2.1%), and gastrointestinal bleeding (1.7%). The top 5 most common AHRQ comorbidities were the same between the IHT and ED populations. A higher proportion of IHTs had at least 1 procedure performed during their hospitalization (68.5% vs 49.8%, P < 0.001). Note that ICD‐9 procedure codes include interventions such as blood transfusions and dialysis (Table 1), which may not be considered procedures in common medical parlance.
As compared with those admitted from the ED, IHTs had a higher proportion of patients categorized with major or extreme admission risk of mortality score (major + extreme, ED 31.1% vs IHT 43.5%, P < 0.001).
Overall Outcomes
IHT patients experienced a 60% longer average LOS, and a higher proportion spent time in the ICU than patients admitted through the ED (Table 2). On average, care for IHT patients cost more per day than for ED patients (Table 2). A lower proportion of IHTs were discharged home (68.6% vs 77.4% of ED patients), and a higher proportion died in the hospital (4.1% vs 1.8%) (P < 0.001 for both). Of the ED or IHT patients who died during their admission, there was no significant difference between the proportion who died within 48 hours of admission (26.4% vs 25.6%, P = 0.3693). After adjusting for age, gender, insurance status, race, ICU utilization and 3M APR‐DRG admission ROM scores, IHT was independently associated with the risk of in‐hospital death (odds ratio [OR]: 1.36, 95% CI: 1.291.43) (Table 3). The C statistic for the in‐hospital mortality model was 0.88.
| ED, n = 809,868 | IHT, n = 75,524 | |
|---|---|---|
| ||
| LOS, mean SD | 5.0 6.9 | 8.0 13.4 |
| ICU days, mean SD | 0.6 2.4 | 1.7 5.2 |
| Patients who spent some time in the ICU | 14.3% | 29.8% |
| % LOS in the ICU (ICU days LOS) | 11.0% | 21.6% |
| Average total cost SD | $10,731 $16,593 | $19,818 $34,665 |
| Average cost per day (total cost LOS) | $2,139 | $2,492 |
| Discharged home | 77.4% | 68.6% |
| Died as inpatient | 14,869 (1.8%) | 3,051 (4.0%) |
| Died within 48 hours of admission (% total deaths) | 3,918 (26.4%) | 780 (25.6%) |
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| ||
| Age, y | 1.00 (1.001.00) | 1.03 (1.031.03) |
| Gender | ||
| Female | Ref. | Ref. |
| Male | 1.13 (1.091.70) | 1.05 (1.011.09) |
| Medicare status | ||
| No | Ref. | Ref. |
| Yes | 2.14 (2.062.22) | 1.39 (1.331.47) |
| Race | ||
| Nonblack | Ref. | Ref. |
| Black | 0.57 (0.550.60) | 0.77 (0.730.81) |
| ICU utilization | ||
| No ICU admission | Ref. | Ref. |
| Direct admission to the ICU | 5.56 (5.295.84) | 2.25 (2.132.38) |
| Delayed ICU admission | 5.48 (5.275.69) | 2.46 (2.362.57) |
| 3M APR‐DRG admission ROM score | ||
| Minor | Ref. | Ref. |
| Moderate | 8.71 (7.5510.05) | 6.28 (5.437.25) |
| Major | 43.97 (38.3150.47) | 25.84 (22.4729.71) |
| Extreme | 238.65 (207.69273.80) | 107.17 (93.07123.40) |
| IHT | ||
| No | Ref. | Ref. |
| Yes | 2.36 (2.262.48) | 1.36 (1.29 1.43) |
Subgroup Analyses
Table 4 demonstrates the unadjusted and adjusted results from our analysis stratified by timing of ICU utilization. IHT remained independently associated with in‐hospital mortality regardless of timing of ICU utilization.
| Subgroup | In‐hospital Mortality, n (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| |||
| No ICU admission, n = 552,171 | |||
| ED, n = 519,421 | 4,913 (0.95%) | Ref. | Ref. |
| IHT, n = 32,750 | 590 (1.80%) | 1.92 [1.762.09] | 1.68 [1.531.84] |
| Direct admission to the ICU, n = 44,537 | |||
| ED, n = 35,614 | 1,733 (4.87%) | Ref. | Ref. |
| IHT, n = 8,923 | 628 (7.04%) | 1.48 [1.351.63] | 1.24 [1.121.37] |
| Delayed ICU admission, n = 110,540 | |||
| ED, n = 95,573 | 4,706 (4.92%) | Ref. | Ref. |
| IHT, n = 14,967 | 1,068 (7.14%) | 1.48 [1.391.59] | 1.25 [1.171.35] |
DISCUSSION
Our study of IHT patients ultimately discharged by hospitalists and general internists at US academic referral centers found significantly increased average LOS, costs, and in‐hospital mortality compared with patients admitted from the ED. The increased risk of mortality persisted after adjustment for patient characteristics and variables representing endogenous risk of mortality, and in more homogeneous subgroups after stratification by presence and timing of ICU utilization. These data confirm findings from single‐center studies and suggest that observations about the difference between IHT and ED populations may be generalizable across US academic hospitals.
Our work builds on 2 single‐center studies that examined mixed medical and surgical academic IHT populations from the late 1980s and early 1990s,[9, 10] and 1 studying surgical ICU patients in 2013.[17] These studies demonstrated longer average LOS, higher costs, and higher mortality rates (in both adjusted and unadjusted analyses). Our work confirmed these findings utilizing a more current, multicenter large dataset of IHT patients ultimately discharged by hospitalists and general internists. Our work is unique from a larger, more recent study[7] in that it focuses on patients transferred to academic health systems, and therefore has particular relevance to those settings. In addition, we divided patients into subpopulations based on the timing of ICU utilization, and found that in each of these populations, IHT remained independently associated with in‐hospital mortality.
Our analysis does not explain why the outcomes of IHTs are worse, but plausible contributing factors include that (1) patients chosen for IHT are at higher risk of death in ways uncaptured by established mortality risk scores, (2) referring, transferring, or accepting providers and institutions have provided inadequate care, (3) the transfer process itself involves harm, (4) socioeconomic bias in selection for IHT,[18] or (5) some combination of the above. Regardless of the causes of the worse outcomes observed in these outside‐hospital transfers, as these patients are colloquially known at accepting hospitals, they present challenges to everyone involved. Referring providers may feel a sense of urgency as these patients' needs exceed their management capabilities. The process is often time consuming and burdensome for referring and accepting providers because of poorly developed systems.[19] The transfer often takes patients further from their home and may make it more difficult for family to participate in their care. The transfer may delay care if the accepting institution cannot immediately accept the patient or if the time in transport is prolonged, which could result in decompensation at a critical juncture. For providers inheriting such patients, the stress of caring for these patients is compounded by the difficulty obtaining records about the prior hospitalization.[20] This frustrating experience is often translated into unfounded judgment of the institution that referred the patient and the care provided there.[21] It is important for hospitalists making decisions throughout the transfer process and for hospital leaders who determine staffing levels, measure the quality of care, manage hospital networks, or write hospital policy to appreciate that the transfer process itself may contribute to the challenges and poor outcomes we observe. Furthermore, regardless of the cause for the increased mortality that we observed, our findings imply that IHT patients require careful evaluation, management, and treatment.
Many accepting institutions have transfer centers that facilitate these transitions, utilizing protocols and templates to standardize the process.[22, 23] Future research should focus on the characteristics of these centers to learn which practices are most efficacious. Interventions to mitigate the known challenges of transfer (including patient selection and triage, handoff communication, and information sharing) could be tested by randomized studies at referring and accepting institutions. There may be a role for health information exchange or the development of enhanced pretransfer evaluation processes using telemedicine models; there is evidence that information sharing may reduce redundant imaging.[24] Perhaps targeted review of IHTs admitted to a non‐ICU portion of the hospital and subsequently transferred to the ICU could identify opportunities to improve triaging protocols and thus avert some of the bad outcomes observed in this subpopulation. A related future direction could be to create protected forumsusing the patient safety organization framework[25]to facilitate the discussion of interhospital transfer outcomes among the referring, transporting, and receiving parties. Lastly, future work should investigate the reasons for the different proportions of black patients in the ED versus IHT cohorts. Our finding that black race was associated with lower risk of mortality has been previously reported but may also benefit from more investigation.[26]
There are several limitations of our work. First, despite extensive adjustment for patient characteristics, due to the observational nature of our study it is still possible that IHTs differ from ED admissions in ways that were unaccounted for in our analysis, and which could be associated with increased mortality independent of the transfer process itself. We are unable to characterize features of the transfer process, such as the reason for transfer, differences in transfer processes among hospitals, or the distance and mode of travel, which may influence outcomes.[27] Because we used administrative data, variations in coding could incorrectly estimate the complexity or severity of illness on admission, which is a previously described risk.[28] In addition, although our dataset was very large, it was limited by incomplete charge data, which limited our ability to measure ICU utilization in our full cohort. The hospitals missing ICU charge data are of variable sizes and are distributed around the country, limiting the chance of systematic bias. Finally, in some settings, hospitalists may serve as the discharging physician for patients admitted to other services such as the ICU, introducing heterogeneity and bias to the sample. We attempted to mitigate such bias through our subgroup analysis, which allowed for comparisons within more homogeneous patient groupings.
In conclusion, our large multicenter study of academic health systems confirms the findings of prior single‐center academic studies and a large general population study that interhospital transfer patients have an increased average LOS, costs, and adjusted in‐hospital mortality than patients admitted from the ED. This difference in mortality persisted even after controlling for several other predictors of mortality. Our findings emphasize the need for future studies designed to clarify the reason for the increased risk and identify targets for interventions to improve outcomes for the interhospital transfer population.
Acknowledgements
The authors gratefully acknowledge Zachary Goldberger and Tom Gallagher for their critical reviews of this article.
Disclosures
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
- . The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2012;40(8):2470–2478.
- . AHRQ WebM23(1):68–75.
- , . Improving the quality of inter‐hospital transfers. J Qual Assur. 1991;13(4):16–20.
- , . Communication errors in dispatch of air medical transport. Prehosp Emerg Care. 2011;15(1):39–43.
- , , , , . Guidelines for the inter‐ and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
- , , , . Interhospital facility transfers in the United States: a nationwide outcomes study [published online November 13, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000148.
- , , , et al. Patients transferred to academic medical centers and other hospitals: characteristics, resource use, and outcomes. Acad Med. 1997;72(10):921–930.
- , , , , . Comparing the hospitalizations of transfer and non‐transfer patients in an academic medical center. Acad Med. 1996;71(3):262–266.
- , . Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34(4):295–309.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- . 3M HIS: APR DRG classification software—overview. Mortality Measurement. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Hughessumm.html. Accessed June 14, 2011.
- , . Risk‐adjusting acute myocardial infarction mortality: are APR‐DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489.
- , , , , . Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk‐adjusted analysis of a large regional database. Arthritis Rheum. 2011;63(8):2531–2539.
- , , , . Examination of hospital characteristics and patient quality outcomes using four inpatient quality indicators and 30‐day all‐cause mortality. Am J Med Qual. 2013;28(1):46–55.
- , , , , . Observed and expected outcomes in transfer and nontransfer patients with a hip fracture. J Orthop Trauma. 2011;25(11):666–669.
- , , , et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79(9):909–913.
- , , , . Insurance status and the transfer of hospitalized patients: an observational study. Ann Intern Med. 2014;160(2):81–90.
- , , . Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592–598.
- . Overwhelmed and uninspired by lack of coordinated care: a call to action for new physicians. Acad Med. 2013;88(11):1600–1602.
- . The outside hospital. Ann Intern Med. 2013;159(7):500–501.
- , , . Untangling the lines: using a transfer center to assist with interfacility transfers. Nurs Econ. 2003;21(2):94–96.
- , , , et al. Ticket to ride: reducing handoff risk during hospital patient transport. J Nurs Care Qual. 2009;24(2):109–115.
- , , . Outside imaging in emergency department transfer patients: CD import reduces rates of subsequent imaging utilization. Radiology. 2011;260(2):408–413.
- Agency for Healthcare Research and Quality. Patient Safety Organization (PSO) Program. Available at: http://www.pso.ahrq.gov. Accessed July 7, 2011.
- , , , , , . Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98–e107.
- , , , . Prognostic factors for mortality following interhospital transfers to the medical intensive care unit of a tertiary referral center. Crit Care Med. 2003;31(7):1981–1986.
- , , , . The accuracy of present‐on‐admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946–1962.
© 2015 Society of Hospital Medicine
Stranger than Fiction
A 65‐year‐old man suffered a myocardial infarction (MI) while traveling in Thailand. After 7 days of recovery, the patient departed for his home in the United States. He developed substernal, nonexertional, inspiratory chest pain and shortness of breath during his return flight and presented directly to an emergency room after arrival.
Initially, the evaluation should focus on life‐threatening diagnoses and not be distracted by the travel history. The immediate diagnostic concerns are active cardiac ischemia, complications of MI, and pulmonary embolus. Other cardiac causes of dyspnea include ischemic mitral regurgitation, postinfarction pericarditis with or without pericardial effusion, and heart failure. Mechanical complications of infarction, such as left ventricular free wall rupture or rupture of the interventricular septum, can occur in this time frame and are associated with significant morbidity. Pneumothorax may be precipitated by air travel, especially in patients with underlying lung disease. The immobilization associated with long airline flights is a risk factor for thromboembolic disease, which is classically associated with pleuritic chest pain. Inspiratory chest pain is also associated with inflammatory processes involving the pericardium or pleura. If pneumonia, pericarditis, or pleural effusion is present, details of his travel history will become more important in his evaluation.
The patient elaborated that he spent 10 days in Thailand. On the third day of his trip he developed severe chest pain while hiking toward a waterfall in a rural northern district. He was transferred to a large private hospital, where he received a stent in the proximal left anterior descending coronary artery 4 hours after symptom onset. At discharge he was prescribed ticagrelor 90 mg twice daily and daily doses of losartan 50 mg, furosemide 20 mg, spironolactone 12.5 mg, aspirin 81 mg, ivabradine 2.5 mg, and pravastatin 40 mg. He had also been taking doxycycline for malaria prophylaxis since departing the United States.
His past medical history was notable for hypertension and hyperlipidemia. The patient was a lifelong nonsmoker, did not use illicit substances, and consumed no more than 2 alcoholic beverages per day. He denied cough, fevers, chills, diaphoresis, weight loss, recent upper respiratory infection, abdominal pain, hematuria, and nausea. However, he reported exertional dyspnea following his MI and nonbloody diarrhea that occurred a few days prior to his return flight and resolved without intervention.
The remainder of his past medical history confirms that he received appropriate post‐MI care, but does not substantially alter the high priority concerns in his differential diagnosis. Diarrhea may occur in up to 50% of international travelers, and is especially common when returning from Southeast Asia or the Indian subcontinent. Disease processes that may explain diarrhea and subsequent dyspnea include intestinal infections that spread to the lung (eg, ascariasis and Loeffler syndrome), infection that precipitates neuromuscular weakness (eg, Campylobacter and Guillain‐Barr syndrome), or infection that precipitates heart failure (eg, coxsackievirus, myocarditis).
On admission, his temperature was 36.2C, heart rate 91 beats per minute, blood pressure 135/81 mm Hg, respiratory rate 16 breaths per minute, and oxygen saturation 98% on room air. Cardiac exam revealed a regular rhythm without rubs, murmurs, or diastolic gallops. He had no jugular venous distention, and no lower extremity edema. His distal pulses were equal and palpable throughout. Pulmonary exam was notable for decreased breath sounds at both bases without wheezing, rhonchi, or crackles noted. He had no rashes, joint effusions, or jaundice. Abdominal and neurologic examinations were unremarkable.
Diminished breath sounds may suggest atelectasis or pleural effusion; the latter could account for the patient's inspiratory chest pain. A chest radiograph is essential to evaluate this finding further. The physical examination is not suggestive of decompensated heart failure; measurement of serum brain natriuretic peptide level would further exclude that diagnosis.
Laboratory evaluation revealed a leukocytosis of 16,000/L, with 76% polymorphonuclear cells and 12% lymphocytes without eosinophils or band forms; a hematocrit of 38%; and a platelet count of 363,000/L. The patient had a creatinine of 1.6 mg/dL, potassium of 2.7 mEq/L, and a troponin‐I of 1.0 ng/mL (normal <0.40 ng/mL), with the remainder of the routine serum chemistries within normal limits. An electrocardiogram (ECG) showed QS complexes in the anteroseptal leads, and a chest radiograph showed bibasilar consolidations and a left pleural effusion. A ventilation‐perfusion scan of the chest was performed to evaluate for pulmonary embolism, and was interpreted as low probability. Transthoracic echocardiography demonstrated severe left ventricular systolic dysfunction with anterior wall akinesis, and an aneurysmal left ventricle with an apical thrombus. No significant valvular pathology or other structural defects were noted.
The ECG and echocardiogram confirm the history of a large anteroseptal infarction with severe left ventricular dysfunction. Serial troponin testing would be reasonable. However, the absence of any acute ischemic ECG changes, typical angina symptoms, and a relatively normal troponin level all suggest his chest pain does not represent active ischemia. His low abnormal troponin‐I is consistent with slow resolution after a large ischemic event in the recent past, and his anterior wall akinesis is consistent with prior infarction in the territory of his culprit left anterior descending coronary artery.
Although acute cardiac conditions appear less likely, the brisk leukocytosis in a returned traveler prompts consideration of infection. His lung consolidations could represent either new or resolving pneumonia. The complete absence of cough and fever is unusual for pneumonia, yet clinical findings are not as sensitive as chest radiograph for this diagnosis. At this point, typical organisms as well as uncommon pathogens associated with diarrhea or his travel history should be included in the differential.
After 24 hours, the patient was discharged on warfarin to treat the apical thrombus and moxifloxacin for a presumed community‐acquired pneumonia. Eight days after discharge, the patient visited his primary care physician with improving, but not resolved, shortness of breath and pleuritic pain despite completing the 7‐day course of moxifloxacin. A chest radiograph showed a large posterior left basal pleural fluid collection, increased from previous.
In the setting of a recent infection, the symptoms and radiographic findings suggest a complicated parapneumonic effusion or empyema. Failure to drain a previously seeded fluid collection leaves bacterial pathogens susceptible to moxifloxacin on the differential, including Streptococcus pneumoniae, Staphylococcus aureus, Legionella species, and other enterobacteriaciae (eg, Klebsiella pneumoniae).
The indolent course should also prompt consideration of more unusual pathogens, including roundworms (such as Ascaris) or lung flukes (Paragonimus), either of which can cause a lung infection without traditional pneumonia symptoms. Tuberculosis tends to present months (or years) after exposure. Older adults may manifest primary pulmonary tuberculosis with lower lobe infiltrates, consistent with this presentation. However, moxifloxacin is quite active against tuberculosis, and although single drug therapy would not be expected to cure the patient, it would be surprising for him to progress this quickly on moxifloxacin.
In northern Thailand, Burkholderia pseudomallei is a common cause of bacteremic pneumonia. The organism often has high‐level resistance to fluoroquinolones, and may present in a more insidious fashion than other causes of community‐acquired pneumonia. Although infection with B pseudomallei (melioidosis) can occasionally mimic apical pulmonary tuberculosis and may present after a prolonged latent period, it most commonly manifests as an acute pneumonia.
The patient was prescribed 10 days of amoxicillin‐clavulanic acid and clindamycin, and decubitus films were ordered to assess the effusion. These films, obtained 5 days later, showed a persistent pleural effusion. Subsequent ultrasound demonstrated loculated fluid, but a thoracentesis was not performed at that time due to the patient's therapeutic international normalized ratio and dual antiplatelet therapy.
The loculation further suggests a complicated parapneumonic effusion or empyema. Clindamycin adds very little to amoxicillin‐clavulanate as far as coverage of oral anaerobes or common pneumonia pathogens and may add to the risk of antibiotic side effects. A susceptible organism might not clear because of failure to drain this collection; if undertreated bacterial infection is suspected, tube thoracentesis is the established standard of care. However, the protracted course of illness makes untreated pyogenic bacterial infections unlikely.
At this point, the top 2 diagnostic considerations are Paragonimus westermani and B pseudomallei. P westermani is initially ingested, usually from an undercooked freshwater crustacean. Infected patients may experience a brief diarrheal illness, as this patient reported. However, infected patients typically have a brisk peripheral eosinophilia.
Melioidosis is thus the leading concern. Amoxicillin‐clavulanate is active against many strains of B pseudomallei, so the failure of the patient to worsen could be seen as a partial treatment and supports this diagnosis. However, as prolonged therapy is necessary for complete eradication of B pseudomallei, a definitive, culture‐based diagnosis should be established before committing the patient to months of antibiotics.
After completing 10 days of clindamycin and amoxicillin‐clavulanate, the patient reported improvement of his pleuritic pain, and repeat physical exam suggested interval decrease in the size of the effusion. Two days later, the patient began experiencing dysuria that persisted despite 3 days of nitrofurantoin.
Melioidosis can also involve the genitourinary tract. Hematogenous spread of B pseudomallei can seed a number of visceral organs including the bladder, joints, and bones. Men with suspected urinary infection should be evaluated for the possibility of prostatitis, an infection with considerable morbidity that requires extended therapy. This gentleman should have a prostate exam, and blood and urine cultures should be collected if prostatitis is suspected. Empiric antibiotics are not recommended without culture in a patient with complicated urinary tract infection.
Prostate exam was unremarkable. A urine culture grew a gram‐negative rod identified as B pseudomallei. Because B pseudomallei can cause fulminant sepsis, the infectious disease consultant requested that he return for admission, further evaluation, and initiation of intravenous antibiotics. Computed tomography (CT) of the chest, abdomen, and pelvis revealed multiple pulmonary nodules, a persistent left pleural effusion, and a rim‐enhancing hypodensity in the prostate consistent with abscess (Figure 1). Blood and pleural fluid cultures were negative.
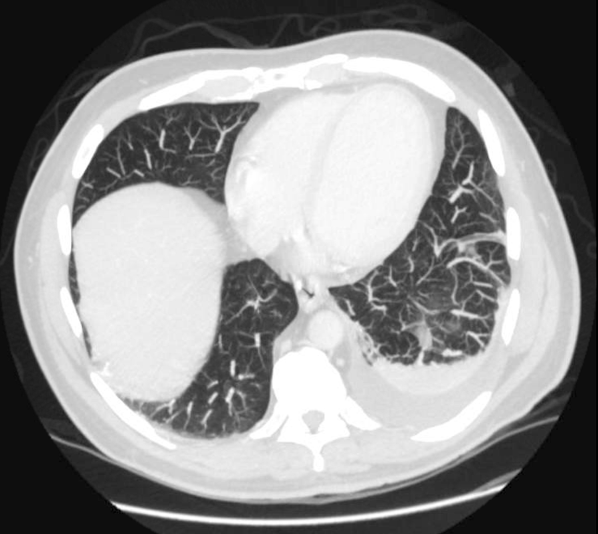
Initial treatment for a patient with severe or metastatic B pseudomallei infection requires high‐dose intravenous antibiotic therapy. Ceftazidime, imipenem, and meropenem are the best studied agents for this purpose. Surgical drainage should be considered for the abscess. Following the completion of intensive intravenous therapy, relapse rates are high unless a longer‐term, consolidation therapy is pursued. Trimethoprim‐sulfamethoxazole is the recommended agent.
The patient was treated with high‐dose ceftazidime for 2 weeks, followed by 6 months of high‐dose oral trimethoprim‐sulfamethoxazole. His symptoms resolved, and 7 months after presentation, he continued to feel well.
DISCUSSION
Melioidosis refers to any infection caused by B pseudomallei, a gram‐negative bacillus found in soil and water, most commonly in Southeast Asia and Australia.[1] It is an important cause of pneumonia in endemic regions; in Thailand, the incidence is as high as 12 cases per 100,000 people, and it is the third leading infectious cause of death, following human immunodeficiency virus and tuberculosis.[2] However, it occurs only as an imported infection in the United States and remains an unfamiliar infection for many US medical practitioners. Melioidosis should be considered in patients returning from endemic regions presenting with sepsis, pneumonia, urinary symptoms, or abscesses.
B pseudomallei can be transmitted to humans through exposure to contaminated soil or water via ingestion, inhalation, or percutaneous inoculation.[1] Outbreaks typically occur during the rainy season and after typhoons.[1, 3] Presumably, this patient's exposure to B pseudomallei occurred while hiking and wading in freshwater lakes and waterfalls. Although hospital‐acquired melioidosis has not been reported, and isolation precautions are not necessary, rare cases of disease acquired via laboratory exposure have been reported among US healthcare workers. Clinicians suspecting melioidosis should alert the receiving laboratory.[4]
The treatment course for melioidosis is lengthy and should involve consultation with an infectious disease specialist. B pseudomallei is known to be resistant to penicillin, first‐ and second‐generation cephalosporins, and moxifloxacin. The standard treatment includes 10 to 14 days of intravenous ceftazidime, meropenem, or imipenem, and then trimethoprim‐sulfamethoxazole for 3 to 6 months.[1] Treatment should be guided by culture susceptibility data when available. There are reports of B pseudomallei having different resistance patterns within the same host; clinicians should culture all drained fluid collections and tailor antibiotics to the most resistant strain recovered.[5, 6] Although melioidosis is a life‐threatening infection, previously healthy patients have an excellent prognosis assuming prompt diagnosis and treatment are provided.[3]
After excluding common causes of chest pain, the discussant identified the need to definitively establish a microbiologic diagnosis by obtaining pleural fluid. Although common clinical scenarios can often be treated with guideline‐supported empiric antibiotics, the use of serial courses of empiric antibiotics should be carefully questioned and is generally discouraged. Specific data to prove or disprove the presence of infection should be obtained before exposing a patient to the risks of multiple drugs or prolonged antibiotic therapy, as well as the risks of delayed (or missed) diagnosis. Unfortunately, a complete evaluation was delayed by clinical contraindications to diagnostic thoracentesis, and a definitive diagnosis was reached only after development of more widespread symptoms.
This patient's protean presentation is not surprising given his ultimate diagnosis. B pseudomallei has been termed the great mimicker, as disease presentation and organ involvement can vary from an indolent localized infection to acute severe sepsis.[7] Pneumonia and genitourinary infections are the most common manifestations, although skin infections, bacteremia, septic arthritis, and neurologic disease are also possible.[1, 3] In addition, melioidosis may develop after a lengthy incubation. In a case series, confirmed incubation periods ranged from 1 to 21 days (mean, 9 days); however, cases of chronic (>2 months) infection, mimicking tuberculosis, are estimated to occur in about 12% of cases.[4] B pseudomallei is also capable of causing reactivation disease, similar to tuberculosis. It was referred to as the Vietnamese time bomb when US Vietnam War veterans, exposed to the disease when helicopters aerosolized the bacteria in the soil, developed the disease only after their return to the United States.[8] Fortunately, only a tiny fraction of the quarter‐million soldiers with serologically confirmed exposure to the bacteria ultimately developed disease.
In The Adventure of the Dying Detective, Sherlock Holmes fakes a serious illness characterized by shortness of breath and weakness to trick an adversary into confessing to murder. The abrupt, crippling infection mimicked by Holmes is thought by some to be melioidosis.[9, 10] Conan Doyle's story was published in 1913, a year after melioidosis was first reported in the medical literature, and the exotic, protean infection may well have sparked Doyle's imagination. However, this patient's case of melioidosis proved stranger than fiction in its untimely concomitant development with an MI. Cracking our case required imagination and nimble thinking to avoid a number of cognitive pitfalls. The patient's recent MI anchored reasoning at his initial presentation, and the initial diagnosis of community‐acquired pneumonia raised the danger of premature closure. Reaching the correct diagnosis required an open mind, a detailed travel history, and firm microbiologic evidence. Hospitalists need not be expert in the health risks of travel to specific foreign destinations, but investigating those risks can hasten proper diagnosis and treatment.
TEACHING POINTS
- Melioidosis should be considered in patients returning from endemic regions who present with sepsis, pneumonia, urinary symptoms, or an abscess.
- For patients with a loculated parapneumonic effusion, tube thoracentesis for culture and drainage is the standard of care for diagnosis and treatment.
- Culture identification and antibiotic sensitivities are critical for management of B pseudomallei, because prolonged antibiotic treatment is needed.
Disclosure
Nothing to report.
- , , . Melioidosis. N Engl J Med. 2012;367(11):1035–1044.
- , , , et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82(6):1113–1117.
- , , . The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):e900.
- , , , et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14(7):e2.
- , , . Variations in ceftazidime and amoxicillin‐clavulanate susceptibilities within a clonal infection of Burkholderia pseudomallei. J Clin Microbiol. 2009;47(5):1556–1558.
- , , , et al. Within‐host evolution of Burkholderia pseudomallei in four cases of acute melioidosis. PLoS Pathog. 2010;6(1):e1000725.
- , , , , . Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4(4):272–282.
- , . Melioidosis. In: Dembeck ZF, ed. Medical Aspects of Biological Warfare. 2nd ed. Washington, DC: Office of the Surgeon General; 2007:146–166.
- . Sherlock Holmes and a biological weapon. J R Soc Med. 2002;95(2):101–103.
- . Sherlock Holmes and tropical medicine: a centennial appraisal. Am J Trop Med Hyg. 1994;50:99–101.
A 65‐year‐old man suffered a myocardial infarction (MI) while traveling in Thailand. After 7 days of recovery, the patient departed for his home in the United States. He developed substernal, nonexertional, inspiratory chest pain and shortness of breath during his return flight and presented directly to an emergency room after arrival.
Initially, the evaluation should focus on life‐threatening diagnoses and not be distracted by the travel history. The immediate diagnostic concerns are active cardiac ischemia, complications of MI, and pulmonary embolus. Other cardiac causes of dyspnea include ischemic mitral regurgitation, postinfarction pericarditis with or without pericardial effusion, and heart failure. Mechanical complications of infarction, such as left ventricular free wall rupture or rupture of the interventricular septum, can occur in this time frame and are associated with significant morbidity. Pneumothorax may be precipitated by air travel, especially in patients with underlying lung disease. The immobilization associated with long airline flights is a risk factor for thromboembolic disease, which is classically associated with pleuritic chest pain. Inspiratory chest pain is also associated with inflammatory processes involving the pericardium or pleura. If pneumonia, pericarditis, or pleural effusion is present, details of his travel history will become more important in his evaluation.
The patient elaborated that he spent 10 days in Thailand. On the third day of his trip he developed severe chest pain while hiking toward a waterfall in a rural northern district. He was transferred to a large private hospital, where he received a stent in the proximal left anterior descending coronary artery 4 hours after symptom onset. At discharge he was prescribed ticagrelor 90 mg twice daily and daily doses of losartan 50 mg, furosemide 20 mg, spironolactone 12.5 mg, aspirin 81 mg, ivabradine 2.5 mg, and pravastatin 40 mg. He had also been taking doxycycline for malaria prophylaxis since departing the United States.
His past medical history was notable for hypertension and hyperlipidemia. The patient was a lifelong nonsmoker, did not use illicit substances, and consumed no more than 2 alcoholic beverages per day. He denied cough, fevers, chills, diaphoresis, weight loss, recent upper respiratory infection, abdominal pain, hematuria, and nausea. However, he reported exertional dyspnea following his MI and nonbloody diarrhea that occurred a few days prior to his return flight and resolved without intervention.
The remainder of his past medical history confirms that he received appropriate post‐MI care, but does not substantially alter the high priority concerns in his differential diagnosis. Diarrhea may occur in up to 50% of international travelers, and is especially common when returning from Southeast Asia or the Indian subcontinent. Disease processes that may explain diarrhea and subsequent dyspnea include intestinal infections that spread to the lung (eg, ascariasis and Loeffler syndrome), infection that precipitates neuromuscular weakness (eg, Campylobacter and Guillain‐Barr syndrome), or infection that precipitates heart failure (eg, coxsackievirus, myocarditis).
On admission, his temperature was 36.2C, heart rate 91 beats per minute, blood pressure 135/81 mm Hg, respiratory rate 16 breaths per minute, and oxygen saturation 98% on room air. Cardiac exam revealed a regular rhythm without rubs, murmurs, or diastolic gallops. He had no jugular venous distention, and no lower extremity edema. His distal pulses were equal and palpable throughout. Pulmonary exam was notable for decreased breath sounds at both bases without wheezing, rhonchi, or crackles noted. He had no rashes, joint effusions, or jaundice. Abdominal and neurologic examinations were unremarkable.
Diminished breath sounds may suggest atelectasis or pleural effusion; the latter could account for the patient's inspiratory chest pain. A chest radiograph is essential to evaluate this finding further. The physical examination is not suggestive of decompensated heart failure; measurement of serum brain natriuretic peptide level would further exclude that diagnosis.
Laboratory evaluation revealed a leukocytosis of 16,000/L, with 76% polymorphonuclear cells and 12% lymphocytes without eosinophils or band forms; a hematocrit of 38%; and a platelet count of 363,000/L. The patient had a creatinine of 1.6 mg/dL, potassium of 2.7 mEq/L, and a troponin‐I of 1.0 ng/mL (normal <0.40 ng/mL), with the remainder of the routine serum chemistries within normal limits. An electrocardiogram (ECG) showed QS complexes in the anteroseptal leads, and a chest radiograph showed bibasilar consolidations and a left pleural effusion. A ventilation‐perfusion scan of the chest was performed to evaluate for pulmonary embolism, and was interpreted as low probability. Transthoracic echocardiography demonstrated severe left ventricular systolic dysfunction with anterior wall akinesis, and an aneurysmal left ventricle with an apical thrombus. No significant valvular pathology or other structural defects were noted.
The ECG and echocardiogram confirm the history of a large anteroseptal infarction with severe left ventricular dysfunction. Serial troponin testing would be reasonable. However, the absence of any acute ischemic ECG changes, typical angina symptoms, and a relatively normal troponin level all suggest his chest pain does not represent active ischemia. His low abnormal troponin‐I is consistent with slow resolution after a large ischemic event in the recent past, and his anterior wall akinesis is consistent with prior infarction in the territory of his culprit left anterior descending coronary artery.
Although acute cardiac conditions appear less likely, the brisk leukocytosis in a returned traveler prompts consideration of infection. His lung consolidations could represent either new or resolving pneumonia. The complete absence of cough and fever is unusual for pneumonia, yet clinical findings are not as sensitive as chest radiograph for this diagnosis. At this point, typical organisms as well as uncommon pathogens associated with diarrhea or his travel history should be included in the differential.
After 24 hours, the patient was discharged on warfarin to treat the apical thrombus and moxifloxacin for a presumed community‐acquired pneumonia. Eight days after discharge, the patient visited his primary care physician with improving, but not resolved, shortness of breath and pleuritic pain despite completing the 7‐day course of moxifloxacin. A chest radiograph showed a large posterior left basal pleural fluid collection, increased from previous.
In the setting of a recent infection, the symptoms and radiographic findings suggest a complicated parapneumonic effusion or empyema. Failure to drain a previously seeded fluid collection leaves bacterial pathogens susceptible to moxifloxacin on the differential, including Streptococcus pneumoniae, Staphylococcus aureus, Legionella species, and other enterobacteriaciae (eg, Klebsiella pneumoniae).
The indolent course should also prompt consideration of more unusual pathogens, including roundworms (such as Ascaris) or lung flukes (Paragonimus), either of which can cause a lung infection without traditional pneumonia symptoms. Tuberculosis tends to present months (or years) after exposure. Older adults may manifest primary pulmonary tuberculosis with lower lobe infiltrates, consistent with this presentation. However, moxifloxacin is quite active against tuberculosis, and although single drug therapy would not be expected to cure the patient, it would be surprising for him to progress this quickly on moxifloxacin.
In northern Thailand, Burkholderia pseudomallei is a common cause of bacteremic pneumonia. The organism often has high‐level resistance to fluoroquinolones, and may present in a more insidious fashion than other causes of community‐acquired pneumonia. Although infection with B pseudomallei (melioidosis) can occasionally mimic apical pulmonary tuberculosis and may present after a prolonged latent period, it most commonly manifests as an acute pneumonia.
The patient was prescribed 10 days of amoxicillin‐clavulanic acid and clindamycin, and decubitus films were ordered to assess the effusion. These films, obtained 5 days later, showed a persistent pleural effusion. Subsequent ultrasound demonstrated loculated fluid, but a thoracentesis was not performed at that time due to the patient's therapeutic international normalized ratio and dual antiplatelet therapy.
The loculation further suggests a complicated parapneumonic effusion or empyema. Clindamycin adds very little to amoxicillin‐clavulanate as far as coverage of oral anaerobes or common pneumonia pathogens and may add to the risk of antibiotic side effects. A susceptible organism might not clear because of failure to drain this collection; if undertreated bacterial infection is suspected, tube thoracentesis is the established standard of care. However, the protracted course of illness makes untreated pyogenic bacterial infections unlikely.
At this point, the top 2 diagnostic considerations are Paragonimus westermani and B pseudomallei. P westermani is initially ingested, usually from an undercooked freshwater crustacean. Infected patients may experience a brief diarrheal illness, as this patient reported. However, infected patients typically have a brisk peripheral eosinophilia.
Melioidosis is thus the leading concern. Amoxicillin‐clavulanate is active against many strains of B pseudomallei, so the failure of the patient to worsen could be seen as a partial treatment and supports this diagnosis. However, as prolonged therapy is necessary for complete eradication of B pseudomallei, a definitive, culture‐based diagnosis should be established before committing the patient to months of antibiotics.
After completing 10 days of clindamycin and amoxicillin‐clavulanate, the patient reported improvement of his pleuritic pain, and repeat physical exam suggested interval decrease in the size of the effusion. Two days later, the patient began experiencing dysuria that persisted despite 3 days of nitrofurantoin.
Melioidosis can also involve the genitourinary tract. Hematogenous spread of B pseudomallei can seed a number of visceral organs including the bladder, joints, and bones. Men with suspected urinary infection should be evaluated for the possibility of prostatitis, an infection with considerable morbidity that requires extended therapy. This gentleman should have a prostate exam, and blood and urine cultures should be collected if prostatitis is suspected. Empiric antibiotics are not recommended without culture in a patient with complicated urinary tract infection.
Prostate exam was unremarkable. A urine culture grew a gram‐negative rod identified as B pseudomallei. Because B pseudomallei can cause fulminant sepsis, the infectious disease consultant requested that he return for admission, further evaluation, and initiation of intravenous antibiotics. Computed tomography (CT) of the chest, abdomen, and pelvis revealed multiple pulmonary nodules, a persistent left pleural effusion, and a rim‐enhancing hypodensity in the prostate consistent with abscess (Figure 1). Blood and pleural fluid cultures were negative.

Initial treatment for a patient with severe or metastatic B pseudomallei infection requires high‐dose intravenous antibiotic therapy. Ceftazidime, imipenem, and meropenem are the best studied agents for this purpose. Surgical drainage should be considered for the abscess. Following the completion of intensive intravenous therapy, relapse rates are high unless a longer‐term, consolidation therapy is pursued. Trimethoprim‐sulfamethoxazole is the recommended agent.
The patient was treated with high‐dose ceftazidime for 2 weeks, followed by 6 months of high‐dose oral trimethoprim‐sulfamethoxazole. His symptoms resolved, and 7 months after presentation, he continued to feel well.
DISCUSSION
Melioidosis refers to any infection caused by B pseudomallei, a gram‐negative bacillus found in soil and water, most commonly in Southeast Asia and Australia.[1] It is an important cause of pneumonia in endemic regions; in Thailand, the incidence is as high as 12 cases per 100,000 people, and it is the third leading infectious cause of death, following human immunodeficiency virus and tuberculosis.[2] However, it occurs only as an imported infection in the United States and remains an unfamiliar infection for many US medical practitioners. Melioidosis should be considered in patients returning from endemic regions presenting with sepsis, pneumonia, urinary symptoms, or abscesses.
B pseudomallei can be transmitted to humans through exposure to contaminated soil or water via ingestion, inhalation, or percutaneous inoculation.[1] Outbreaks typically occur during the rainy season and after typhoons.[1, 3] Presumably, this patient's exposure to B pseudomallei occurred while hiking and wading in freshwater lakes and waterfalls. Although hospital‐acquired melioidosis has not been reported, and isolation precautions are not necessary, rare cases of disease acquired via laboratory exposure have been reported among US healthcare workers. Clinicians suspecting melioidosis should alert the receiving laboratory.[4]
The treatment course for melioidosis is lengthy and should involve consultation with an infectious disease specialist. B pseudomallei is known to be resistant to penicillin, first‐ and second‐generation cephalosporins, and moxifloxacin. The standard treatment includes 10 to 14 days of intravenous ceftazidime, meropenem, or imipenem, and then trimethoprim‐sulfamethoxazole for 3 to 6 months.[1] Treatment should be guided by culture susceptibility data when available. There are reports of B pseudomallei having different resistance patterns within the same host; clinicians should culture all drained fluid collections and tailor antibiotics to the most resistant strain recovered.[5, 6] Although melioidosis is a life‐threatening infection, previously healthy patients have an excellent prognosis assuming prompt diagnosis and treatment are provided.[3]
After excluding common causes of chest pain, the discussant identified the need to definitively establish a microbiologic diagnosis by obtaining pleural fluid. Although common clinical scenarios can often be treated with guideline‐supported empiric antibiotics, the use of serial courses of empiric antibiotics should be carefully questioned and is generally discouraged. Specific data to prove or disprove the presence of infection should be obtained before exposing a patient to the risks of multiple drugs or prolonged antibiotic therapy, as well as the risks of delayed (or missed) diagnosis. Unfortunately, a complete evaluation was delayed by clinical contraindications to diagnostic thoracentesis, and a definitive diagnosis was reached only after development of more widespread symptoms.
This patient's protean presentation is not surprising given his ultimate diagnosis. B pseudomallei has been termed the great mimicker, as disease presentation and organ involvement can vary from an indolent localized infection to acute severe sepsis.[7] Pneumonia and genitourinary infections are the most common manifestations, although skin infections, bacteremia, septic arthritis, and neurologic disease are also possible.[1, 3] In addition, melioidosis may develop after a lengthy incubation. In a case series, confirmed incubation periods ranged from 1 to 21 days (mean, 9 days); however, cases of chronic (>2 months) infection, mimicking tuberculosis, are estimated to occur in about 12% of cases.[4] B pseudomallei is also capable of causing reactivation disease, similar to tuberculosis. It was referred to as the Vietnamese time bomb when US Vietnam War veterans, exposed to the disease when helicopters aerosolized the bacteria in the soil, developed the disease only after their return to the United States.[8] Fortunately, only a tiny fraction of the quarter‐million soldiers with serologically confirmed exposure to the bacteria ultimately developed disease.
In The Adventure of the Dying Detective, Sherlock Holmes fakes a serious illness characterized by shortness of breath and weakness to trick an adversary into confessing to murder. The abrupt, crippling infection mimicked by Holmes is thought by some to be melioidosis.[9, 10] Conan Doyle's story was published in 1913, a year after melioidosis was first reported in the medical literature, and the exotic, protean infection may well have sparked Doyle's imagination. However, this patient's case of melioidosis proved stranger than fiction in its untimely concomitant development with an MI. Cracking our case required imagination and nimble thinking to avoid a number of cognitive pitfalls. The patient's recent MI anchored reasoning at his initial presentation, and the initial diagnosis of community‐acquired pneumonia raised the danger of premature closure. Reaching the correct diagnosis required an open mind, a detailed travel history, and firm microbiologic evidence. Hospitalists need not be expert in the health risks of travel to specific foreign destinations, but investigating those risks can hasten proper diagnosis and treatment.
TEACHING POINTS
- Melioidosis should be considered in patients returning from endemic regions who present with sepsis, pneumonia, urinary symptoms, or an abscess.
- For patients with a loculated parapneumonic effusion, tube thoracentesis for culture and drainage is the standard of care for diagnosis and treatment.
- Culture identification and antibiotic sensitivities are critical for management of B pseudomallei, because prolonged antibiotic treatment is needed.
Disclosure
Nothing to report.
A 65‐year‐old man suffered a myocardial infarction (MI) while traveling in Thailand. After 7 days of recovery, the patient departed for his home in the United States. He developed substernal, nonexertional, inspiratory chest pain and shortness of breath during his return flight and presented directly to an emergency room after arrival.
Initially, the evaluation should focus on life‐threatening diagnoses and not be distracted by the travel history. The immediate diagnostic concerns are active cardiac ischemia, complications of MI, and pulmonary embolus. Other cardiac causes of dyspnea include ischemic mitral regurgitation, postinfarction pericarditis with or without pericardial effusion, and heart failure. Mechanical complications of infarction, such as left ventricular free wall rupture or rupture of the interventricular septum, can occur in this time frame and are associated with significant morbidity. Pneumothorax may be precipitated by air travel, especially in patients with underlying lung disease. The immobilization associated with long airline flights is a risk factor for thromboembolic disease, which is classically associated with pleuritic chest pain. Inspiratory chest pain is also associated with inflammatory processes involving the pericardium or pleura. If pneumonia, pericarditis, or pleural effusion is present, details of his travel history will become more important in his evaluation.
The patient elaborated that he spent 10 days in Thailand. On the third day of his trip he developed severe chest pain while hiking toward a waterfall in a rural northern district. He was transferred to a large private hospital, where he received a stent in the proximal left anterior descending coronary artery 4 hours after symptom onset. At discharge he was prescribed ticagrelor 90 mg twice daily and daily doses of losartan 50 mg, furosemide 20 mg, spironolactone 12.5 mg, aspirin 81 mg, ivabradine 2.5 mg, and pravastatin 40 mg. He had also been taking doxycycline for malaria prophylaxis since departing the United States.
His past medical history was notable for hypertension and hyperlipidemia. The patient was a lifelong nonsmoker, did not use illicit substances, and consumed no more than 2 alcoholic beverages per day. He denied cough, fevers, chills, diaphoresis, weight loss, recent upper respiratory infection, abdominal pain, hematuria, and nausea. However, he reported exertional dyspnea following his MI and nonbloody diarrhea that occurred a few days prior to his return flight and resolved without intervention.
The remainder of his past medical history confirms that he received appropriate post‐MI care, but does not substantially alter the high priority concerns in his differential diagnosis. Diarrhea may occur in up to 50% of international travelers, and is especially common when returning from Southeast Asia or the Indian subcontinent. Disease processes that may explain diarrhea and subsequent dyspnea include intestinal infections that spread to the lung (eg, ascariasis and Loeffler syndrome), infection that precipitates neuromuscular weakness (eg, Campylobacter and Guillain‐Barr syndrome), or infection that precipitates heart failure (eg, coxsackievirus, myocarditis).
On admission, his temperature was 36.2C, heart rate 91 beats per minute, blood pressure 135/81 mm Hg, respiratory rate 16 breaths per minute, and oxygen saturation 98% on room air. Cardiac exam revealed a regular rhythm without rubs, murmurs, or diastolic gallops. He had no jugular venous distention, and no lower extremity edema. His distal pulses were equal and palpable throughout. Pulmonary exam was notable for decreased breath sounds at both bases without wheezing, rhonchi, or crackles noted. He had no rashes, joint effusions, or jaundice. Abdominal and neurologic examinations were unremarkable.
Diminished breath sounds may suggest atelectasis or pleural effusion; the latter could account for the patient's inspiratory chest pain. A chest radiograph is essential to evaluate this finding further. The physical examination is not suggestive of decompensated heart failure; measurement of serum brain natriuretic peptide level would further exclude that diagnosis.
Laboratory evaluation revealed a leukocytosis of 16,000/L, with 76% polymorphonuclear cells and 12% lymphocytes without eosinophils or band forms; a hematocrit of 38%; and a platelet count of 363,000/L. The patient had a creatinine of 1.6 mg/dL, potassium of 2.7 mEq/L, and a troponin‐I of 1.0 ng/mL (normal <0.40 ng/mL), with the remainder of the routine serum chemistries within normal limits. An electrocardiogram (ECG) showed QS complexes in the anteroseptal leads, and a chest radiograph showed bibasilar consolidations and a left pleural effusion. A ventilation‐perfusion scan of the chest was performed to evaluate for pulmonary embolism, and was interpreted as low probability. Transthoracic echocardiography demonstrated severe left ventricular systolic dysfunction with anterior wall akinesis, and an aneurysmal left ventricle with an apical thrombus. No significant valvular pathology or other structural defects were noted.
The ECG and echocardiogram confirm the history of a large anteroseptal infarction with severe left ventricular dysfunction. Serial troponin testing would be reasonable. However, the absence of any acute ischemic ECG changes, typical angina symptoms, and a relatively normal troponin level all suggest his chest pain does not represent active ischemia. His low abnormal troponin‐I is consistent with slow resolution after a large ischemic event in the recent past, and his anterior wall akinesis is consistent with prior infarction in the territory of his culprit left anterior descending coronary artery.
Although acute cardiac conditions appear less likely, the brisk leukocytosis in a returned traveler prompts consideration of infection. His lung consolidations could represent either new or resolving pneumonia. The complete absence of cough and fever is unusual for pneumonia, yet clinical findings are not as sensitive as chest radiograph for this diagnosis. At this point, typical organisms as well as uncommon pathogens associated with diarrhea or his travel history should be included in the differential.
After 24 hours, the patient was discharged on warfarin to treat the apical thrombus and moxifloxacin for a presumed community‐acquired pneumonia. Eight days after discharge, the patient visited his primary care physician with improving, but not resolved, shortness of breath and pleuritic pain despite completing the 7‐day course of moxifloxacin. A chest radiograph showed a large posterior left basal pleural fluid collection, increased from previous.
In the setting of a recent infection, the symptoms and radiographic findings suggest a complicated parapneumonic effusion or empyema. Failure to drain a previously seeded fluid collection leaves bacterial pathogens susceptible to moxifloxacin on the differential, including Streptococcus pneumoniae, Staphylococcus aureus, Legionella species, and other enterobacteriaciae (eg, Klebsiella pneumoniae).
The indolent course should also prompt consideration of more unusual pathogens, including roundworms (such as Ascaris) or lung flukes (Paragonimus), either of which can cause a lung infection without traditional pneumonia symptoms. Tuberculosis tends to present months (or years) after exposure. Older adults may manifest primary pulmonary tuberculosis with lower lobe infiltrates, consistent with this presentation. However, moxifloxacin is quite active against tuberculosis, and although single drug therapy would not be expected to cure the patient, it would be surprising for him to progress this quickly on moxifloxacin.
In northern Thailand, Burkholderia pseudomallei is a common cause of bacteremic pneumonia. The organism often has high‐level resistance to fluoroquinolones, and may present in a more insidious fashion than other causes of community‐acquired pneumonia. Although infection with B pseudomallei (melioidosis) can occasionally mimic apical pulmonary tuberculosis and may present after a prolonged latent period, it most commonly manifests as an acute pneumonia.
The patient was prescribed 10 days of amoxicillin‐clavulanic acid and clindamycin, and decubitus films were ordered to assess the effusion. These films, obtained 5 days later, showed a persistent pleural effusion. Subsequent ultrasound demonstrated loculated fluid, but a thoracentesis was not performed at that time due to the patient's therapeutic international normalized ratio and dual antiplatelet therapy.
The loculation further suggests a complicated parapneumonic effusion or empyema. Clindamycin adds very little to amoxicillin‐clavulanate as far as coverage of oral anaerobes or common pneumonia pathogens and may add to the risk of antibiotic side effects. A susceptible organism might not clear because of failure to drain this collection; if undertreated bacterial infection is suspected, tube thoracentesis is the established standard of care. However, the protracted course of illness makes untreated pyogenic bacterial infections unlikely.
At this point, the top 2 diagnostic considerations are Paragonimus westermani and B pseudomallei. P westermani is initially ingested, usually from an undercooked freshwater crustacean. Infected patients may experience a brief diarrheal illness, as this patient reported. However, infected patients typically have a brisk peripheral eosinophilia.
Melioidosis is thus the leading concern. Amoxicillin‐clavulanate is active against many strains of B pseudomallei, so the failure of the patient to worsen could be seen as a partial treatment and supports this diagnosis. However, as prolonged therapy is necessary for complete eradication of B pseudomallei, a definitive, culture‐based diagnosis should be established before committing the patient to months of antibiotics.
After completing 10 days of clindamycin and amoxicillin‐clavulanate, the patient reported improvement of his pleuritic pain, and repeat physical exam suggested interval decrease in the size of the effusion. Two days later, the patient began experiencing dysuria that persisted despite 3 days of nitrofurantoin.
Melioidosis can also involve the genitourinary tract. Hematogenous spread of B pseudomallei can seed a number of visceral organs including the bladder, joints, and bones. Men with suspected urinary infection should be evaluated for the possibility of prostatitis, an infection with considerable morbidity that requires extended therapy. This gentleman should have a prostate exam, and blood and urine cultures should be collected if prostatitis is suspected. Empiric antibiotics are not recommended without culture in a patient with complicated urinary tract infection.
Prostate exam was unremarkable. A urine culture grew a gram‐negative rod identified as B pseudomallei. Because B pseudomallei can cause fulminant sepsis, the infectious disease consultant requested that he return for admission, further evaluation, and initiation of intravenous antibiotics. Computed tomography (CT) of the chest, abdomen, and pelvis revealed multiple pulmonary nodules, a persistent left pleural effusion, and a rim‐enhancing hypodensity in the prostate consistent with abscess (Figure 1). Blood and pleural fluid cultures were negative.

Initial treatment for a patient with severe or metastatic B pseudomallei infection requires high‐dose intravenous antibiotic therapy. Ceftazidime, imipenem, and meropenem are the best studied agents for this purpose. Surgical drainage should be considered for the abscess. Following the completion of intensive intravenous therapy, relapse rates are high unless a longer‐term, consolidation therapy is pursued. Trimethoprim‐sulfamethoxazole is the recommended agent.
The patient was treated with high‐dose ceftazidime for 2 weeks, followed by 6 months of high‐dose oral trimethoprim‐sulfamethoxazole. His symptoms resolved, and 7 months after presentation, he continued to feel well.
DISCUSSION
Melioidosis refers to any infection caused by B pseudomallei, a gram‐negative bacillus found in soil and water, most commonly in Southeast Asia and Australia.[1] It is an important cause of pneumonia in endemic regions; in Thailand, the incidence is as high as 12 cases per 100,000 people, and it is the third leading infectious cause of death, following human immunodeficiency virus and tuberculosis.[2] However, it occurs only as an imported infection in the United States and remains an unfamiliar infection for many US medical practitioners. Melioidosis should be considered in patients returning from endemic regions presenting with sepsis, pneumonia, urinary symptoms, or abscesses.
B pseudomallei can be transmitted to humans through exposure to contaminated soil or water via ingestion, inhalation, or percutaneous inoculation.[1] Outbreaks typically occur during the rainy season and after typhoons.[1, 3] Presumably, this patient's exposure to B pseudomallei occurred while hiking and wading in freshwater lakes and waterfalls. Although hospital‐acquired melioidosis has not been reported, and isolation precautions are not necessary, rare cases of disease acquired via laboratory exposure have been reported among US healthcare workers. Clinicians suspecting melioidosis should alert the receiving laboratory.[4]
The treatment course for melioidosis is lengthy and should involve consultation with an infectious disease specialist. B pseudomallei is known to be resistant to penicillin, first‐ and second‐generation cephalosporins, and moxifloxacin. The standard treatment includes 10 to 14 days of intravenous ceftazidime, meropenem, or imipenem, and then trimethoprim‐sulfamethoxazole for 3 to 6 months.[1] Treatment should be guided by culture susceptibility data when available. There are reports of B pseudomallei having different resistance patterns within the same host; clinicians should culture all drained fluid collections and tailor antibiotics to the most resistant strain recovered.[5, 6] Although melioidosis is a life‐threatening infection, previously healthy patients have an excellent prognosis assuming prompt diagnosis and treatment are provided.[3]
After excluding common causes of chest pain, the discussant identified the need to definitively establish a microbiologic diagnosis by obtaining pleural fluid. Although common clinical scenarios can often be treated with guideline‐supported empiric antibiotics, the use of serial courses of empiric antibiotics should be carefully questioned and is generally discouraged. Specific data to prove or disprove the presence of infection should be obtained before exposing a patient to the risks of multiple drugs or prolonged antibiotic therapy, as well as the risks of delayed (or missed) diagnosis. Unfortunately, a complete evaluation was delayed by clinical contraindications to diagnostic thoracentesis, and a definitive diagnosis was reached only after development of more widespread symptoms.
This patient's protean presentation is not surprising given his ultimate diagnosis. B pseudomallei has been termed the great mimicker, as disease presentation and organ involvement can vary from an indolent localized infection to acute severe sepsis.[7] Pneumonia and genitourinary infections are the most common manifestations, although skin infections, bacteremia, septic arthritis, and neurologic disease are also possible.[1, 3] In addition, melioidosis may develop after a lengthy incubation. In a case series, confirmed incubation periods ranged from 1 to 21 days (mean, 9 days); however, cases of chronic (>2 months) infection, mimicking tuberculosis, are estimated to occur in about 12% of cases.[4] B pseudomallei is also capable of causing reactivation disease, similar to tuberculosis. It was referred to as the Vietnamese time bomb when US Vietnam War veterans, exposed to the disease when helicopters aerosolized the bacteria in the soil, developed the disease only after their return to the United States.[8] Fortunately, only a tiny fraction of the quarter‐million soldiers with serologically confirmed exposure to the bacteria ultimately developed disease.
In The Adventure of the Dying Detective, Sherlock Holmes fakes a serious illness characterized by shortness of breath and weakness to trick an adversary into confessing to murder. The abrupt, crippling infection mimicked by Holmes is thought by some to be melioidosis.[9, 10] Conan Doyle's story was published in 1913, a year after melioidosis was first reported in the medical literature, and the exotic, protean infection may well have sparked Doyle's imagination. However, this patient's case of melioidosis proved stranger than fiction in its untimely concomitant development with an MI. Cracking our case required imagination and nimble thinking to avoid a number of cognitive pitfalls. The patient's recent MI anchored reasoning at his initial presentation, and the initial diagnosis of community‐acquired pneumonia raised the danger of premature closure. Reaching the correct diagnosis required an open mind, a detailed travel history, and firm microbiologic evidence. Hospitalists need not be expert in the health risks of travel to specific foreign destinations, but investigating those risks can hasten proper diagnosis and treatment.
TEACHING POINTS
- Melioidosis should be considered in patients returning from endemic regions who present with sepsis, pneumonia, urinary symptoms, or an abscess.
- For patients with a loculated parapneumonic effusion, tube thoracentesis for culture and drainage is the standard of care for diagnosis and treatment.
- Culture identification and antibiotic sensitivities are critical for management of B pseudomallei, because prolonged antibiotic treatment is needed.
Disclosure
Nothing to report.
- , , . Melioidosis. N Engl J Med. 2012;367(11):1035–1044.
- , , , et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82(6):1113–1117.
- , , . The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):e900.
- , , , et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14(7):e2.
- , , . Variations in ceftazidime and amoxicillin‐clavulanate susceptibilities within a clonal infection of Burkholderia pseudomallei. J Clin Microbiol. 2009;47(5):1556–1558.
- , , , et al. Within‐host evolution of Burkholderia pseudomallei in four cases of acute melioidosis. PLoS Pathog. 2010;6(1):e1000725.
- , , , , . Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4(4):272–282.
- , . Melioidosis. In: Dembeck ZF, ed. Medical Aspects of Biological Warfare. 2nd ed. Washington, DC: Office of the Surgeon General; 2007:146–166.
- . Sherlock Holmes and a biological weapon. J R Soc Med. 2002;95(2):101–103.
- . Sherlock Holmes and tropical medicine: a centennial appraisal. Am J Trop Med Hyg. 1994;50:99–101.
- , , . Melioidosis. N Engl J Med. 2012;367(11):1035–1044.
- , , , et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82(6):1113–1117.
- , , . The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):e900.
- , , , et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008;14(7):e2.
- , , . Variations in ceftazidime and amoxicillin‐clavulanate susceptibilities within a clonal infection of Burkholderia pseudomallei. J Clin Microbiol. 2009;47(5):1556–1558.
- , , , et al. Within‐host evolution of Burkholderia pseudomallei in four cases of acute melioidosis. PLoS Pathog. 2010;6(1):e1000725.
- , , , , . Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4(4):272–282.
- , . Melioidosis. In: Dembeck ZF, ed. Medical Aspects of Biological Warfare. 2nd ed. Washington, DC: Office of the Surgeon General; 2007:146–166.
- . Sherlock Holmes and a biological weapon. J R Soc Med. 2002;95(2):101–103.
- . Sherlock Holmes and tropical medicine: a centennial appraisal. Am J Trop Med Hyg. 1994;50:99–101.