User login
Long-Term Surgical Management of Severe Pelvic Injury and Resulting Neurogenic Bladder From an Improvised Explosive Device
More than 52,000 soldiers have been injured and 6,800 have been killed during the wars in Iraq and Afghanistan.1 Blast injuries from improvised explosive devices (IEDs) account for 70% to 79% of combat-related injuries and deaths in these wars.2 Advances in personal body armor, rapid and advanced surgical treatment, and the changing nature of combat in Iraq and Afghanistan have changed injury patterns and survival compared with prior military conflicts such as those in Vietnam and Korea.3
The most common combat-related injuries in the recent wars are extremity, facial, brain, and gastrointestinal injuries. Pelvic and genitourinary injuries are also common, accounting for about 8% of total injuries.2 Pelvic and genitourinary injury can cause long-term disability from nerve injury (neurogenic bladder, neurogenic bowel, sexual dysfunction, urethral injury), as well as general loss of genital structures from blast injuries.
The usual care for bladder dysfunction from pelvic or genitourinary injury ranges from the use of chronic indwelling catheters to reconstructive surgery. However, there is no standard of care for long-term treatment of patients with pelvic or genitourinary injury who experience bladder dysfunction. Reconstructive surgery has the potential to improve quality of life (QOL) and eliminate chronic indwelling catheters, which are prone to cause infection and long-term kidney problems in patients with bladder dysfunction from traumatic injury.
This case report evaluates the efficacy of reconstructive surgery for bladder dysfunction to improve independence and QOL and decrease complications associated with chronic indwelling urinary catheters. The authors hope to raise awareness regarding this option for patients with pelvic, spinal cord, or genitourinary injury who are young and face long-term disability from their injuries.
Case Presentation
A 22-year-old man presented to the George E. Wahlen VAMC Urology Clinic in Salt Lake City, Utah with a complicated history related to combat injuries. During combat operations 3 years earlier, he was injured by an IED blast while on foot patrol. His injuries included bilateral severe extremity injury, perineal and genital blast wounds, a bladder injury, pelvic fracture, colorectal injury, and extensive soft tissue loss. He underwent multiple abdominal explorations, left leg amputation below the knee, multiple skin grafts, soft tissue debridements, left-side orchiectomy, bladder repair, and diverting colostomy. He survived the injuries and was eventually discharged from active military service and returned home.
Upon presentation to the VAMC, the patient had a diverting colostomy, suprapubic bladder catheter, and bladder and bowel function consistent with cauda equina syndrome (pelvic nerve injury). Given the lack of rectal tone, fecal incontinence was likely with colostomy reversal. His bladder had low volume and poor compliance (elasticity). In addition, the patient had no volitional control of urination or defecation.
The patient previously performed intermittent self-catheterization but experienced total urinary incontinence (UI) between catheterizations, due to his bladder dynamics and a lack of urinary sphincter tone. A suprapubic bladder catheter was previously placed to control UI. However, the patient remained incontinent, and urinary leakage, need for diapers, and urinary tract infections (UTIs) negatively impacted QOL. The patient ambulated well and was physically active. His priority was to reduce incontinence and improve QOL.
Catheterizable Ileal Cecocystoplasty
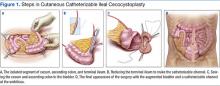
The patient underwent cutaneous catheterizable ileal cecocystoplasty (CCIC) (Figure 1). In this surgery, a segment of the cecum and ascending colon with attached terminal ileum is used to increase the size of the bladder (augmentation cystoplasty) and create a channel for catheterization from the umbilicus. The cecum and colon are detubularized, and a large rectangular plate of large bowel is formed, which is then sewn to the bladder, expanding its volume. About 10 to 15 cm of the terminal ileum is tapered to the diameter of a pencil and brought through the base of the umbilicus, creating a small stoma for intermittent bladder catheterization. The ileocecal valve is tightened and serves as a continence mechanism to prevent urinary leakage through the small stoma in the umbilicus.4
A perineal urethral mesh sling was placed at the time of the patient’s surgery to bolster the deinnervated urinary sphincter and prevent urethral leakage. The goal of reconstructive surgery for this patient was to create a small bowel channel connecting the umbilicus and bladder that could be catheterized every 4 to 6 hours, increase bladder capacity, and increase sphincteric resistance to reduce urethral leakage through the penis. Because there can be damage from passing a catheter through mesh slings and the urethra over time, including stenosis or erosion of the sling, an alternative catheterizable channel was needed in this patient.
The patient recovered after the surgery and was able to self-catheterize without difficulty. However, the urethral mesh sling did not place enough pressure on the urethra to prevent leakage, and he had persistent incontinence from the penis. Three months after the original surgery the patient had exploration of the perineum, which revealed that the mesh sling was loose and exerting inadequate pressure on the urethra. It was likely the sling slipped postoperatively—a known complication of urethral slings. An artificial urinary sphincter (AUS) was placed around the urethra during the second surgery to address the patient’s UI.
More than 1 year after the original surgery, the patient self-catheterizes about 4 to 5 times daily via the catheterizable channel using a single-use catheter. His bladder holds at least 500 mL. The patient does not have significant leakage from the channel or the penis. He is no longer dependent on a chronic indwelling catheter and is free of the problems associated with severe UI, including foul odor, UTIs, and social isolation.
Discussion
Patients with spinal cord or pelvic nerve injury often develop spastic bladders with low capacities. This is similar to muscle spasticity that may occur with a neurologic injury, below the level of the injury, such as in the lower extremities. The powerful uncontrolled bladder spasms and small bladder capacity most often lead to incontinence. Additionally, neurologic control of the urinary sphincter is affected, leading to either uncontrolled spasms or poor tone. Patients with these injuries have no volitional control of bladder functions and are forced to catheterize intermittently, use a condom-type catheter, or have a chronic indwelling catheter (a Foley catheter or suprapubic catheter).
Intermittent catheterization is the preferred management option for neurogenic bladder. When compared with chronic indwelling catheters, intermittent catheterization is associated with lower rates of UTI and upper tract abnormalities and with the loss of renal function.5 Unfortunately, patients do not often stay on intermittent catheterization. A recent study showed that up to 70% of patients with spinal cord injuries who used clean intermittent catheterization when discharged from acute rehabilitation discontinue use and are subsequently managed by chronic indwelling catheters.6 Although the reasons why intermittent catheterization is discontinued are unclear, patient dissatisfaction with catheterization, anatomic problems, such as urethral scarring, or continued leakage despite medical treatments, such as anticholinergic medicines, may be factors.
Uncontrolled leakage and UI significantly impacts QOL and may cause patients to choose chronic indwelling catheters over intermittent catheterization. Several treatments are available to control incontinence associated with intermittent catheterization. Anticholinergic medications and more recently onabotulinum toxin A may help improve bladder spasticity. In 2011, the FDA approved onabotulinum toxin A for transurethral bladder injections. It has been shown to increase functional bladder capacity and decrease spasticity.7,8 Onabotulinum toxin A treatment will not enlarge a small, contracted bladder.
Onabotulinum toxin A treatment would not be ideal for the patient in this case study. His absolute bladder capacity was 200 mL, and onabotulinum toxin A treatment would not significantly improve capacity or make intermittent catheterization practical. Additionally, the patient had poor urinary sphincter function, and he would continue to leak regardless of improvements in the bladder spasticity or tone.
Augmentation enterocystoplasty is surgical enlargement of the bladder, using a piece of the bowel and is indicated in patients with low bladder volumes. With this procedure the native bladder becomes defunctionalized, and patients experience a dramatic improvement in bladder volumes and a reduction in bladder spasms and leakage. The use of the colon and terminal ileum for bladder augmentation, or CCIC, was first reported by Sarosdy in 2 patients in 1992.9 In 1996, King and colleagues demonstrated successful outcomes with CCIC in a cohort of 8 patients after 34 months of follow-up.10 Seven patients successfully used clean intermittent catheterization, and 1 patient chose an indwelling catheter because of progressive upper extremity weakness. No patients experienced worsened renal function or pyelonephritis suggestive of upper urinary tract deterioration. A single patient had mild stomal stenosis, which was successfully revised under local anesthesia.
In another study, Sutton and colleagues reported at 27 months an improvement of 276 mL in bladder capacity, no metabolic complications, and a 95% continence rate in a cohort of 23 patients with neurogenic bladder who underwent CCIC.4 Sutton and colleagues later reported outcomes for 34 patients with a median of 31 months follow-up.11 The most common complications were recurrent UTIs (12%) and stomal stenosis (12%). Only 3 patients (9%) required surgical revisions for stomal stenosis.
Altered bowel function and metabolic abnormalities are a concern after bowel resection and reconstruction. However, a study has found no subjective change in bowel function following ileal resection of up to 60 cm for urinary diversion for bladder malignancy.12 Rates of hyperchloremic hypokalemic metabolic acidosis are low, and most changes in electrolytes are subclinical.13,14 Long-term vitamin B12 deficiency is seen with larger (> 50 cm) ileal resections but is rare with CCIC, given the small segment used for reconstruction.15 Overall, CCIC is shown to have excellent surgical outcomes in carefully selected patients with neurogenic bladder.
In addition to low bladder capacity, the case study patient also had intrinsic sphincteric deficiency (very low urinary sphincter tone), which is common with pelvic nerve injury but unusual with spinal cord injury. He initially received a suburethral mesh sling that supported and compressed the urethra and buttressed the natural urinary sphincter. However, patients can develop catheterization issues with a suburethral sling due to mechanical compression of the urethra and traversing the compressed area with a urinary catheter. Given the indication for augmentation cystoplasty in this patient, he additionally elected to undergo catheterization channel creation to avoid long-term issues of urethral catheterization through the urethra compressed by the sling.
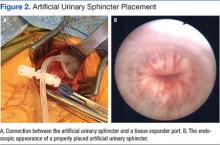
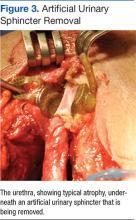
Unfortunately, this patient had postoperative issues with his suburethral sling, and a modified AUS was inserted rather than a second sling. Normally, an AUS is attached to a pump mechanism in the scrotum. The pump allows the patient to cycle fluid from the sphincter cuff to a reservoir in the abdomen, removing compression on the urethra and allowing normal urination. Because this patient could not effectively urinate from the penis, the authors wanted to obstruct the urethra to prevent leakage without closing it permanently. The AUS was connected to a tissue expander port placed subcutaneously in the lower abdomen rather than to a pump mechanism. This modified approach used fewer mechanical parts compared with the pump mechanism, possibly reducing rates of mechanical failure. Additionally, a lower cuff pressure could be used to obstruct the urethra and prevent leakage, reducing the likelihood of urethral atrophy. Fewer mechanical parts and a lower cuff pressure could theoretically improve longevity of the AUS (Figure 3). This modified method of AUS placement has been described in patients with sphincteric deficiency and spinal cord injury.16
These 2 reconstructive surgeries freed the patient from indwelling catheter dependence and significantly improved his incontinence and QOL. Many patients with spinal cord injury or pelvic injury could benefit from similar reconstructive surgeries if conservative measures such as anticholinergic medications or onabotulinum toxin A treatments do not control incontinence.
Conclusion
Blast injuries in soldiers often cause pelvic and genitourinary injuries. These injuries can lead to chronic urinary problems and profound social and physical disability. These young veterans need innovative, individualized approaches to best manage their long-term urinary issues. Reconstructive surgery may improve QOL and decrease disability from bladder dysfunction for carefully selected patients. Clinicians caring for veterans with pelvic and genitourinary injury should strive to create a system where these options are available when they are appropriate.
1. U.S. Department of Defense. U.S. Casualty Status. U.S. Department of Defense Website. http://www.defense.gov/casualty.pdf. Updated November 3, 2015. Accessed November 4, 2015.
2. Schoenfeld AJ, Dunn JC, Bader JO, Belmont PJ Jr. The nature and extent of war injuries sustained by combat specialty personnel killed and wounded in Afghanistan and Iraq, 2003-2011. J Trauma Acute Care Surg. 2013;75(2):287-291.
3. Pannell D, Brisebois R, Talbot M, et al. Causes of death in Canadian Forces members deployed to Afghanistan and implications on tactical combat casualty care provision. J Trauma. 2011;71(5)(suppl 1):S401-S407.
4. Sutton MA, Hinson JL, Nickell KG, Boone TB. Continent ileocecal augmentation cystoplasty. Spinal Cord. 1998;36(4):246-251.
5. Weld KJ, Wall BM, Mangold TA, Steere EL, Dmochowski RR. Influences on renal function in chronic spinal cord injured patients. J Urol. 2000;164(5):1490-1493.
6. Cameron AP, Wallner LP, Tate DG, Sarma AV, Rodriguez GM, Clemens JQ. Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol. 2010;184(1):213-217.
7. Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60(4):742-750.
8. Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187(6):2131-2139.
9. Sarosdy MF. Continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1992;40(2):102-106.
10. King DH, Hlavinka TC, Sarosdy MF. Additional experience with continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1996;47(4):471-475.
11. Khavari R, Fletcher SG, Liu J, Boone TB. A modification to augmentation cystoplasty with catheterizable stoma for neurogenic patients: technique and long-term results. Urology. 2012;80(2):460-464.
12. Fung B, Kessler TM, Haeni K, Burkhard FC, Studer UE. Bowel function remains subjectively unchanged after ileal resection for construction of continent ileal reservoirs. Eur Urol. 2011;60(3):585-590.
13. Adams RC, Vachha B, Samuelson ML, Keefover-Hicks A, Snodgrass WT. Incidence of new onset metabolic acidosis following enteroplasty for myelomeningocele. J Urol. 2010;183(1):302-305.
14. Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep. 2007;8(2):157-162.
15. Pannek J, Haupt G, Schulze H, Senge T. Influence of continent ileal urinary diversion on vitamin B12 absorption. J Urol. 1996;155(4):1206-1208.
16. Bersch U, Göcking K, Pannek J. The artificial urinary sphincter in patients with spinal cord lesion: description of a modified technique and clinical results. Eur Urol. 2009;55(3):687-693.
More than 52,000 soldiers have been injured and 6,800 have been killed during the wars in Iraq and Afghanistan.1 Blast injuries from improvised explosive devices (IEDs) account for 70% to 79% of combat-related injuries and deaths in these wars.2 Advances in personal body armor, rapid and advanced surgical treatment, and the changing nature of combat in Iraq and Afghanistan have changed injury patterns and survival compared with prior military conflicts such as those in Vietnam and Korea.3
The most common combat-related injuries in the recent wars are extremity, facial, brain, and gastrointestinal injuries. Pelvic and genitourinary injuries are also common, accounting for about 8% of total injuries.2 Pelvic and genitourinary injury can cause long-term disability from nerve injury (neurogenic bladder, neurogenic bowel, sexual dysfunction, urethral injury), as well as general loss of genital structures from blast injuries.
The usual care for bladder dysfunction from pelvic or genitourinary injury ranges from the use of chronic indwelling catheters to reconstructive surgery. However, there is no standard of care for long-term treatment of patients with pelvic or genitourinary injury who experience bladder dysfunction. Reconstructive surgery has the potential to improve quality of life (QOL) and eliminate chronic indwelling catheters, which are prone to cause infection and long-term kidney problems in patients with bladder dysfunction from traumatic injury.
This case report evaluates the efficacy of reconstructive surgery for bladder dysfunction to improve independence and QOL and decrease complications associated with chronic indwelling urinary catheters. The authors hope to raise awareness regarding this option for patients with pelvic, spinal cord, or genitourinary injury who are young and face long-term disability from their injuries.
Case Presentation
A 22-year-old man presented to the George E. Wahlen VAMC Urology Clinic in Salt Lake City, Utah with a complicated history related to combat injuries. During combat operations 3 years earlier, he was injured by an IED blast while on foot patrol. His injuries included bilateral severe extremity injury, perineal and genital blast wounds, a bladder injury, pelvic fracture, colorectal injury, and extensive soft tissue loss. He underwent multiple abdominal explorations, left leg amputation below the knee, multiple skin grafts, soft tissue debridements, left-side orchiectomy, bladder repair, and diverting colostomy. He survived the injuries and was eventually discharged from active military service and returned home.
Upon presentation to the VAMC, the patient had a diverting colostomy, suprapubic bladder catheter, and bladder and bowel function consistent with cauda equina syndrome (pelvic nerve injury). Given the lack of rectal tone, fecal incontinence was likely with colostomy reversal. His bladder had low volume and poor compliance (elasticity). In addition, the patient had no volitional control of urination or defecation.
The patient previously performed intermittent self-catheterization but experienced total urinary incontinence (UI) between catheterizations, due to his bladder dynamics and a lack of urinary sphincter tone. A suprapubic bladder catheter was previously placed to control UI. However, the patient remained incontinent, and urinary leakage, need for diapers, and urinary tract infections (UTIs) negatively impacted QOL. The patient ambulated well and was physically active. His priority was to reduce incontinence and improve QOL.
Catheterizable Ileal Cecocystoplasty
The patient underwent cutaneous catheterizable ileal cecocystoplasty (CCIC) (Figure 1). In this surgery, a segment of the cecum and ascending colon with attached terminal ileum is used to increase the size of the bladder (augmentation cystoplasty) and create a channel for catheterization from the umbilicus. The cecum and colon are detubularized, and a large rectangular plate of large bowel is formed, which is then sewn to the bladder, expanding its volume. About 10 to 15 cm of the terminal ileum is tapered to the diameter of a pencil and brought through the base of the umbilicus, creating a small stoma for intermittent bladder catheterization. The ileocecal valve is tightened and serves as a continence mechanism to prevent urinary leakage through the small stoma in the umbilicus.4
A perineal urethral mesh sling was placed at the time of the patient’s surgery to bolster the deinnervated urinary sphincter and prevent urethral leakage. The goal of reconstructive surgery for this patient was to create a small bowel channel connecting the umbilicus and bladder that could be catheterized every 4 to 6 hours, increase bladder capacity, and increase sphincteric resistance to reduce urethral leakage through the penis. Because there can be damage from passing a catheter through mesh slings and the urethra over time, including stenosis or erosion of the sling, an alternative catheterizable channel was needed in this patient.
The patient recovered after the surgery and was able to self-catheterize without difficulty. However, the urethral mesh sling did not place enough pressure on the urethra to prevent leakage, and he had persistent incontinence from the penis. Three months after the original surgery the patient had exploration of the perineum, which revealed that the mesh sling was loose and exerting inadequate pressure on the urethra. It was likely the sling slipped postoperatively—a known complication of urethral slings. An artificial urinary sphincter (AUS) was placed around the urethra during the second surgery to address the patient’s UI.
More than 1 year after the original surgery, the patient self-catheterizes about 4 to 5 times daily via the catheterizable channel using a single-use catheter. His bladder holds at least 500 mL. The patient does not have significant leakage from the channel or the penis. He is no longer dependent on a chronic indwelling catheter and is free of the problems associated with severe UI, including foul odor, UTIs, and social isolation.
Discussion
Patients with spinal cord or pelvic nerve injury often develop spastic bladders with low capacities. This is similar to muscle spasticity that may occur with a neurologic injury, below the level of the injury, such as in the lower extremities. The powerful uncontrolled bladder spasms and small bladder capacity most often lead to incontinence. Additionally, neurologic control of the urinary sphincter is affected, leading to either uncontrolled spasms or poor tone. Patients with these injuries have no volitional control of bladder functions and are forced to catheterize intermittently, use a condom-type catheter, or have a chronic indwelling catheter (a Foley catheter or suprapubic catheter).
Intermittent catheterization is the preferred management option for neurogenic bladder. When compared with chronic indwelling catheters, intermittent catheterization is associated with lower rates of UTI and upper tract abnormalities and with the loss of renal function.5 Unfortunately, patients do not often stay on intermittent catheterization. A recent study showed that up to 70% of patients with spinal cord injuries who used clean intermittent catheterization when discharged from acute rehabilitation discontinue use and are subsequently managed by chronic indwelling catheters.6 Although the reasons why intermittent catheterization is discontinued are unclear, patient dissatisfaction with catheterization, anatomic problems, such as urethral scarring, or continued leakage despite medical treatments, such as anticholinergic medicines, may be factors.
Uncontrolled leakage and UI significantly impacts QOL and may cause patients to choose chronic indwelling catheters over intermittent catheterization. Several treatments are available to control incontinence associated with intermittent catheterization. Anticholinergic medications and more recently onabotulinum toxin A may help improve bladder spasticity. In 2011, the FDA approved onabotulinum toxin A for transurethral bladder injections. It has been shown to increase functional bladder capacity and decrease spasticity.7,8 Onabotulinum toxin A treatment will not enlarge a small, contracted bladder.
Onabotulinum toxin A treatment would not be ideal for the patient in this case study. His absolute bladder capacity was 200 mL, and onabotulinum toxin A treatment would not significantly improve capacity or make intermittent catheterization practical. Additionally, the patient had poor urinary sphincter function, and he would continue to leak regardless of improvements in the bladder spasticity or tone.
Augmentation enterocystoplasty is surgical enlargement of the bladder, using a piece of the bowel and is indicated in patients with low bladder volumes. With this procedure the native bladder becomes defunctionalized, and patients experience a dramatic improvement in bladder volumes and a reduction in bladder spasms and leakage. The use of the colon and terminal ileum for bladder augmentation, or CCIC, was first reported by Sarosdy in 2 patients in 1992.9 In 1996, King and colleagues demonstrated successful outcomes with CCIC in a cohort of 8 patients after 34 months of follow-up.10 Seven patients successfully used clean intermittent catheterization, and 1 patient chose an indwelling catheter because of progressive upper extremity weakness. No patients experienced worsened renal function or pyelonephritis suggestive of upper urinary tract deterioration. A single patient had mild stomal stenosis, which was successfully revised under local anesthesia.
In another study, Sutton and colleagues reported at 27 months an improvement of 276 mL in bladder capacity, no metabolic complications, and a 95% continence rate in a cohort of 23 patients with neurogenic bladder who underwent CCIC.4 Sutton and colleagues later reported outcomes for 34 patients with a median of 31 months follow-up.11 The most common complications were recurrent UTIs (12%) and stomal stenosis (12%). Only 3 patients (9%) required surgical revisions for stomal stenosis.
Altered bowel function and metabolic abnormalities are a concern after bowel resection and reconstruction. However, a study has found no subjective change in bowel function following ileal resection of up to 60 cm for urinary diversion for bladder malignancy.12 Rates of hyperchloremic hypokalemic metabolic acidosis are low, and most changes in electrolytes are subclinical.13,14 Long-term vitamin B12 deficiency is seen with larger (> 50 cm) ileal resections but is rare with CCIC, given the small segment used for reconstruction.15 Overall, CCIC is shown to have excellent surgical outcomes in carefully selected patients with neurogenic bladder.
In addition to low bladder capacity, the case study patient also had intrinsic sphincteric deficiency (very low urinary sphincter tone), which is common with pelvic nerve injury but unusual with spinal cord injury. He initially received a suburethral mesh sling that supported and compressed the urethra and buttressed the natural urinary sphincter. However, patients can develop catheterization issues with a suburethral sling due to mechanical compression of the urethra and traversing the compressed area with a urinary catheter. Given the indication for augmentation cystoplasty in this patient, he additionally elected to undergo catheterization channel creation to avoid long-term issues of urethral catheterization through the urethra compressed by the sling.
Unfortunately, this patient had postoperative issues with his suburethral sling, and a modified AUS was inserted rather than a second sling. Normally, an AUS is attached to a pump mechanism in the scrotum. The pump allows the patient to cycle fluid from the sphincter cuff to a reservoir in the abdomen, removing compression on the urethra and allowing normal urination. Because this patient could not effectively urinate from the penis, the authors wanted to obstruct the urethra to prevent leakage without closing it permanently. The AUS was connected to a tissue expander port placed subcutaneously in the lower abdomen rather than to a pump mechanism. This modified approach used fewer mechanical parts compared with the pump mechanism, possibly reducing rates of mechanical failure. Additionally, a lower cuff pressure could be used to obstruct the urethra and prevent leakage, reducing the likelihood of urethral atrophy. Fewer mechanical parts and a lower cuff pressure could theoretically improve longevity of the AUS (Figure 3). This modified method of AUS placement has been described in patients with sphincteric deficiency and spinal cord injury.16
These 2 reconstructive surgeries freed the patient from indwelling catheter dependence and significantly improved his incontinence and QOL. Many patients with spinal cord injury or pelvic injury could benefit from similar reconstructive surgeries if conservative measures such as anticholinergic medications or onabotulinum toxin A treatments do not control incontinence.
Conclusion
Blast injuries in soldiers often cause pelvic and genitourinary injuries. These injuries can lead to chronic urinary problems and profound social and physical disability. These young veterans need innovative, individualized approaches to best manage their long-term urinary issues. Reconstructive surgery may improve QOL and decrease disability from bladder dysfunction for carefully selected patients. Clinicians caring for veterans with pelvic and genitourinary injury should strive to create a system where these options are available when they are appropriate.
More than 52,000 soldiers have been injured and 6,800 have been killed during the wars in Iraq and Afghanistan.1 Blast injuries from improvised explosive devices (IEDs) account for 70% to 79% of combat-related injuries and deaths in these wars.2 Advances in personal body armor, rapid and advanced surgical treatment, and the changing nature of combat in Iraq and Afghanistan have changed injury patterns and survival compared with prior military conflicts such as those in Vietnam and Korea.3
The most common combat-related injuries in the recent wars are extremity, facial, brain, and gastrointestinal injuries. Pelvic and genitourinary injuries are also common, accounting for about 8% of total injuries.2 Pelvic and genitourinary injury can cause long-term disability from nerve injury (neurogenic bladder, neurogenic bowel, sexual dysfunction, urethral injury), as well as general loss of genital structures from blast injuries.
The usual care for bladder dysfunction from pelvic or genitourinary injury ranges from the use of chronic indwelling catheters to reconstructive surgery. However, there is no standard of care for long-term treatment of patients with pelvic or genitourinary injury who experience bladder dysfunction. Reconstructive surgery has the potential to improve quality of life (QOL) and eliminate chronic indwelling catheters, which are prone to cause infection and long-term kidney problems in patients with bladder dysfunction from traumatic injury.
This case report evaluates the efficacy of reconstructive surgery for bladder dysfunction to improve independence and QOL and decrease complications associated with chronic indwelling urinary catheters. The authors hope to raise awareness regarding this option for patients with pelvic, spinal cord, or genitourinary injury who are young and face long-term disability from their injuries.
Case Presentation
A 22-year-old man presented to the George E. Wahlen VAMC Urology Clinic in Salt Lake City, Utah with a complicated history related to combat injuries. During combat operations 3 years earlier, he was injured by an IED blast while on foot patrol. His injuries included bilateral severe extremity injury, perineal and genital blast wounds, a bladder injury, pelvic fracture, colorectal injury, and extensive soft tissue loss. He underwent multiple abdominal explorations, left leg amputation below the knee, multiple skin grafts, soft tissue debridements, left-side orchiectomy, bladder repair, and diverting colostomy. He survived the injuries and was eventually discharged from active military service and returned home.
Upon presentation to the VAMC, the patient had a diverting colostomy, suprapubic bladder catheter, and bladder and bowel function consistent with cauda equina syndrome (pelvic nerve injury). Given the lack of rectal tone, fecal incontinence was likely with colostomy reversal. His bladder had low volume and poor compliance (elasticity). In addition, the patient had no volitional control of urination or defecation.
The patient previously performed intermittent self-catheterization but experienced total urinary incontinence (UI) between catheterizations, due to his bladder dynamics and a lack of urinary sphincter tone. A suprapubic bladder catheter was previously placed to control UI. However, the patient remained incontinent, and urinary leakage, need for diapers, and urinary tract infections (UTIs) negatively impacted QOL. The patient ambulated well and was physically active. His priority was to reduce incontinence and improve QOL.
Catheterizable Ileal Cecocystoplasty
The patient underwent cutaneous catheterizable ileal cecocystoplasty (CCIC) (Figure 1). In this surgery, a segment of the cecum and ascending colon with attached terminal ileum is used to increase the size of the bladder (augmentation cystoplasty) and create a channel for catheterization from the umbilicus. The cecum and colon are detubularized, and a large rectangular plate of large bowel is formed, which is then sewn to the bladder, expanding its volume. About 10 to 15 cm of the terminal ileum is tapered to the diameter of a pencil and brought through the base of the umbilicus, creating a small stoma for intermittent bladder catheterization. The ileocecal valve is tightened and serves as a continence mechanism to prevent urinary leakage through the small stoma in the umbilicus.4
A perineal urethral mesh sling was placed at the time of the patient’s surgery to bolster the deinnervated urinary sphincter and prevent urethral leakage. The goal of reconstructive surgery for this patient was to create a small bowel channel connecting the umbilicus and bladder that could be catheterized every 4 to 6 hours, increase bladder capacity, and increase sphincteric resistance to reduce urethral leakage through the penis. Because there can be damage from passing a catheter through mesh slings and the urethra over time, including stenosis or erosion of the sling, an alternative catheterizable channel was needed in this patient.
The patient recovered after the surgery and was able to self-catheterize without difficulty. However, the urethral mesh sling did not place enough pressure on the urethra to prevent leakage, and he had persistent incontinence from the penis. Three months after the original surgery the patient had exploration of the perineum, which revealed that the mesh sling was loose and exerting inadequate pressure on the urethra. It was likely the sling slipped postoperatively—a known complication of urethral slings. An artificial urinary sphincter (AUS) was placed around the urethra during the second surgery to address the patient’s UI.
More than 1 year after the original surgery, the patient self-catheterizes about 4 to 5 times daily via the catheterizable channel using a single-use catheter. His bladder holds at least 500 mL. The patient does not have significant leakage from the channel or the penis. He is no longer dependent on a chronic indwelling catheter and is free of the problems associated with severe UI, including foul odor, UTIs, and social isolation.
Discussion
Patients with spinal cord or pelvic nerve injury often develop spastic bladders with low capacities. This is similar to muscle spasticity that may occur with a neurologic injury, below the level of the injury, such as in the lower extremities. The powerful uncontrolled bladder spasms and small bladder capacity most often lead to incontinence. Additionally, neurologic control of the urinary sphincter is affected, leading to either uncontrolled spasms or poor tone. Patients with these injuries have no volitional control of bladder functions and are forced to catheterize intermittently, use a condom-type catheter, or have a chronic indwelling catheter (a Foley catheter or suprapubic catheter).
Intermittent catheterization is the preferred management option for neurogenic bladder. When compared with chronic indwelling catheters, intermittent catheterization is associated with lower rates of UTI and upper tract abnormalities and with the loss of renal function.5 Unfortunately, patients do not often stay on intermittent catheterization. A recent study showed that up to 70% of patients with spinal cord injuries who used clean intermittent catheterization when discharged from acute rehabilitation discontinue use and are subsequently managed by chronic indwelling catheters.6 Although the reasons why intermittent catheterization is discontinued are unclear, patient dissatisfaction with catheterization, anatomic problems, such as urethral scarring, or continued leakage despite medical treatments, such as anticholinergic medicines, may be factors.
Uncontrolled leakage and UI significantly impacts QOL and may cause patients to choose chronic indwelling catheters over intermittent catheterization. Several treatments are available to control incontinence associated with intermittent catheterization. Anticholinergic medications and more recently onabotulinum toxin A may help improve bladder spasticity. In 2011, the FDA approved onabotulinum toxin A for transurethral bladder injections. It has been shown to increase functional bladder capacity and decrease spasticity.7,8 Onabotulinum toxin A treatment will not enlarge a small, contracted bladder.
Onabotulinum toxin A treatment would not be ideal for the patient in this case study. His absolute bladder capacity was 200 mL, and onabotulinum toxin A treatment would not significantly improve capacity or make intermittent catheterization practical. Additionally, the patient had poor urinary sphincter function, and he would continue to leak regardless of improvements in the bladder spasticity or tone.
Augmentation enterocystoplasty is surgical enlargement of the bladder, using a piece of the bowel and is indicated in patients with low bladder volumes. With this procedure the native bladder becomes defunctionalized, and patients experience a dramatic improvement in bladder volumes and a reduction in bladder spasms and leakage. The use of the colon and terminal ileum for bladder augmentation, or CCIC, was first reported by Sarosdy in 2 patients in 1992.9 In 1996, King and colleagues demonstrated successful outcomes with CCIC in a cohort of 8 patients after 34 months of follow-up.10 Seven patients successfully used clean intermittent catheterization, and 1 patient chose an indwelling catheter because of progressive upper extremity weakness. No patients experienced worsened renal function or pyelonephritis suggestive of upper urinary tract deterioration. A single patient had mild stomal stenosis, which was successfully revised under local anesthesia.
In another study, Sutton and colleagues reported at 27 months an improvement of 276 mL in bladder capacity, no metabolic complications, and a 95% continence rate in a cohort of 23 patients with neurogenic bladder who underwent CCIC.4 Sutton and colleagues later reported outcomes for 34 patients with a median of 31 months follow-up.11 The most common complications were recurrent UTIs (12%) and stomal stenosis (12%). Only 3 patients (9%) required surgical revisions for stomal stenosis.
Altered bowel function and metabolic abnormalities are a concern after bowel resection and reconstruction. However, a study has found no subjective change in bowel function following ileal resection of up to 60 cm for urinary diversion for bladder malignancy.12 Rates of hyperchloremic hypokalemic metabolic acidosis are low, and most changes in electrolytes are subclinical.13,14 Long-term vitamin B12 deficiency is seen with larger (> 50 cm) ileal resections but is rare with CCIC, given the small segment used for reconstruction.15 Overall, CCIC is shown to have excellent surgical outcomes in carefully selected patients with neurogenic bladder.
In addition to low bladder capacity, the case study patient also had intrinsic sphincteric deficiency (very low urinary sphincter tone), which is common with pelvic nerve injury but unusual with spinal cord injury. He initially received a suburethral mesh sling that supported and compressed the urethra and buttressed the natural urinary sphincter. However, patients can develop catheterization issues with a suburethral sling due to mechanical compression of the urethra and traversing the compressed area with a urinary catheter. Given the indication for augmentation cystoplasty in this patient, he additionally elected to undergo catheterization channel creation to avoid long-term issues of urethral catheterization through the urethra compressed by the sling.
Unfortunately, this patient had postoperative issues with his suburethral sling, and a modified AUS was inserted rather than a second sling. Normally, an AUS is attached to a pump mechanism in the scrotum. The pump allows the patient to cycle fluid from the sphincter cuff to a reservoir in the abdomen, removing compression on the urethra and allowing normal urination. Because this patient could not effectively urinate from the penis, the authors wanted to obstruct the urethra to prevent leakage without closing it permanently. The AUS was connected to a tissue expander port placed subcutaneously in the lower abdomen rather than to a pump mechanism. This modified approach used fewer mechanical parts compared with the pump mechanism, possibly reducing rates of mechanical failure. Additionally, a lower cuff pressure could be used to obstruct the urethra and prevent leakage, reducing the likelihood of urethral atrophy. Fewer mechanical parts and a lower cuff pressure could theoretically improve longevity of the AUS (Figure 3). This modified method of AUS placement has been described in patients with sphincteric deficiency and spinal cord injury.16
These 2 reconstructive surgeries freed the patient from indwelling catheter dependence and significantly improved his incontinence and QOL. Many patients with spinal cord injury or pelvic injury could benefit from similar reconstructive surgeries if conservative measures such as anticholinergic medications or onabotulinum toxin A treatments do not control incontinence.
Conclusion
Blast injuries in soldiers often cause pelvic and genitourinary injuries. These injuries can lead to chronic urinary problems and profound social and physical disability. These young veterans need innovative, individualized approaches to best manage their long-term urinary issues. Reconstructive surgery may improve QOL and decrease disability from bladder dysfunction for carefully selected patients. Clinicians caring for veterans with pelvic and genitourinary injury should strive to create a system where these options are available when they are appropriate.
1. U.S. Department of Defense. U.S. Casualty Status. U.S. Department of Defense Website. http://www.defense.gov/casualty.pdf. Updated November 3, 2015. Accessed November 4, 2015.
2. Schoenfeld AJ, Dunn JC, Bader JO, Belmont PJ Jr. The nature and extent of war injuries sustained by combat specialty personnel killed and wounded in Afghanistan and Iraq, 2003-2011. J Trauma Acute Care Surg. 2013;75(2):287-291.
3. Pannell D, Brisebois R, Talbot M, et al. Causes of death in Canadian Forces members deployed to Afghanistan and implications on tactical combat casualty care provision. J Trauma. 2011;71(5)(suppl 1):S401-S407.
4. Sutton MA, Hinson JL, Nickell KG, Boone TB. Continent ileocecal augmentation cystoplasty. Spinal Cord. 1998;36(4):246-251.
5. Weld KJ, Wall BM, Mangold TA, Steere EL, Dmochowski RR. Influences on renal function in chronic spinal cord injured patients. J Urol. 2000;164(5):1490-1493.
6. Cameron AP, Wallner LP, Tate DG, Sarma AV, Rodriguez GM, Clemens JQ. Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol. 2010;184(1):213-217.
7. Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60(4):742-750.
8. Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187(6):2131-2139.
9. Sarosdy MF. Continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1992;40(2):102-106.
10. King DH, Hlavinka TC, Sarosdy MF. Additional experience with continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1996;47(4):471-475.
11. Khavari R, Fletcher SG, Liu J, Boone TB. A modification to augmentation cystoplasty with catheterizable stoma for neurogenic patients: technique and long-term results. Urology. 2012;80(2):460-464.
12. Fung B, Kessler TM, Haeni K, Burkhard FC, Studer UE. Bowel function remains subjectively unchanged after ileal resection for construction of continent ileal reservoirs. Eur Urol. 2011;60(3):585-590.
13. Adams RC, Vachha B, Samuelson ML, Keefover-Hicks A, Snodgrass WT. Incidence of new onset metabolic acidosis following enteroplasty for myelomeningocele. J Urol. 2010;183(1):302-305.
14. Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep. 2007;8(2):157-162.
15. Pannek J, Haupt G, Schulze H, Senge T. Influence of continent ileal urinary diversion on vitamin B12 absorption. J Urol. 1996;155(4):1206-1208.
16. Bersch U, Göcking K, Pannek J. The artificial urinary sphincter in patients with spinal cord lesion: description of a modified technique and clinical results. Eur Urol. 2009;55(3):687-693.
1. U.S. Department of Defense. U.S. Casualty Status. U.S. Department of Defense Website. http://www.defense.gov/casualty.pdf. Updated November 3, 2015. Accessed November 4, 2015.
2. Schoenfeld AJ, Dunn JC, Bader JO, Belmont PJ Jr. The nature and extent of war injuries sustained by combat specialty personnel killed and wounded in Afghanistan and Iraq, 2003-2011. J Trauma Acute Care Surg. 2013;75(2):287-291.
3. Pannell D, Brisebois R, Talbot M, et al. Causes of death in Canadian Forces members deployed to Afghanistan and implications on tactical combat casualty care provision. J Trauma. 2011;71(5)(suppl 1):S401-S407.
4. Sutton MA, Hinson JL, Nickell KG, Boone TB. Continent ileocecal augmentation cystoplasty. Spinal Cord. 1998;36(4):246-251.
5. Weld KJ, Wall BM, Mangold TA, Steere EL, Dmochowski RR. Influences on renal function in chronic spinal cord injured patients. J Urol. 2000;164(5):1490-1493.
6. Cameron AP, Wallner LP, Tate DG, Sarma AV, Rodriguez GM, Clemens JQ. Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol. 2010;184(1):213-217.
7. Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60(4):742-750.
8. Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187(6):2131-2139.
9. Sarosdy MF. Continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1992;40(2):102-106.
10. King DH, Hlavinka TC, Sarosdy MF. Additional experience with continent urinary diversion using cutaneous ileocecocystoplasty. Urology. 1996;47(4):471-475.
11. Khavari R, Fletcher SG, Liu J, Boone TB. A modification to augmentation cystoplasty with catheterizable stoma for neurogenic patients: technique and long-term results. Urology. 2012;80(2):460-464.
12. Fung B, Kessler TM, Haeni K, Burkhard FC, Studer UE. Bowel function remains subjectively unchanged after ileal resection for construction of continent ileal reservoirs. Eur Urol. 2011;60(3):585-590.
13. Adams RC, Vachha B, Samuelson ML, Keefover-Hicks A, Snodgrass WT. Incidence of new onset metabolic acidosis following enteroplasty for myelomeningocele. J Urol. 2010;183(1):302-305.
14. Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep. 2007;8(2):157-162.
15. Pannek J, Haupt G, Schulze H, Senge T. Influence of continent ileal urinary diversion on vitamin B12 absorption. J Urol. 1996;155(4):1206-1208.
16. Bersch U, Göcking K, Pannek J. The artificial urinary sphincter in patients with spinal cord lesion: description of a modified technique and clinical results. Eur Urol. 2009;55(3):687-693.