User login
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
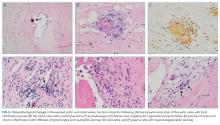
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
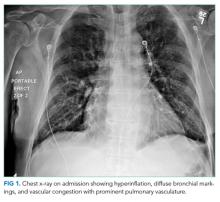
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
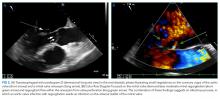
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.
Due to concern for IE, blood cultures were repeated, and IV vancomycin, IV ceftriaxone, and IV gentamicin were initiated. Azithromycin and prednisone were discontinued. His respiratory status continued to improve with IV furosemide, albuterol, ipratropium, and supportive care.
TTE inadequately visualizes the mitral valve, but is useful for tricuspid valve assessment because the right ventricle is closer to the chest wall. Transesophageal echocardiography (TEE) is indicated for a more detailed assessment of the left heart valves for vegetations and perivalvar abscesses. The new regurgitant murmurs satisfy a major criterion of the modified Duke criteria, and valvar vegetations suggests IE. He does not yet fulfill the other major modified Duke criterion for IE, nor does he satisfy enough minor criteria because there are no diagnostic vascular, microbiologic, or immunologic phenomena. However, no diagnostic rubric is perfect, and these results should not supersede clinical judgment. Despite the absence of positive cultures, the concern for bacterial IE remains high. The absence of embolic phenomena fits best with subacute rather than acute IE. Three negative blood cultures to date suggest a fastidious organism is responsible, although oral flora remain on the differential.
There is rarely a need to “hold” blood cultures for prolonged periods because modern instruments typically yield positive results within 7 days for most bacteria, including the HACEK group. Blood culture-negative endocarditis (BCNE) is considered when 3 sets of cultures are negative for at least 5 days. In this situation, one should consider other microorganisms based on the patient’s exposure history. Only certain species with complex growth requirements, such as Brucella and Bartonella, require prolonged holds. Revisiting his exposure history would be helpful in deciding whether serologic testing warranted. If he recalls exposure to parturient animals, then Coxiella is worth pursuing; if he has been bitten by lice, then B. quintana rises as a possibility; if the scratches on his limbs are from recent cat scratches, then B. henselae becomes more likely. Both C. burnetti and Bartonella endocarditis might be partially treated by his courses of azithromycin, confounding the picture.
If the infectious work-up is ultimately negative, one could then consider other etiologies of endocarditis, such as nonbacterial thrombotic endocarditis, which is seen in the context of malignancy and systemic lupus erythematosus (Libman-Sacks endocarditis). Other mimickers of IE include myxomatous valve degeneration, ruptured mitral chordae, and eosinophilic heart disease (Löffler’s endocarditis).
A transesophageal echocardiogram confirmed the presence of small echodensities on the aortic valve’s right and left coronary cusps, consistent with vegetations. The vegetation on the anterior leaflet of the mitral valve from the TTE also showed an aneurysm with a small perforation (Figure 2).
The combination of aortic regurgitation and the mitral valve aneurysm supports IE, because the aortic regurgitant jet directly strikes the anterior mitral valve leaflet, seeding the valve with infection from the aortic cusps. A positive serum PCR is diagnostic, but if it had been negative or unavailable, the serology would remain very helpful. In this context, the elevated IgG titer implicates B. henselae, the agent responsible for cat scratch disease (CSD). Out of context, these titers would not be diagnostic, because anti-Bartonella IgG may be increased due to a prior subclinical episode of CSD. Anti-Bartonella IgM is an unreliable indicator of recent infection because it may wane within weeks, and this IgG titer is higher than what is observed with most remote infections.
Revisiting previous cat exposure is warranted. He lost his cat to an illness 3 years prior, however it would be appropriate to inquire about other animals, such as a stray kitten with fleas, which his skin scratches suggest. Up to 50% of all cats in flea endemic regions harbor Bartonella and are asymptomatic. Rarely, dogs can serve as reservoirs of this organism, with a presumed transmission route via flea, louse, or tick. Regardless of the route of infection, treatment should be focused on B. henselae IE.
Azithromycin can treat CSD, and its use for his presumed COPD exacerbation may have temporized his infection. However, azithromycin monotherapy is not recommended for B. henselae IE. Treatment is usually with 2 antibiotics, including an aminoglycoside (gentamicin) for the first 2 weeks, combined with either a tetracycline, a macrolide, or a beta-lactam for a minimum of 4-6 weeks. Oral rifampin can be considered if gentamicin is not tolerated. After completing IV treatment, an additional 6 months of oral doxycycline or azithromycin should be considered, especially for those who have not undergone valve surgery.
The mitral valve aneurysm, abscesses, and heart failure warranted valve replacement. Surgery should be considered for all patients with Bartonella IE, primarily because delayed diagnosis often leads to irreversible valve damage. Ideally, surgically explanted tissue should be divided into 2 portions: half should be sent to pathology and stained with H&E, Warthin-Starry, and Steiner staining procedures, while the other half should be sent for culture, and then PCR if stains are negative.
His symptoms are compatible with subacute IE, which is typically more difficult to diagnose than acute IE due to its insidious onset. He meets criteria for blood culture negative IE based on 3 sets of negative blood cultures for greater than 5 days and major criteria for IE. The pathologic changes are consistent with B. henselae infection.
DISCUSSION
The incidence of IE in the United States is 40,000 cases per year1 with an in-hospital mortality of 15%-20% and a 1-year mortality of up to 40%.2,3 Five to 20% of patients with IE never develop positive blood cultures4 due to receipt of antibiotics prior to culture, inadequate microbiologic testing, or infection caused by noncultivable bacteria (eg, Tropheryma whipplei), fastidious extracellular bacteria (eg, HACEK group and nutritionally variant streptococci), or by intracellular pathogens with complex nutrient requirements (eg, Bartonella, Chlamydia, Brucella, or Coxiella). Previous administration of antibiotics reduces the likelihood of isolating an organism by 35%-40%.5 Patients meeting criteria for BCNE should prompt consideration of serologic testing. The most prevalent pathogens vary globally, and incidence data in the US is scarce. Worldwide, the majority of BCNE cases are caused by Coxiella, Bartonella, and Brucella species.6,7
When clinical suspicion for IE remains high despite negative cultures, detailed history can uncover clues and guide additional testing. For example, contact with contaminated milk products or farm animals are associated with Brucella, Coxiella, and Erysipelothrix species IE.7,8 Bartonella species are zoonotic gram-negative bacilli with a tropism for endothelial cells and are transmitted by arthropod vectors (ie, fleas, lice, ticks, and sandflies), cat scratches, or cat bites. Bartonella may account for 3%-4% of all cases of IE, most of which are due to B. henselae and B. quintana.7, 9 Underlying heart valve disease, alcoholism, cirrhosis, and homelessness are associated with B. henselae endocarditis.10
Diagnostic criteria are lacking for B. henselae IE, and the modified Duke criteria is of limited utility for diagnosing Bartonella IE because blood cultures are often negative and echocardiographic evidence of vegetation is not always apparent. Serology plays a critical role in the diagnosis of Bartonella infections. The addition of positive serology, Western blot or PCR for B. henselae and B. quintana as a major criterion in the modified Duke criteria for IE has been proposed but has not yet been formally accepted.9 For B. henselae IE, an IgG titer of ≥1:800 has been recommended as a cutoff for subacute IE because it combines a high specificity and positive predictive value along with reasonable sensitivity and negative predictive value in this situation.9 The humoral immune response rises over time, and thus acute IE due to Bartonella may not generate a substantial IgG titer. Interestingly, because of the indolent nature of this pathogen, most cases of IE present once IgG titers have begun to rise. Serum PCR testing has shown a sensitivity and specificity of 58% and 100%, respectively.11 Isolation by blood culture requires specific growth media and prolonged incubation, with a sensitivity as low as 20% and 30% for blood and tissue, respectively.10 The microbiology laboratory should be notified of suspected Bartonella to intensify efforts to cultivate this organism. If infection with Coxiella or Brucella is suspected, the lab should also be informed, both to increase diagnostic yield and to trigger enhanced biosafety precautions when handling the specimens. Despite attempts to optimize the yield, up to 75% of Bartonella IE may remain culture negative,12,13 making it difficult to meet the current major modified Duke criterion of positive blood cultures. H&E staining of valve tissue infected with Bartonella commonly reveals increased inflammation, fibrosis, and calcified granulomas relative to endocarditis from other causes.14 The Warthin-Starry silver stain can identify small, darkly staining bacteria in more than 75% of Bartonella endocarditis; however, this stain is not specific for Bartonella species.9
This case highlights the challenge of diagnosing subacute IE because this patient received antibiotics and steroids prior to presentation, clouding the clinical picture. Although he did not exhibit textbook signs of endocarditis, his symptoms (new onset heart failure and new regurgitant murmurs) prioritized the diagnosis. The combination of elevated serum titers, positive PCR, valve granulomas and abscesses on TEE, and pathology findings led the discussant to the correct diagnosis. Scratching beneath the surface revealed his penchant for cats, but this was only considered a key epidemiological feature later in his clinical course.
TEACHING POINTS
- Subacute IE typically presents with indolent constitutional symptoms over a course of weeks to months, whereas acute IE causes a rapid onset of fevers, rigors, and is more likely to exhibit embolic phenomena.
- Epidemiologic features specific to Bartonella species include alcoholism, cirrhosis, dog or cat exposure, homelessness, and body lice, and should be considered in suspected cases of BCNE.
- If suspicion for endocarditis remains high and animal exposure is elicited, then serologic and PCR testing for fastidious organisms should be strongly considered. The most common causes of BCNE include Coxiella, Bartonella, and Brucella species.
- The modified Duke criteria do not incorporate Bartonella within the diagnostic schema. Presentation is usually late and often requires valve replacement.
Acknowledgments
The authors thank Dr. Michael Pfeiffer from the Pennsylvania State Hershey Heart and Vascular Institute for providing his expertise in diagnostic echocardiography.
Disclosure
There are no conflicts of interest or financial disclosures to report.
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893. PubMed
2. Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13(3):428-438. PubMed
3. Heller R, Artois M, Xemar V, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35(6):1327-1331. PubMed
4. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelsein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. PubMed
5. Bashore TM, Cabell C, Fowler, V Jr., Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274-352. PubMed
6. Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. PubMed
7. Baddour LM, Wilson WR, Bayer AS, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015; 132(15):1435-1486. PubMed
8. Tunkel AR, Kaye D. Endocarditis with negative blood cultures. N Engl J Med. 1992;326(18):1215-1217. PubMed
9. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev. 2017;30(3):709-746. PubMed
10. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84(3):162-173. PubMed
11. Sanogo YO, Zeaiter Z, Caruso G, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg Infect Dis. 2003;9(3):329-332. PubMed
12. Raoult D, Fournier PE, DrancourtM, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646-652. PubMed
13. La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998). J Clin Microbiol. 1999;37(6):1899-1905. PubMed
14. Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114(6):880-889. PubMed
© 2018 Society of Hospital Medicine