User login
Update on nonhormonal approaches to menopausal management
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
Individualizing the evaluation is imperative
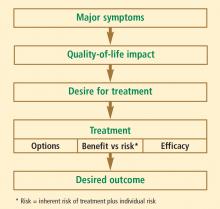
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
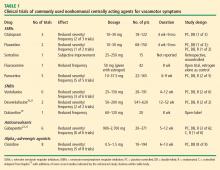
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
Individualizing the evaluation is imperative
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
As the life expectancy of women in the United States now exceeds 80 years,1 many millions of US women will spend more than one-third to even one-half of their lives beyond menopause. While hormone therapy (HT) can effectively address many of the symptoms of menopause, women who are unwilling or unable to take HT need nonhormonal alternatives for treatment of menopausal symptoms as well as the estrogen-deficiency bone loss that ensues in many women. This article reviews current and experimental nonhormonal therapies for menopausal symptoms and related issues, such as midlife sexual dysfunction and maintenance of bone health.
DEFINING THE TERMINOLOGY OF MENOPAUSE
We begin the discussion of menopausal health with a clarification of some terms.2
Menopause refers to the final menstrual period and simply represents a point in time. Menopause can be diagnosed only a year after it occurs, when it is clear that the last menstrual period was truly the final one.
Perimenopause consists of three components: the period shortly before menopause (when the biological and clinical features of impending menopause begin), menopause itself (final menstrual period), and the year following menopause. Perimenopause is synonymous with menopausal transition.
Postmenopause is the period beginning at the time of the final menstrual period (menopause), although it is recognized only after a year of amenorrhea. The early postmenopausal phase is the first 5 years after menopause, whereas all the time thereafter is referred to as the late menopausal phase.
MENOPAUSAL ASSESSMENT
Symptoms
The primary symptoms of perimenopause are:
- Vasomotor symptoms (eg, hot flashes, night sweats)
- Menstrual cycle changes (ie, oligomenorrhea, amenorrhea)
- Vaginal dryness.
Secondary symptoms include sleep disturbance, low sex drive and/or reduced sexual arousal, stress or urge urinary incontinence, mood changes, and somatic complaints.
Vasomotor symptoms, vaginal dryness, and dyspareunia (painful intercourse) contributing to sexual dysfunction have been correlated with the loss of sex hormones (particularly estrogen) associated with menopause, whereas the other symptoms listed above (sleep disturbance, urinary symptoms, mood changes, somatic symptoms) have not been linked definitively to menopause and may be a function of aging.2
Vasomotor symptoms are the predominant reason that women seek medical treatment around the time of menopause.3 More than 75% of women report hot flashes within the 2 years surrounding menopause. Among these women who have hot flashes, 25% report that these symptoms remain for greater than 5 years, and 10% report that they remain for more than 10 years.3 Vasomotor symptoms may be associated with sleep disturbance, mood swings, cognitive deficits, social impairment, a reduction in productivity, embarrassment, anxiety, and fatigue.
Individualizing the evaluation is imperative
The overall patient must be considered in this assessment, which includes her personal history, family history, social history, and current medication use. Common factors affecting postmenopausal health—such as bone density; vaginal, bladder, and sexual function; cardiovascular health (including lipid profile, blood pressure, and tobacco use); thromboembolic risk; and cancer risk, including breast cancer—should be included in the assessment. For most women under the age of 60 years who have menopausal symptoms, HT remains the gold standard and recommended treatment, according to both the American Association of Clinical Endocrinologists4 and the North American Menopause Society.5 However, for women who cannot or will not take HT, there are other treatment options to consider.
ALTERNATIVE TREATMENTS FOR VASOMOTOR SYMPTOMS: FOCUS ON NONHORMONAL OPTIONS
Options for the treatment of vasomotor symptoms include lifestyle modification, HT, nonhormonal centrally acting agents, and complementary and alternative medicine. Lifestyle modifications to cope with hot flashes include dressing in layers, adjusting room temperature, and deep breathing and relaxation exercises. Complementary and alternative medical approaches to vasomotor symptoms have generally not been evaluated in well-designed studies or have been found ineffective, so they will not be discussed further here. HT was discussed at length in the previous articles in this supplement, and because of its perceived risks, some women are unwilling to use HT. For these women, and particularly for those with contraindications to HT—especially those with breast cancer treated with medications that promote severe vasomotor symptoms—nonhormonal alternatives for vasomotor symptom treatment clearly are needed. Centrally acting agents show the most promise in this regard.
The rationale for a nonhormonal approach
Development of vasomotor symptoms seems to be related to the withdrawal of gonadotropins and the instability of serotonin and norepinephrine in the hypothalamus.6–9 A small increase in core temperature precedes a vasomotor symptom episode in approximately 70% of women. A narrowing of the hypothalamic thermoregulatory set point is followed by an increasing sensation of intense heat and peripheral vasodilation, leading to an exaggerated response (ie, severe sweating and flashing) to the very small rise in core temperature. This pathophysiology of vasomotor symptoms is the basis for the use of alternatives to HT, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs
As detailed in Table 1, studies of SSRIs have usually been only weeks in duration, have often been uncontrolled or retrospective in design, and have generally enrolled small numbers of patients, making it difficult to draw valid conclusions from their data.10–13 Overall, the results with SSRIs are mixed with respect to efficacy in reducing the incidence and severity of vasomotor symptoms.
Most studies of SSRIs for this indication have been performed with paroxetine, which has the highest affinity for the norepinephrine receptor among the SSRIs. Fluoxetine and paroxetine have each been studied in randomized controlled trials in menopausal women with vasomotor symptoms, and each has resulted in a reduction in the frequency and severity of those symptoms compared with placebo.11,12 The North American Menopause Society (NAMS), in its 2004 position statement on management of menopause-related vasomotor symptoms,5 and the National Institutes of Health2 have recognized fluoxetine and paroxetine as possible alternatives to HT for the treatment of vasomotor symptoms.
One cautionary note is required with SSRI use in this setting: because SSRIs are strong inhibitors of CYP2D6, an enzyme important in the metabolism of tamoxifen,14 the potential for interactions between SSRIs and tamoxifen must be recognized. In breast cancer patients with the CYP2D6 genotype, paroxetine reduced plasma levels of the active metabolite of tamoxifen.15
SNRIs
Studies of SNRIs have also enrolled few patients, with treatment durations of 4 to 52 weeks (Table 1).10,13
Venlafaxine has been the most widely studied of the SNRIs, but the longest follow-up in studies of venlafaxine has been only 12 weeks.10,13,16–18 It has been shown to reduce the frequency and severity of vasomotor symptoms in several studies, and two of its studies had randomized controlled designs.16,18 In its 2004 position statement, NAMS recognizes low-dose venlafaxine (37.5 to 75.0 mg) as a nonhormonal alternative for the treatment of vasomotor symptoms.5
Duloxetine has been assessed in a single published clinical trial for vasomotor symptoms, a small, 8week, open-label investigation that demonstrated a small reduction in the frequency of vasomotor symptoms with its use.19
Desvenlafaxine succinate, the active metabolite of venlafaxine, was approved by the US Food and Drug Administration (FDA) in February 2008 for the treatment of major depressive disorder. It is currently under FDA review for treatment of menopause-related vasomotor symptoms and is expected to be the first FDA-approved nonhormonal agent for the treatment of menopausal vasomotor symptoms. Among the centrally acting agents studied for treatment of vasomotor symptoms, desvenlafaxine has been assessed in the largest randomized controlled trials to date.
In a randomized trial of 541 menopausal women with hot flashes, both dosages of desvenlafaxine tested (100 and 150 mg/day) were associated with a sustained significant reduction in the incidence of moderate to severe vasomotor symptoms compared with placebo over the 12 to 26 weeks of treatment.20 Withdrawal of desvenlafaxine was associated with a recurrence of symptoms, which the study authors argue is proof that the drug was responsible for the reduced incidence of vasomotor symptoms, despite the large placebo effect observed in the study.
Another randomized trial compared four dosages of desvenlafaxine (50, 100, 150, or 200 mg/day) with placebo in 707 healthy postmenopausal women who experienced at least 50 moderate to severe hot flashes per week.21 Among the 620 evaluable women, the best results overall were seen with the 100-mg dose of desvenlafaxine, which was associated with a 64% reduction from baseline in the average daily number of hot flashes at week 12. Compared with the placebo group, significantly greater percentages of patients achieved a 75% or greater reduction in the number of hot flashes from baseline in the 100-, 150-, and 200mg dose groups at week 4, and in the 100- and 200-mg dose groups at week 12.
The most common side effects associated with desvenlafaxine were nausea, dizziness, and insomnia. The most common symptoms that occurred upon discontinuation were dizziness, nausea, and headache.21 The rate of discontinuation with desvenlafaxine was lowest in the group assigned to 50 mg, which suggests a dose-related effect in terms of side effects. It should be noted that desvenlafaxine in this study was started at full dosage without titration and was discontinued abruptly, practices that are not typical with the use of venlafaxine and may account for the above-mentioned side effects.
SNRIs are weak inhibitors of CYP2D6 and therefore represent a good nonhormonal alternative for vasomotor symptoms in breast cancer patients being treated with tamoxifen.
Anticonvulsants
Gabapentin is an anticonvulsant that has been assessed in several trials for the treatment of vasomotor symptoms, showing superior efficacy to placebo in all placebo-controlled trials (Table 1). Its mechanism of action against hot flashes is uncertain, but it has been theorized that gabapentin may modulate calcium currents.5
In addition to the placebo-controlled trials mentioned above, gabapentin has been assessed in comparison with estrogen22 and in combination with antidepressants.23 One study randomized 60 postmenopausal women with moderate to severe hot flashes to treatment with conjugated estrogens (0.625 mg/day), gabapentin (titrated to 2,400 mg/day), or placebo for 12 weeks.22 Gabapentin and estrogen were similarly effective in reducing the study’s primary outcome measure—hot flash composite score at 12 weeks—and each was significantly superior to placebo in this regard.
Another study assessed gabapentin in combination with antidepressants (mostly venlafaxine or paroxetine) in 118 women with inadequate hot flash control, 91 of whom were evaluable at 5 weeks.23 Three-fourths of the study population had a history of breast cancer, and two-thirds were taking tamoxifen or an aromatase inhibitor at entry. Women were randomized either to remain on their antidepressant and have gabapentin added or to be weaned off their antidepressant and switched to gabapentin monotherapy. Gabapentin alone was associated with a statistically significant 50% median reduction in hot flash frequency from baseline, with no additional efficacy induced by continuation of the antidepressant. Negative mood changes and nervousness by week 2 were noted in the women who discontinued their antidepressants, although there was no change in quality-of-life evaluations.
The most common side effects of gabapentin in this clinical setting have been somnolence, disorientation, and headache. Notably, the effective dosages of gabapentin studied in women with vasomotor symptoms were higher (900 to 2,700 mg/day) than is sometimes possible to achieve in real-world practice, so the clinical relevance of these studies may be somewhat limited. Nevertheless, the NAMS position statement recognizes gabapentin as an alternative to HT for treating vasomotor symptoms.5
Alpha2-adrenergic receptor agonists
The alpha2-adrenergic receptor agonist clonidine has been used for treatment of hot flashes, but its efficacy has been modest at best in small trials of short duration (Table 1). The total daily doses used ranged from 0.5 mg to 1.5 mg, and side effects of dry mouth and dizziness were reported to cause relatively high discontinuation rates. While clonidine is an option, it should be reserved for patients who are intolerant of the other nonhormonal options discussed above.
Special considerations in breast cancer patients
Women with breast cancer merit special consideration, for several reasons. First, their cancer constitutes a contraindication to HT, so they are leading candidates for nonhormonal approaches to vasomotor symptom control. Second, chemotherapy itself may induce menopausal symptoms. Finally, vasomotor symptoms are often induced by other common (and longer-term) breast cancer therapies, including aromatase inhibitors (ie, anastrozole, exemestane, letrozole) in addition to tamoxifen, as mentioned above. Because SSRIs are strong inhibitors of CYP2D6, which is critical to tamoxifen metabolism, the SNRIs or gabapentin are preferred nonhormonal options in women taking tamoxifen.
Vitamin E: Scant evidence for symptom improvement, but a role in VTE prevention?
Vitamin E was frequently recommended in the past as a possible nonhormonal alternative to treat vasomotor symptoms, but small clinical trials have shown that it is not much more likely than placebo to be effective for this indication. Evidence from the Women’s Health Study indicates, however, that any value of vitamin E supplementation in this population may lie in reducing the risk of venous thromboembolism (VTE).24 In this large placebo-controlled trial, randomization to 600 IU of alpha-tocopherol every other day was associated with modest reductions in VTE overall and more significant reductions among the subgroup of women at highest risk for VTE—ie, those with a history of prior VTE or a prothrombotic mutation.
ALTERNATIVES TO SYSTEMIC ESTROGEN FOR OTHER MENOPAUSAL HEALTH ISSUES
Vaginal atrophy
New research is helping to define just how “low” low-dose topical therapy can go while still providing efficacy. A recent placebo-controlled trial compared 10-µg and 25-µg strengths of estradiol-containing vaginal tablets for vaginal atrophy in 230 postmenopausal women.26 Over the 12-week study, both doses of estradiol significantly improved vaginal maturation, lowered vaginal pH, and reduced the severity of vaginal symptoms compared with placebo. Although improvements were greater with the 25-µg dose, the results suggest that 10-µg topical estradiol is an effective option for women with vaginal atrophy who wish to minimize their exposure to estrogen.
Although there is insufficient evidence to support endometrial surveillance in asymptomatic women using vaginal estrogen, such surveillance may be indicated in women at high risk for endometrial cancer, in those requiring a higher dose for vaginal atrophy relief, and in those with spotting or breakthrough bleeding.25
Sexual dysfunction
Sexual dysfunction is not directly caused by the menopausal transition but is multifactorial, involving physical health, mental health, relationship dynamics, and partner availability, among other factors. The two most common complaints relating to sexual dysfunction in women at midlife are lack of desire and hypoarousal.
A number of therapies are currently under investigation for treatment of female sexual dysfunction at midlife. These include the same low-dose vaginal estrogen preparations used for vaginal atrophy as well as newer approaches currently in clinical testing, such as the melanocortin receptor agonist bremelanotide27,28 and topical alprostadil,29 both of which act by inducing sexual arousal. In a study of premenopausal women with sexual arousal disorder, bremelanotide increased both genital arousal and sexual desire.30
The most widely studied pharmacotherapy approach to sexual dysfunction has been testosterone replacement. A recent Cochrane review assessed the results of 23 trials that evaluated the addition of testosterone to HT (estrogen with or without progestogen) in 1,957 peri- and postmenopausal women.31 It found that adding testosterone to HT has a beneficial effect on sexual function in postmenopausal women but also confers the adverse effect of reducing levels of high-density lipoprotein cholesterol. The authors concluded that the impact of testosterone therapy on other health outcomes in estrogenized postmenopausal women is unclear, as is the existence of a benefit in sexual function for perimenopausal women.
In addition to systemic testosterone, a transdermal testosterone patch has been studied for treatment of sexual dysfunction in postmenopausal women; FDA evaluation of the patch is awaiting the availability of long-term safety data, although the testosterone patch for women is available in Europe.
According to a 2005 NAMS position statement on testosterone therapy,32 postmenopausal women presenting with symptoms of decreased sexual desire that causes personal distress may be candidates for testosterone therapy. The NAMS statement further clarifies that all other identifiable causes of sexual dysfunction should be considered and ruled out as appropriate. Because of a lack of safety and efficacy data on the use of testosterone therapy in unestrogenized women, testosterone therapy alone cannot be recommended in women not taking concomitant estrogen.32
Bone health
Many alternatives to systemic HT exist for maintaining bone health, including calcium and vitamin D supplementation, bisphosphonates, selective estrogen receptor modulators, calcitonin, recombinant human parathyroid hormone, and ultralow-dose transdermal estradiol. Beyond calcium and vitamin D supplementation, the most appropriate options for most women will likely be bisphosphonates or transdermal estradiol.
Ultralow-dose (0.014 mg/day) transdermal estradiol has been shown to significantly increase bone mineral density (BMD) at both the hip and the spine in post-menopausal women compared with placebo.33 However, the only agent that has demonstrated fracture risk reduction in women who do not otherwise have osteoporosis is standard-dose estrogen therapy (eg, 0.625 mg conjugated equine estrogens). Lower doses of estrogen, which may maintain bone density, do not have data on fracture risk reduction. Although lower doses of estrogen have been found to increase BMD, there is a dose-dependent response, with higher doses producing more of an increase.34,35
Bisphosphonates. The oral bisphosphonates, alendronate and risedronate, have proven efficacy in reducing hip fracture rates in women who already have osteoporosis. Risedronate recently gained FDA approval for administration in a regimen involving two 75-mg tablets taken on a monthly (consecutive-day) basis, and a 150-mg monthly risedronate tablet is expected soon.
Zoledronic acid, an injectable bisphosphonate, recently gained FDA approval for administration as a once-yearly intravenous infusion after this regimen was shown in a large 3-year placebo-controlled trial to significantly reduce the risk of morphometric spine, hip, nonvertebral, wrist, and rib fractures in postmenopausal women with osteoporosis.36 In this trial, atrial fibrillation was more common in women treated with zoledronic acid than in those who received placebo. However, any link between zoledronic acid and atrial fibrillation is uncertain, since episodes of atrial fibrillation tended to occur more than 30 days after the infusion and since circulating active levels of zoledronic acid persist for only up to 1 week. (A history of arrhythmia or atrial fibrillation is not listed in FDA-approved labeling as a contraindication to zoledronic acid.) Also, there was no increased risk of jaw osteonecrosis in subjects treated with zoledronic acid.36
Intravenous dosing can be helpful when patients have intolerable gastrointestinal side effects or other contraindications to oral dosing, as well as to ensure adherence.
Ibandronate is another bisphosphonate that has been shown to reduce the risk of vertebral fractures. It is administered as a once-monthly oral dose or as an intravenous injection given every 3 months. Although these less-frequent dosing regimens can be more convenient for patients and the injectable form can eliminate gastrointestinal side effects, widespread use of ibandronate has been limited somewhat by a lack of evidence for reduction of nonvertebral and hip fractures.37
Raloxifene is the only selective estrogen receptor modulator (SERMs, which the FDA recently requested be called “estrogen agonists/estrogen antagonists”) approved for the prevention and treatment of osteoporosis in postmenopausal women. Raloxifene reduced the vertebral fracture rate by 40% to 50% over 2 to 4 years of use but did not reduce nonvertebral fracture rates. Raloxifene also reduces the risk of invasive breast cancer development.38,39 It has not been shown to lower the risk of coronary events or overall stroke risk but was instead associated with an increased risk of VTE and fatal stroke.
Synthetic recombinant human parathyroid hormone (PTH[1–34]; teriparatide) is currently the sole available agent in the new class of bone anabolic agents, although others are on the horizon. PTH(1–34) is given as a once-daily subcutaneous injection for up to 2 years of therapy. It is associated with a reduction in the risk of vertebral and nonvertebral fractures and is indicated for postmenopausal women (and men) with osteoporosis who are at high risk for fracture, as well as those in whom other medications have failed or are not tolerated.
Although rat studies revealed a potential increased risk for osteosarcoma with PTH(1–34) use, this has not been seen in any human studies or in postmarketing surveillance. As the risk was dependent on dose and duration of therapy, use of PTH(1–34) is not recommended for more than 2 years or in patients at increased risk for osteosarcoma.
Concomitant use of PTH(1–34) with a bisphosphonate seems to blunt its effect and is therefore to be avoided. Resumption of bisphosphonate use after 2 years of PTH(1–34) therapy seems to prevent the loss of densitometric gains that ensues upon cessation of PTH(1–34).40
Calcitonin is an older agent administered mainly as a nasal spray. It reduces vertebral fracture risk in postmenopausal women and is FDA-approved for the treatment, but not prevention, of osteoporosis. Calcitonin has questionable mild analgesic effects in compression fracture treatment. Because of its expense and inferior efficacy relative to other therapies, it is generally reserved for patients who cannot tolerate other agents.41
Therapies on the horizon for osteoporosis prevention and/or treatment in postmenopausal women include strontium ranelate, third-generation SERMs or estrogen agonists/antagonists (ie, bazedoxifene and lasofoxifene), and combination estrogen/SERM therapies.
SUMMARY
The risk-benefit assessment for management of vasomotor symptoms and other menopause-related health issues should be tailored to formulate the most efficacious and safe treatment plan for each individual woman. The most appropriate management is guided by the individual patient’s own assessment of her most bothersome symptom(s) and her preferences and comfort level regarding various risks and quality-of-life issues. To best inform these patient choices, physicians must strive to clearly and accurately present the risks and benefits of the various available treatment options.
For most symptomatic menopausal women, HT remains the best treatment. However, for women unable or unwilling to take HT, there are alternatives for the treatment of vasomotor symptoms and bone loss. Low doses of local vaginal estrogen remain an option for treatment of genitourinary atrophy, even in women in whom systemic HT may be contraindicated.
Reassessment of current data and ongoing clinical trials will assist clinicians and patients in decision-making regarding menopausal HT. Nonhormonal therapies for menopausal symptoms should be used to provide effective treatment options for those menopausal patients unwilling or unable to take HT.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.
- Anderson RN. United States life tables, 1997. Natl Vital Stat Rep 1999; 47:1–37.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: management of menopause-related symptoms. Ann Intern Med 2005; 142:1003–1013.
- Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47.
- American Association of Clinical Endocrinologists (AACE) postion statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause 2004; 11:11–33.
- Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14:435–448.
- Bachmann GA. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005; 50:155–165.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med 2005; 23:117–125.
- Berendsen HH. The role of serotonin in hot flashes. Maturitas 2000; 36:155–164.
- Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071.
- Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002; 20:1578–1583.
- Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834.
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol 2007; 196:97–106.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97:30–39.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95:1758–1764.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000; 356:2059–2063.
- Barton D, La VB, Loprinzi C, Novotny P, Wilwerding MB, Sloan J. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002; 29:33–40.
- Evans ML, Pritts E, Vittinghoff E, et al. Management of postmenopausal hot flushes with venlafaxine hydrochloride: a randomized, controlled trial. Obstet Gynecol 2005; 105:161–166.
- Joffe H, Soares CN, Petrillo LF, et al. Treatment of depression and menopause-related symptoms with the serotonin-norepinephrine reuptake inhibitor duloxetine. J Clin Psychiatry 2007; 68:943–950.
- Kagan R, Constantine G, Olivier S. Treatment with desvenlafaxine succinate (DVS) results in a sustained reduction in number of severe hot flushes (HFs) in menopausal women [abstract]. Menopause 2007; 14:1084. Abstract S-13.
- Speroff L, Gass M, Constantine G, Olivier S, for the Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108:41–48.
- Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25:308–312.
- Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation 2007; 116:1497–1503.
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–369.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76.
- Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual dysfunction. J Sex Med 2007; 4(Suppl 4):269–279.
- Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med 2008; 5:887–897.
- Heiman JR, Gittelman M, Costabile R, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol 2006; 27:31–41.
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med 2006; 3:628–638.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4):CD004509.
- North American Menopause Society. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 2005; 12:497–511.
- Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 2004; 104:443–451.
- Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157:2609–2615.
- Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002; 287:2668–2676.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008; 148:197–213.
- Vogel VG, Constantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 2005; 353:555–565.
- Chesnut CH III, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000; 109:267–276.
Case studies and clinical considerations in menopausal management
Dr. Holly Thacker: In light of the updates that Drs. Hodis and Gass have presented on hormone therapy (HT) for menopausal women and that Drs. Jenkins and Sikon have presented on nonhormonal options for menopausal management, let’s start our roundtable by considering a couple of case studies that will give us the chance to apply the latest data in a practical way.
CASE 1: A SYMPTOMATIC 67-YEAR-OLD IN WHOM HORMONE THERAPY WAS ABRUPTLY STOPPED
Dr. Margaret McKenzie: A 67-year-old menopausal woman presents for the evaluation of hot flashes, vaginal dryness causing dyspareunia, and decreased libido. She was previously on estrogen-progestogen therapy (EPT), consisting of daily conjugated equine estrogens and medroxyprogesterone acetate, for 15 years starting at the time of natural menopause. Her gynecologist discontinued this HT abruptly at the time of the initial release of data from the Women’s Health Initiative trial, and the patient is now seeking another opinion about resuming HT. When she presents, she has been off HT for less than 6 months. Would you restart HT in this patient?
Dr. Andrea Sikon: The abrupt discontinuation certainly contributes to her symptoms. The short duration of time off HT is important, and would lead me to restart HT after an updated review of risk factors. She had been on it for 15 years and has done fine, so she appears to be an ideal candidate to restart.
Dr. McKenzie: What specific questions would you ask when doing your risk assessment? How would you evaluate this patient to determine whether she is a good candidate to continue HT?
Dr. Thacker: I would obtain a family history. Using a population-based risk assessment such as the Gail model, I would calculate her absolute risk of breast cancer based on her duration of EPT use. I might offer her a lower-dose regimen. A conjugated equine estrogen dosage of 0.3 mg/day may be as effective in a 67-year-old woman as 0.625 mg/day is in a younger woman in terms of relieving vasomotor symptoms, depending on individual metabolism. We do have evidence from the HOPE trial that 0.3 mg/day is effective for relief of vasomotor symptoms.1 In addition, data from the Nurses’ Health Study show no increased risk of stroke with 0.3 mg/day, as opposed to the increased risk with 0.625 mg/day, and there are other data showing that the risk of stroke is possibly related to dosage.2
At the same time, we do not yet have long-term data to show that the lower dose is necessarily safer and we do not have data on bone fracture risk with the lower dose, so I would want to know this patient’s bone mineral density (BMD). I would also want to know about her cardiovascular risk profile, including her lipid profile, and I would want more details about her sexual function.
Dr. McKenzie: I will supply a few more case details. This patient’s body mass index (BMI) was 24 kg/m2. She exercised regularly. Her BMD was normal for her age. She was taking a statin to treat hyperlipidemia. She was a nonsmoker, and her family history was unremarkable. Does any of this information change the way that you would counsel her?
Dr. Howard Hodis: Knowing her BMI and that she was on a statin, I would have even less of a problem reinitiating HT.
Case continued
Dr. McKenzie: Well, EPT was reinitiated in this patient at a lower dose (0.45/1.5 mg), and she was satisfied. At her most recent visit, several years later, the possibility of reducing her dose of HT was offered; however, the patient is happy with her quality of life and accepts whatever risk that continued HT may bring. She inquired about transdermal testosterone to restore her sex drive, and it was agreed that if it receives US marketing approval for women with decreased libido, a 24-week trial would be attempted.
Dr. Hodis: If you look at the data, this patient not only may enhance her quality of life by continuing HT but might extend it as well.
Dr. Thacker: Many patients inquire about using testosterone only, without estrogen, for treating dyspareunia and low libido. Clinicians must understand that testosterone is aromatized to estrogen. If a patient is on a high dose of oral estrogen, I would consider switching to transdermal estrogen before trying testosterone, whose use in women remains off-label in the United States. But the patient in our case has been doing well for several years on low-dose estrogen and she still has her ovaries.
Dr. Margery Gass: Some colleagues and I completed a study, which was presented at a recent Endocrine Society meeting,3 in which transdermal testosterone was just as effective without estrogen in increasing libido. But this remains moot for general clinical practice unless the transdermal testosterone patch is approved in this country (as it is already approved for use in women in Europe).
Dr. Hodis: Would any of you be worried about this patient’s fracture risk after having HT stopped following 15 years of use? Data show that the rate of bone loss after abrupt cessation of HT is just as great as when a woman is going through menopause.
Dr. Gass: Yes, and that is exactly the point. The woman should be assessed under these circumstances just as she would be at menopause, using the same risk factors.
Dr. Thacker: I think that underscores that there is risk in stopping treatment, just as there is in taking treatment or not taking treatment, and all of these risks should be considered. Many times, once a patient is off HT, some clinicians forget to check the patient’s BMD or to do a complete genital examination.
Dr. Sikon: Many providers who do not specialize in women’s health may forget that when HT is stopped, it is as if a newly menopausal state is being created. Providers need to think about ensuing changes in bone and genitourinary status as well as quality-of-life concerns.
Dr. McKenzie: In today’s clinical environment, there is awareness of the importance of long-term bone health because patients are living longer. The use of BMD measurements in practice is clearly expanding.
Dr. Thacker: It is worth noting that all of the drugs used to treat osteoporosis have been studied primarily in women who already have osteoporosis. The therapy with the most data to support a reduction in the risk of all types of fracture is HT. These data are very impressive, and although fracture prevention would not be the sole reason for using HT, it can make the overall risk-benefit assessment easier, particularly if it can be determined whether or not an individual patient is at high risk of venous thromboembolism (VTE).
CASE 2: A SYMPTOMATIC 47-YEAR-OLD WITH A HISTORY OF BREAST CANCER
Dr. McKenzie: A 47-year-old postmenopausal woman with a 7-year history of breast cancer presents for the management of hot flashes, irritability, and reduced sleep. In addition, recent onset of vaginal dryness is causing dyspareunia that is not alleviated by lubricants. Her breast cancer was estrogen receptor (ER)/progesterone receptor (PR)–positive, and she received tamoxifen therapy for 5 years (now completed) following the initial diagnosis and management. How would you approach the management of this patient?
Dr. Marjorie Jenkins: I would first try to determine the severity of her hot flashes and how much her symptoms are affecting her quality of life. She may say that she is still having hot flashes, but how frequent are they? Do they cause nighttime awakening and subsequent fatigue?
Dr. McKenzie: The reason this patient presented was to ask for some form of relief because her symptoms were affecting her quality of life. Her dyspareunia was getting worse. She was trying various lubricants without success. At the same time, she expressed fear because her tumor was ER/PR-positive. Most cancer survivors have recurrence in the back of their mind even though they are in remission.
Dr. Jenkins: Was she asking specifically about HT or about help to relieve her symptoms?
Dr. McKenzie: She was asking for help for her symptoms.
Dr. Jenkins: I would consider low-dose vaginal estrogen to address her dyspareunia. I would also consider a serotonin-norepinephrine reuptake inhibitor, such as venlafaxine, to treat her hot flashes and irritability, along with lifestyle modifications, although the latter do not have evidence-based support. If these measures failed to offer relief, I would reconsider the risks and benefits of low-dose HT. I would call her oncologist for input if I planned to start vaginal estrogen, low-dose topical testosterone, or any type of hormonal treatment. I would make sure that the patient knew that her oncologist was working as part of the team responsible for her management.
Dr. Thacker: I concur. When I see hormonally sensitive breast cancer patients with vaginal symptoms, particularly when they are taking tamoxifen, I often talk to them about local estrogen before vaginal atrophy becomes severe. Once the atrophy becomes severe, local estrogen, even in low doses, may be absorbed systemically (increasing the risk of endometrial hyperplasia) until the the vagina becomes re-estrogenized and stratified with healthy squamous epithelium. Once this restratification takes place, there are generally no systemic hormonal effects with low-dose local vaginal estrogen, but it is best to avoid severe atrophy in the first place. I like to start local estrogen early if I know that the oncologist wants to use an aromatase inhibitor in a breast cancer survivor. I prescribe the low-dose vaginal form frequently in my practice, and order transvaginal ultrasonography liberally if there is concern about the endometrium.
Additionally, I would offer this patient venlafaxine or, more specifically, desvenlafaxine, as the literature has shown that the latter agent is associated with an improvement in sleep.4 (Currently, desvenlafaxine has been approved by the US Food and Drug Administration [FDA] solely to treat major depression; however, it has been studied in nondepressed women with hot flashes and is expected to be the first nonhormonal agent to receive FDA approval specifically to treat hot flashes.)
Dr. Gass: This patient still has her uterus in place. Data show that estrogens have a first-pass uterine effect, and this gives me pause because estrogen levels could well be higher in the uterus than in the bloodstream. Because of those data, as well as the absence of long-term safety data, the use of estrogen in this patient would cause me concern.
With breast cancer patients in particular, I try everything else to treat vaginal dryness before adding estrogen. I believe that if a patient is having dyspareunia despite the use of adequate lubricants, something else is the problem. In many cases these women have not had intercourse for months because they have been undergoing treatment for breast cancer, and they can have hallmark pain syndromes or constriction of the vagina that may require treatments besides just estrogen. If all else failed, I would prescribe vaginal estrogen on a temporary basis. Women who stay sexually active after menopause can do perfectly fine without treatment, but those who have periods of abstinence and then try to resume sexual activity typically run into problems.
Dr. Hodis: Would you have concern about breast cancer recurrence with estrogen reinitiation, based on the literature?
Dr. Gass: I have seen recurrences out to 20 years after the initial cancer. If the cancer does recur, the woman will always have a nagging doubt that it could have been avoided if she had not used estrogen.
Dr. Thacker: We have many breast cancer survivors in our practice. It is an easier decision to give estrogen to women who have had bilateral mastectomy with reconstruction for a stage I breast cancer and have already had a hysterectomy or bilateral salpingooophorectomy. But we otherwise try to avoid it unless the patient has first tried everything else and is miserable from her vaginal symptoms.
Dr. Gass: When a patient is diagnosed with breast cancer, I gently encourage her to continue sexual activity through the course of treatment, explaining that she may be far better off later. It may avoid a lot of problems if we can proactively get that message across.
Dr. Jenkins: That is a great point, and there is evidence that increased sexual activity decreases vaginal atrophy and assists in maintaining vaginal elasticity and the ability of urogenital congestion with arousal. Although lubricants and moisturizers do work well, they may require repeated application, and inelasticity is still a problem for some patients. It is somewhat of a “use it or lose it” proposition.
Dr. Thacker: Recurrent urinary tract infections (UTIs) are a problem as well. Patients see urologists, undergo multiple endoscopies, and are treated with antibiotics, sometimes chronically, yet often they are not even offered local vaginal estrogen, which reduces recurrent UTIs. Local estrogen should be considered in any woman with vaginal atrophy and recurrent UTIs.
Case continued
Dr. McKenzie: To return to our case, this patient’s vagina was, in fact, severely constricted because she had not been sexually active for a while. Her BMI was initially greater than 25 kg/m2, but she lost weight and became more symptomatic as she did so. She then stopped having sex because it was painful.
When she presented initially, she had a package of black cohosh with her and was willing to try it for her symptoms but was apprehensive after reading the disclaimers in the package. A 47-year-old who has already been diagnosed with ER-positive breast cancer is generally anxious about using any therapies that may be associated with an increased risk of breast cancer.
After examination of her vagina, her oncologist was consulted and suggested that her serum estradiol levels be measured; they were less than 20 pg/mL. We then agreed to a trial of estradiol vaginal tablets, vaginal dilators, and an increase in her sexual activity. She has been on vaginal estradiol for 2 years and is functioning very well. Her hot flashes improved spontaneously as her body adapted to her new weight.
BEYOND THE CASES: OTHER CHALLENGES IN MENOPAUSAL MANAGEMENT
HT discontinuation, dose reduction in real-world practice
Dr. Thacker: The first case we covered touched on both discontinuation of HT and HT dosage reduction. These are issues that come up often in clinical practice; what lessons does the panel have to share on these issues?
Dr. Gass: I find that there is a small subset of women who are highly symptomatic and are probably always going to be miserable whenever they try to go off HT. For some, if they are highly symptomatic at menopause, they tend to stay that way. They may try to go off, but a year later, they come back and say, “I am just too miserable.”
Dr. McKenzie: In my practice, I have noticed patients who end up staying on higher doses of HT for a long period because they do not tolerate weaning. If you take them down to 0.625 mg of estrogen, their hot flashes resume, so they seem to require 0.9 mg.
Dr. Thacker: I have a very small subset of those women too. I wonder if their metabolism is different; maybe 0.9 mg of conjugated equine estrogens is to them what 0.3 mg is to other women. As women get older, perhaps metabolic changes are one reason that many can reduce their hormone dosage. It is very challenging. I tell my students that it is much easier trying to determine how much thyroid hormone replacement to give a patient than how much estrogen.
When does transdermal estrogen make sense?
Dr. Sikon: I would be interested in the rest of the panel’s views on management of a woman in her early postmenopausal years who is symptomatic and has been on oral HT and is also a smoker. Would you switch her to transdermal estrogen or continue her oral HT and continue aggressive smoking cessation counseling?
Dr. Thacker: Many practitioners think that a smoker cannot take any type of HT because they equate it to oral/hormonal contraceptives, which increase the risk of heart attack in smokers over age 35, but hormonal contraceptives are different in that they involve a much higher dose of hormone. In my practice, the cases when I will offer transdermal estrogen are generally when a patient has gastrointestinal upset, known gallbladder disease, elevated triglycerides, or a prior deep vein thrombosis (DVT), even though I will tell the patient that the HT-associated risks are still possible with transdermal therapies. Many women are inappropriately and inaccurately told that compounded transdermal therapies are “risk free.”
Dr. McKenzie: Transdermal estrogen is also an option for patients who are poor pill takers or are already taking too many pills. Many of my patients are on transdermal estrogen for the convenience that it offers. It is unfortunate that transdermal progesterone cream does not protect the endometrium in all patients; for women with a uterus, an oral progestogen needs to be prescribed. However, two transdermal patches containing progestogens have been shown to be efficacious in protecting the endometrium.
How should a history of DVT affect decision-making?
Dr. McKenzie: What do you do when a patient has a history of postoperative DVT and is already on HT? How many of the panel would discontinue the HT as opposed to continuing it?
Dr. Hodis: If it were a history of spontaneous DVT, I would feel uncomfortable continuing HT. A few years ago, a clinical trial was stopped early because women with a prior spontaneous DVT who were randomized to HT had a substantial increase in DVT incidence relative to those randomized to placebo.5 In the case of provoked or postoperative DVT, it may be a tougher call.
Dr. Thacker: I think that DVT is the greatest risk with HT, even though the media are more focused on breast cancer risk. The risk of breast cancer with estrogen alone is debatable, at least with oral conjugated estrogen, which was associated with a decreased risk in women who had undergone hysterectomy in the Women’s Health Initiative (WHI).6
When I see a woman with a history of DVT in my practice, I check her homocysteine levels and check for factor V Leiden and prothrombin gene mutation. If I find an inherited hypercoagulability disorder, I tell the patient that her risk of DVT with any type of hormone product is not just multiplicative, it is logarithmic. If the patient already requires lifelong anticoagulation, then I am a bit more comfortable with prescribing HT and I usually will try the transdermal route first; however, I always consider nonhormonal treatment alternatives first.
Dr. Gass: The WHI was supposed to have excluded women with a history of DVT, but a few such women were enrolled, and it was demonstrated that they were at higher risk of DVT recurrence if randomized to HT. The majority of DVT episodes in the WHI were spontaneous, not related to surgical procedures.
Dr. Jenkins: I have a patient who had been on low-dose HT for 30 years and underwent lumbar spine surgery. She had a somewhat prolonged recovery, so her lack of mobility and her age clearly increased her risk of DVT. So she was taken off HT and became miserable from the resulting hot flashes and sleep disturbance. We thoroughly discussed the risks and benefits of restarting HT, and because she was taking warfarin, we felt comfortable restarting the HT.
Women with spontaneous DVT are a different case, however, and I have an issue with restarting oral or transdermal HT in those cases. However, if we discuss the data with these patients and document the significant risks of HT in their cases, some may want to accept the increased risk in order to improve their quality of life, and that may be reasonable if they are truly fully informed.
Dr. Thacker: What about a woman who has been on oral contraceptives for several years and has not had a DVT? Is the safe use of oral/ hormonal contraceptives something you take into account, Dr. Gass, in your decision whether to prescribe HT?
Dr. Gass: Yes, that can be reassuring. Twenty-seven percent of EPT participants and 49% of estrogen therapy (ET) participants in the WHI randomized trial had used hormones in the past, so it was as if they were already tested for an early risk of blood clots.7
What role for SERMs (estrogen agonists/antagonists)?
Dr. Thacker: I would like to discuss the use of estrogen agonists/estogen antagonists, formerly known as selective estrogen receptor modulators (SERMs), such as raloxifene. Raloxifene now has an indication for breast cancer prevention as well as for reduction of vertebral fractures. I don’t know if there is adequate recognition among practitioners that SERMs appear to be associated with the same risk of DVT that estrogen is, and a greater risk of fatal stroke.
Dr. Jenkins: I find the lack of hip fracture data with raloxifene concerning, because hip fracture carries the highest 5-year mortality of any type of fracture. Raloxifene therefore is not a first-line agent for bone loss in my practice. But we also have to consider the patient’s risk of breast cancer and whether or not she has been on tamoxifen and now needs an agent to protect her against fracture. The question is whether we should consider starting these patients on raloxifene versus a bisphosphonate.
Dr. Thacker: Dr. Gass, do you think that raloxifene is safe for the endometrium? For years, we did not know the full effects of tamoxifen on the endometrium; it took experience with millions of patients to find out that tamoxifen increases the risk of endometrial cancer.
Dr. Gass: I do think that raloxifene is safer. I use it in my practice primarily for women younger than age 65 who are not yet at high risk for hip fracture but are still concerned about breast cancer. Although this concern diminishes as women age without having developed breast cancer, for younger women, who may see their friends getting breast cancer, it is a major concern. So if a patient is a good candidate for a bone loss agent and also has concern about breast cancer, raloxifene can be a good option, especially since we do not know what the implications are of taking bisphosphonates for 30 years. Questions about that are starting to be raised, so I think it is good to consider a sequential approach for some of these patients. A sequential approach might involve use of HT in a symptomatic menopausal woman, followed by use of raloxifene after the woman no longer has menopausal symptoms but is concerned about spine fracture protection and breast cancer risk reduction, followed by bisphosphonate use as she gets older and is at increased risk for stroke/VTE and for hip fracture.
The challenge of educating younger doctors about HT
Dr. Thacker: I think we need to find ways to translate the data on HT to younger generations of physicians, because the closer one is to graduating from medical school, the less likely he or she is to offer HT to an otherwise healthy, severely symptomatic woman younger than age 60.
Dr. McKenzie: Absolutely, and I think the real challenge is to reach younger physicians who go into private practice, who generally have the fewest opportunities to stay on top of the latest evidence. We must offer evidence-based education programs on this topic to physicians in the community to ensure that they are equipped to understand and explain the real risks and benefits of HT in order to individualize treatment decisions.
As a physician at a tertiary care center, I am surprised at the number of women referred to me who should have already been on HT for menopausal symptoms, but their physicians were unduly influenced by the initial WHI publication. They need to thoroughly evaluate their patients, assess their risks, assess any new medical problems, try to educate them, and then tailor therapy to improve their patients’ quality of life.
Correcting misperceptions: WHI was not a treatment trial
Dr. Thacker: I believe that many practitioners and especially students do not realize that the WHI was a trial designed to assess prevention of chronic diseases. It was not a menopausal treatment trial, and often its data are being misapplied to women who are different from the ones enrolled in the WHI, in that they are younger and more symptomatic.
Dr. Gass: It is correct that the WHI was not a treatment trial, but that was how HT was being used by some physicians and patients prior to the WHI. Physicians in this country were giving some 65-year-old women HT for osteoporosis and dementia. These practices needed to be supported with data, and that was the impetus behind the trial. Along the same lines, it is important how we present the risks to patients. If HT is being used as a therapy for a woman suffering from menopausal symptoms, she might be willing to accept more risk than if it is being used like a vitamin pill, to promote general health, in which case the risks should be virtually nil because the woman is healthy and without complaints.
Dr. Thacker: Yes, and that is why I think the earlier discussion of comparable risks of breast cancer, stroke, and VTE with aspirin, SERMs, fibric acid derivatives, and statins helps to put the risks of HT in perspective. It appears that physicians and patients tolerate very similar risks with commonly used nonhormonal medicines in women but do not tolerate any risks with HT, even in symptomatic women. In my opinion, this is a medical travesty. It is important to recognize that there are few absolutes in medicine that apply to all patients. The only universal recommendations I make to all patients are to wear seatbelts and not to smoke.
Dr. Hodis: What I find notable is that with HT we see a reduction in mortality regardless of the risks that we have described. As the observational data show, if we start HT and continue it, there is a reduction in mortality of 30% or even greater, and the clinical trial data tend to support this benefit. So why do we shy away from HT? Because we are worried about a small increase in breast cancer diagnoses or a small increase in DVT? That is an issue I am grappling with.
Dr. Thacker: Similarly, how do you reconcile the observational data with aspirin? In the Nurses’ Health Study, the aspirin users had lower mortality, but in all of the randomized controlled trials in midlife women, we do not see a reduction in cardiovascular risk with aspirin, let alone a reduction in mortality. So, the people who self-select for treatment are obviously different from those enrolled in randomized trials. The randomized controlled trial may be our gold standard, but it is not necessarily the only evidence to consider.
Dr. Hodis: But there is a concordance between observational studies and randomized trials with respect to overall mortality and HT. The data from a meta-analysis of 30 randomized trials8 are consistent with the data from observational studies, even though they do identify risks. I wonder how many more women would select HT if we told them about the 30% reduction in mortality despite the possibility of breast cancer diagnosis and DVT risks.
Dr. Gass: Women will select according to their own agenda.
Dr. Hodis: Yes, in the end, it is all individualized.
Dr. Gass: Indeed, because women have specific concerns, such as breast cancer, fracture risk, or Alzheimer disease, and they base their personal decisions on these specific concerns. I educate them about the risks and benefits, and they pretty much decide for themselves. They know their priorities.
Age and the risk-benefit assessment with HT
Dr. Thacker: Does the panel have any comments on the recent position statement from the American Association of Clinical Endocrinologists concluding that the benefits of HT exceed the risks in symptomatic women younger than age 60?
Dr. Hodis: My only comment is to ask why it took them so long to come to that conclusion.
Dr. Thacker: We could say the same for the North American Menopause Society (NAMS). It was not until its 2007 position statement on the use of ET and EPT in perimenopausal and postmenopausal women that NAMS moved beyond the issue of a time limit for HT—the “lowest dose for the shortest amount of time” mantra—and recommended the type of reevaluation used with any other treatment.
It seems as if practitioners are less willing to tolerate the risks of HT in older women than to tolerate the risks of hormonal contraceptives in younger women. Perhaps that is because hormonal contraceptives prevent pregnancy and the risks associated with it, yet this same value is not afforded to the symptoms and other effects suffered by postmenopausal women. I think we did afford HT similar value as hormonal contraceptives in the 1980s and 1990s, when we were trying to promote the potential health benefits of HT before we had bisphosphonates and statins and before we realized the risks of VTE with HT, risks that were identified much sooner with hormonal contraceptives. Since then the medical community has overcorrected by too often dismissing HT, overemphasizing the risks and ignoring very important quality-of-life issues, including sex and sleep disturbances, in the process.
Dr. Jenkins: Also, too many people associate menopause only with hot flashes, without taking into account the increased risk of serious diseases that may occur at this time, such as osteoporosis and heart disease.
Dr. Thacker: That may be because menopause is a normal event. It can be a great time of life for many women; in fact, it is associated with lower rates of depression, unless there is a prolonged symptomatic perimenopause. Menopause is certainly not a disease, and NAMS has been very good at recognizing and promoting it as a normal phase of life. But to neglect treating a woman going through menopausal symptoms just because menopause is a normal life event would be akin to withholding assistance for women during childbirth, which is another natural event.
We fail from a medical perspective if we do not take care of symptomatic women, because they will then turn to people who are not physicians and who offer unregulated therapies. These people may deliver the right message—that menopausal women deserve to feel well and look good—but the way they tell women to treat menopausal symptoms is not science-based.
At one time, we were overtreating women and not individualizing therapy, but to me it is even more worrisome to withhold therapies unless women are so highly symptomatic that they consider ending their life. We are continuing to discover the risks and benefits of HT and how to further tailor it. We have many newer, lower-dose HT options, and we are expecting the first nonhormonal therapy for menopausal vasomotor symptoms, desvenlafaxine. We are fortunate to have bone agents and local vaginal therapies for women without vasomotor symptoms. With both hormonal and nonhormonal options, we must keep the risks and benefits of any therapy in perspective.

CONCLUSIONS
Dr. Thacker: This has been a great discussion, and although we do not all agree on every point, I would like to conclude by summarizing some key points on which I think we do all agree (see sidebar above). Menopause is a normal life event, but for some women who are symptomatic, and for a smaller percentage who will be symptomatic for the rest of their lives, HT is the gold standard, although it does not treat all symptoms and has some well-defined risks. We do have other options on the horizon for relief of vasomotor symptoms, for bone health, and for urogenital atrophy.
Following the data on the effects of HT on cardiovascular health will be particularly interesting. Although there is not support for using HT specifically for cardiovascular prevention, there are provocative data that in the symptomatic woman who has self-selected it, HT has cardiovascular benefit and reduces the risk of diabetes.
A woman on HT who has not had “early harm” does not need to arbitrarily discontinue therapy based on any time limit, as long as she is being periodically reevaluated and is offered individualized options.
- Utian WH, Shoupe D, Backmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 2001; 75:1065–1079.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Davis S, Studd J, Bouchard C, Kroll R, Moufarege A, Von Schoultz B. The effect of a testosterone transdermal system on hypoactive sexual desire disorder in postmenopausal women not receiving systemic estrogen therapy, the APHRODITE study. Abstract presented at: 86th Annual Meeting of the Endocrine Society; June 16–19, 2004; New Orleans, LA.
- Speroff L, Gass M, Constantine G, Olivier S; Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Høibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrøm E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy: results of the randomized, double-blind, placebo-controlled Estrogen in Venous Thromboembolism Trial (EVTET). Thromb Haemost 2000; 84:961–967.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003; 13:S78–S86.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.
Dr. Holly Thacker: In light of the updates that Drs. Hodis and Gass have presented on hormone therapy (HT) for menopausal women and that Drs. Jenkins and Sikon have presented on nonhormonal options for menopausal management, let’s start our roundtable by considering a couple of case studies that will give us the chance to apply the latest data in a practical way.
CASE 1: A SYMPTOMATIC 67-YEAR-OLD IN WHOM HORMONE THERAPY WAS ABRUPTLY STOPPED
Dr. Margaret McKenzie: A 67-year-old menopausal woman presents for the evaluation of hot flashes, vaginal dryness causing dyspareunia, and decreased libido. She was previously on estrogen-progestogen therapy (EPT), consisting of daily conjugated equine estrogens and medroxyprogesterone acetate, for 15 years starting at the time of natural menopause. Her gynecologist discontinued this HT abruptly at the time of the initial release of data from the Women’s Health Initiative trial, and the patient is now seeking another opinion about resuming HT. When she presents, she has been off HT for less than 6 months. Would you restart HT in this patient?
Dr. Andrea Sikon: The abrupt discontinuation certainly contributes to her symptoms. The short duration of time off HT is important, and would lead me to restart HT after an updated review of risk factors. She had been on it for 15 years and has done fine, so she appears to be an ideal candidate to restart.
Dr. McKenzie: What specific questions would you ask when doing your risk assessment? How would you evaluate this patient to determine whether she is a good candidate to continue HT?
Dr. Thacker: I would obtain a family history. Using a population-based risk assessment such as the Gail model, I would calculate her absolute risk of breast cancer based on her duration of EPT use. I might offer her a lower-dose regimen. A conjugated equine estrogen dosage of 0.3 mg/day may be as effective in a 67-year-old woman as 0.625 mg/day is in a younger woman in terms of relieving vasomotor symptoms, depending on individual metabolism. We do have evidence from the HOPE trial that 0.3 mg/day is effective for relief of vasomotor symptoms.1 In addition, data from the Nurses’ Health Study show no increased risk of stroke with 0.3 mg/day, as opposed to the increased risk with 0.625 mg/day, and there are other data showing that the risk of stroke is possibly related to dosage.2
At the same time, we do not yet have long-term data to show that the lower dose is necessarily safer and we do not have data on bone fracture risk with the lower dose, so I would want to know this patient’s bone mineral density (BMD). I would also want to know about her cardiovascular risk profile, including her lipid profile, and I would want more details about her sexual function.
Dr. McKenzie: I will supply a few more case details. This patient’s body mass index (BMI) was 24 kg/m2. She exercised regularly. Her BMD was normal for her age. She was taking a statin to treat hyperlipidemia. She was a nonsmoker, and her family history was unremarkable. Does any of this information change the way that you would counsel her?
Dr. Howard Hodis: Knowing her BMI and that she was on a statin, I would have even less of a problem reinitiating HT.
Case continued
Dr. McKenzie: Well, EPT was reinitiated in this patient at a lower dose (0.45/1.5 mg), and she was satisfied. At her most recent visit, several years later, the possibility of reducing her dose of HT was offered; however, the patient is happy with her quality of life and accepts whatever risk that continued HT may bring. She inquired about transdermal testosterone to restore her sex drive, and it was agreed that if it receives US marketing approval for women with decreased libido, a 24-week trial would be attempted.
Dr. Hodis: If you look at the data, this patient not only may enhance her quality of life by continuing HT but might extend it as well.
Dr. Thacker: Many patients inquire about using testosterone only, without estrogen, for treating dyspareunia and low libido. Clinicians must understand that testosterone is aromatized to estrogen. If a patient is on a high dose of oral estrogen, I would consider switching to transdermal estrogen before trying testosterone, whose use in women remains off-label in the United States. But the patient in our case has been doing well for several years on low-dose estrogen and she still has her ovaries.
Dr. Margery Gass: Some colleagues and I completed a study, which was presented at a recent Endocrine Society meeting,3 in which transdermal testosterone was just as effective without estrogen in increasing libido. But this remains moot for general clinical practice unless the transdermal testosterone patch is approved in this country (as it is already approved for use in women in Europe).
Dr. Hodis: Would any of you be worried about this patient’s fracture risk after having HT stopped following 15 years of use? Data show that the rate of bone loss after abrupt cessation of HT is just as great as when a woman is going through menopause.
Dr. Gass: Yes, and that is exactly the point. The woman should be assessed under these circumstances just as she would be at menopause, using the same risk factors.
Dr. Thacker: I think that underscores that there is risk in stopping treatment, just as there is in taking treatment or not taking treatment, and all of these risks should be considered. Many times, once a patient is off HT, some clinicians forget to check the patient’s BMD or to do a complete genital examination.
Dr. Sikon: Many providers who do not specialize in women’s health may forget that when HT is stopped, it is as if a newly menopausal state is being created. Providers need to think about ensuing changes in bone and genitourinary status as well as quality-of-life concerns.
Dr. McKenzie: In today’s clinical environment, there is awareness of the importance of long-term bone health because patients are living longer. The use of BMD measurements in practice is clearly expanding.
Dr. Thacker: It is worth noting that all of the drugs used to treat osteoporosis have been studied primarily in women who already have osteoporosis. The therapy with the most data to support a reduction in the risk of all types of fracture is HT. These data are very impressive, and although fracture prevention would not be the sole reason for using HT, it can make the overall risk-benefit assessment easier, particularly if it can be determined whether or not an individual patient is at high risk of venous thromboembolism (VTE).
CASE 2: A SYMPTOMATIC 47-YEAR-OLD WITH A HISTORY OF BREAST CANCER
Dr. McKenzie: A 47-year-old postmenopausal woman with a 7-year history of breast cancer presents for the management of hot flashes, irritability, and reduced sleep. In addition, recent onset of vaginal dryness is causing dyspareunia that is not alleviated by lubricants. Her breast cancer was estrogen receptor (ER)/progesterone receptor (PR)–positive, and she received tamoxifen therapy for 5 years (now completed) following the initial diagnosis and management. How would you approach the management of this patient?
Dr. Marjorie Jenkins: I would first try to determine the severity of her hot flashes and how much her symptoms are affecting her quality of life. She may say that she is still having hot flashes, but how frequent are they? Do they cause nighttime awakening and subsequent fatigue?
Dr. McKenzie: The reason this patient presented was to ask for some form of relief because her symptoms were affecting her quality of life. Her dyspareunia was getting worse. She was trying various lubricants without success. At the same time, she expressed fear because her tumor was ER/PR-positive. Most cancer survivors have recurrence in the back of their mind even though they are in remission.
Dr. Jenkins: Was she asking specifically about HT or about help to relieve her symptoms?
Dr. McKenzie: She was asking for help for her symptoms.
Dr. Jenkins: I would consider low-dose vaginal estrogen to address her dyspareunia. I would also consider a serotonin-norepinephrine reuptake inhibitor, such as venlafaxine, to treat her hot flashes and irritability, along with lifestyle modifications, although the latter do not have evidence-based support. If these measures failed to offer relief, I would reconsider the risks and benefits of low-dose HT. I would call her oncologist for input if I planned to start vaginal estrogen, low-dose topical testosterone, or any type of hormonal treatment. I would make sure that the patient knew that her oncologist was working as part of the team responsible for her management.
Dr. Thacker: I concur. When I see hormonally sensitive breast cancer patients with vaginal symptoms, particularly when they are taking tamoxifen, I often talk to them about local estrogen before vaginal atrophy becomes severe. Once the atrophy becomes severe, local estrogen, even in low doses, may be absorbed systemically (increasing the risk of endometrial hyperplasia) until the the vagina becomes re-estrogenized and stratified with healthy squamous epithelium. Once this restratification takes place, there are generally no systemic hormonal effects with low-dose local vaginal estrogen, but it is best to avoid severe atrophy in the first place. I like to start local estrogen early if I know that the oncologist wants to use an aromatase inhibitor in a breast cancer survivor. I prescribe the low-dose vaginal form frequently in my practice, and order transvaginal ultrasonography liberally if there is concern about the endometrium.
Additionally, I would offer this patient venlafaxine or, more specifically, desvenlafaxine, as the literature has shown that the latter agent is associated with an improvement in sleep.4 (Currently, desvenlafaxine has been approved by the US Food and Drug Administration [FDA] solely to treat major depression; however, it has been studied in nondepressed women with hot flashes and is expected to be the first nonhormonal agent to receive FDA approval specifically to treat hot flashes.)
Dr. Gass: This patient still has her uterus in place. Data show that estrogens have a first-pass uterine effect, and this gives me pause because estrogen levels could well be higher in the uterus than in the bloodstream. Because of those data, as well as the absence of long-term safety data, the use of estrogen in this patient would cause me concern.
With breast cancer patients in particular, I try everything else to treat vaginal dryness before adding estrogen. I believe that if a patient is having dyspareunia despite the use of adequate lubricants, something else is the problem. In many cases these women have not had intercourse for months because they have been undergoing treatment for breast cancer, and they can have hallmark pain syndromes or constriction of the vagina that may require treatments besides just estrogen. If all else failed, I would prescribe vaginal estrogen on a temporary basis. Women who stay sexually active after menopause can do perfectly fine without treatment, but those who have periods of abstinence and then try to resume sexual activity typically run into problems.
Dr. Hodis: Would you have concern about breast cancer recurrence with estrogen reinitiation, based on the literature?
Dr. Gass: I have seen recurrences out to 20 years after the initial cancer. If the cancer does recur, the woman will always have a nagging doubt that it could have been avoided if she had not used estrogen.
Dr. Thacker: We have many breast cancer survivors in our practice. It is an easier decision to give estrogen to women who have had bilateral mastectomy with reconstruction for a stage I breast cancer and have already had a hysterectomy or bilateral salpingooophorectomy. But we otherwise try to avoid it unless the patient has first tried everything else and is miserable from her vaginal symptoms.
Dr. Gass: When a patient is diagnosed with breast cancer, I gently encourage her to continue sexual activity through the course of treatment, explaining that she may be far better off later. It may avoid a lot of problems if we can proactively get that message across.
Dr. Jenkins: That is a great point, and there is evidence that increased sexual activity decreases vaginal atrophy and assists in maintaining vaginal elasticity and the ability of urogenital congestion with arousal. Although lubricants and moisturizers do work well, they may require repeated application, and inelasticity is still a problem for some patients. It is somewhat of a “use it or lose it” proposition.
Dr. Thacker: Recurrent urinary tract infections (UTIs) are a problem as well. Patients see urologists, undergo multiple endoscopies, and are treated with antibiotics, sometimes chronically, yet often they are not even offered local vaginal estrogen, which reduces recurrent UTIs. Local estrogen should be considered in any woman with vaginal atrophy and recurrent UTIs.
Case continued
Dr. McKenzie: To return to our case, this patient’s vagina was, in fact, severely constricted because she had not been sexually active for a while. Her BMI was initially greater than 25 kg/m2, but she lost weight and became more symptomatic as she did so. She then stopped having sex because it was painful.
When she presented initially, she had a package of black cohosh with her and was willing to try it for her symptoms but was apprehensive after reading the disclaimers in the package. A 47-year-old who has already been diagnosed with ER-positive breast cancer is generally anxious about using any therapies that may be associated with an increased risk of breast cancer.
After examination of her vagina, her oncologist was consulted and suggested that her serum estradiol levels be measured; they were less than 20 pg/mL. We then agreed to a trial of estradiol vaginal tablets, vaginal dilators, and an increase in her sexual activity. She has been on vaginal estradiol for 2 years and is functioning very well. Her hot flashes improved spontaneously as her body adapted to her new weight.
BEYOND THE CASES: OTHER CHALLENGES IN MENOPAUSAL MANAGEMENT
HT discontinuation, dose reduction in real-world practice
Dr. Thacker: The first case we covered touched on both discontinuation of HT and HT dosage reduction. These are issues that come up often in clinical practice; what lessons does the panel have to share on these issues?
Dr. Gass: I find that there is a small subset of women who are highly symptomatic and are probably always going to be miserable whenever they try to go off HT. For some, if they are highly symptomatic at menopause, they tend to stay that way. They may try to go off, but a year later, they come back and say, “I am just too miserable.”
Dr. McKenzie: In my practice, I have noticed patients who end up staying on higher doses of HT for a long period because they do not tolerate weaning. If you take them down to 0.625 mg of estrogen, their hot flashes resume, so they seem to require 0.9 mg.
Dr. Thacker: I have a very small subset of those women too. I wonder if their metabolism is different; maybe 0.9 mg of conjugated equine estrogens is to them what 0.3 mg is to other women. As women get older, perhaps metabolic changes are one reason that many can reduce their hormone dosage. It is very challenging. I tell my students that it is much easier trying to determine how much thyroid hormone replacement to give a patient than how much estrogen.
When does transdermal estrogen make sense?
Dr. Sikon: I would be interested in the rest of the panel’s views on management of a woman in her early postmenopausal years who is symptomatic and has been on oral HT and is also a smoker. Would you switch her to transdermal estrogen or continue her oral HT and continue aggressive smoking cessation counseling?
Dr. Thacker: Many practitioners think that a smoker cannot take any type of HT because they equate it to oral/hormonal contraceptives, which increase the risk of heart attack in smokers over age 35, but hormonal contraceptives are different in that they involve a much higher dose of hormone. In my practice, the cases when I will offer transdermal estrogen are generally when a patient has gastrointestinal upset, known gallbladder disease, elevated triglycerides, or a prior deep vein thrombosis (DVT), even though I will tell the patient that the HT-associated risks are still possible with transdermal therapies. Many women are inappropriately and inaccurately told that compounded transdermal therapies are “risk free.”
Dr. McKenzie: Transdermal estrogen is also an option for patients who are poor pill takers or are already taking too many pills. Many of my patients are on transdermal estrogen for the convenience that it offers. It is unfortunate that transdermal progesterone cream does not protect the endometrium in all patients; for women with a uterus, an oral progestogen needs to be prescribed. However, two transdermal patches containing progestogens have been shown to be efficacious in protecting the endometrium.
How should a history of DVT affect decision-making?
Dr. McKenzie: What do you do when a patient has a history of postoperative DVT and is already on HT? How many of the panel would discontinue the HT as opposed to continuing it?
Dr. Hodis: If it were a history of spontaneous DVT, I would feel uncomfortable continuing HT. A few years ago, a clinical trial was stopped early because women with a prior spontaneous DVT who were randomized to HT had a substantial increase in DVT incidence relative to those randomized to placebo.5 In the case of provoked or postoperative DVT, it may be a tougher call.
Dr. Thacker: I think that DVT is the greatest risk with HT, even though the media are more focused on breast cancer risk. The risk of breast cancer with estrogen alone is debatable, at least with oral conjugated estrogen, which was associated with a decreased risk in women who had undergone hysterectomy in the Women’s Health Initiative (WHI).6
When I see a woman with a history of DVT in my practice, I check her homocysteine levels and check for factor V Leiden and prothrombin gene mutation. If I find an inherited hypercoagulability disorder, I tell the patient that her risk of DVT with any type of hormone product is not just multiplicative, it is logarithmic. If the patient already requires lifelong anticoagulation, then I am a bit more comfortable with prescribing HT and I usually will try the transdermal route first; however, I always consider nonhormonal treatment alternatives first.
Dr. Gass: The WHI was supposed to have excluded women with a history of DVT, but a few such women were enrolled, and it was demonstrated that they were at higher risk of DVT recurrence if randomized to HT. The majority of DVT episodes in the WHI were spontaneous, not related to surgical procedures.
Dr. Jenkins: I have a patient who had been on low-dose HT for 30 years and underwent lumbar spine surgery. She had a somewhat prolonged recovery, so her lack of mobility and her age clearly increased her risk of DVT. So she was taken off HT and became miserable from the resulting hot flashes and sleep disturbance. We thoroughly discussed the risks and benefits of restarting HT, and because she was taking warfarin, we felt comfortable restarting the HT.
Women with spontaneous DVT are a different case, however, and I have an issue with restarting oral or transdermal HT in those cases. However, if we discuss the data with these patients and document the significant risks of HT in their cases, some may want to accept the increased risk in order to improve their quality of life, and that may be reasonable if they are truly fully informed.
Dr. Thacker: What about a woman who has been on oral contraceptives for several years and has not had a DVT? Is the safe use of oral/ hormonal contraceptives something you take into account, Dr. Gass, in your decision whether to prescribe HT?
Dr. Gass: Yes, that can be reassuring. Twenty-seven percent of EPT participants and 49% of estrogen therapy (ET) participants in the WHI randomized trial had used hormones in the past, so it was as if they were already tested for an early risk of blood clots.7
What role for SERMs (estrogen agonists/antagonists)?
Dr. Thacker: I would like to discuss the use of estrogen agonists/estogen antagonists, formerly known as selective estrogen receptor modulators (SERMs), such as raloxifene. Raloxifene now has an indication for breast cancer prevention as well as for reduction of vertebral fractures. I don’t know if there is adequate recognition among practitioners that SERMs appear to be associated with the same risk of DVT that estrogen is, and a greater risk of fatal stroke.
Dr. Jenkins: I find the lack of hip fracture data with raloxifene concerning, because hip fracture carries the highest 5-year mortality of any type of fracture. Raloxifene therefore is not a first-line agent for bone loss in my practice. But we also have to consider the patient’s risk of breast cancer and whether or not she has been on tamoxifen and now needs an agent to protect her against fracture. The question is whether we should consider starting these patients on raloxifene versus a bisphosphonate.
Dr. Thacker: Dr. Gass, do you think that raloxifene is safe for the endometrium? For years, we did not know the full effects of tamoxifen on the endometrium; it took experience with millions of patients to find out that tamoxifen increases the risk of endometrial cancer.
Dr. Gass: I do think that raloxifene is safer. I use it in my practice primarily for women younger than age 65 who are not yet at high risk for hip fracture but are still concerned about breast cancer. Although this concern diminishes as women age without having developed breast cancer, for younger women, who may see their friends getting breast cancer, it is a major concern. So if a patient is a good candidate for a bone loss agent and also has concern about breast cancer, raloxifene can be a good option, especially since we do not know what the implications are of taking bisphosphonates for 30 years. Questions about that are starting to be raised, so I think it is good to consider a sequential approach for some of these patients. A sequential approach might involve use of HT in a symptomatic menopausal woman, followed by use of raloxifene after the woman no longer has menopausal symptoms but is concerned about spine fracture protection and breast cancer risk reduction, followed by bisphosphonate use as she gets older and is at increased risk for stroke/VTE and for hip fracture.
The challenge of educating younger doctors about HT
Dr. Thacker: I think we need to find ways to translate the data on HT to younger generations of physicians, because the closer one is to graduating from medical school, the less likely he or she is to offer HT to an otherwise healthy, severely symptomatic woman younger than age 60.
Dr. McKenzie: Absolutely, and I think the real challenge is to reach younger physicians who go into private practice, who generally have the fewest opportunities to stay on top of the latest evidence. We must offer evidence-based education programs on this topic to physicians in the community to ensure that they are equipped to understand and explain the real risks and benefits of HT in order to individualize treatment decisions.
As a physician at a tertiary care center, I am surprised at the number of women referred to me who should have already been on HT for menopausal symptoms, but their physicians were unduly influenced by the initial WHI publication. They need to thoroughly evaluate their patients, assess their risks, assess any new medical problems, try to educate them, and then tailor therapy to improve their patients’ quality of life.
Correcting misperceptions: WHI was not a treatment trial
Dr. Thacker: I believe that many practitioners and especially students do not realize that the WHI was a trial designed to assess prevention of chronic diseases. It was not a menopausal treatment trial, and often its data are being misapplied to women who are different from the ones enrolled in the WHI, in that they are younger and more symptomatic.
Dr. Gass: It is correct that the WHI was not a treatment trial, but that was how HT was being used by some physicians and patients prior to the WHI. Physicians in this country were giving some 65-year-old women HT for osteoporosis and dementia. These practices needed to be supported with data, and that was the impetus behind the trial. Along the same lines, it is important how we present the risks to patients. If HT is being used as a therapy for a woman suffering from menopausal symptoms, she might be willing to accept more risk than if it is being used like a vitamin pill, to promote general health, in which case the risks should be virtually nil because the woman is healthy and without complaints.
Dr. Thacker: Yes, and that is why I think the earlier discussion of comparable risks of breast cancer, stroke, and VTE with aspirin, SERMs, fibric acid derivatives, and statins helps to put the risks of HT in perspective. It appears that physicians and patients tolerate very similar risks with commonly used nonhormonal medicines in women but do not tolerate any risks with HT, even in symptomatic women. In my opinion, this is a medical travesty. It is important to recognize that there are few absolutes in medicine that apply to all patients. The only universal recommendations I make to all patients are to wear seatbelts and not to smoke.
Dr. Hodis: What I find notable is that with HT we see a reduction in mortality regardless of the risks that we have described. As the observational data show, if we start HT and continue it, there is a reduction in mortality of 30% or even greater, and the clinical trial data tend to support this benefit. So why do we shy away from HT? Because we are worried about a small increase in breast cancer diagnoses or a small increase in DVT? That is an issue I am grappling with.
Dr. Thacker: Similarly, how do you reconcile the observational data with aspirin? In the Nurses’ Health Study, the aspirin users had lower mortality, but in all of the randomized controlled trials in midlife women, we do not see a reduction in cardiovascular risk with aspirin, let alone a reduction in mortality. So, the people who self-select for treatment are obviously different from those enrolled in randomized trials. The randomized controlled trial may be our gold standard, but it is not necessarily the only evidence to consider.
Dr. Hodis: But there is a concordance between observational studies and randomized trials with respect to overall mortality and HT. The data from a meta-analysis of 30 randomized trials8 are consistent with the data from observational studies, even though they do identify risks. I wonder how many more women would select HT if we told them about the 30% reduction in mortality despite the possibility of breast cancer diagnosis and DVT risks.
Dr. Gass: Women will select according to their own agenda.
Dr. Hodis: Yes, in the end, it is all individualized.
Dr. Gass: Indeed, because women have specific concerns, such as breast cancer, fracture risk, or Alzheimer disease, and they base their personal decisions on these specific concerns. I educate them about the risks and benefits, and they pretty much decide for themselves. They know their priorities.
Age and the risk-benefit assessment with HT
Dr. Thacker: Does the panel have any comments on the recent position statement from the American Association of Clinical Endocrinologists concluding that the benefits of HT exceed the risks in symptomatic women younger than age 60?
Dr. Hodis: My only comment is to ask why it took them so long to come to that conclusion.
Dr. Thacker: We could say the same for the North American Menopause Society (NAMS). It was not until its 2007 position statement on the use of ET and EPT in perimenopausal and postmenopausal women that NAMS moved beyond the issue of a time limit for HT—the “lowest dose for the shortest amount of time” mantra—and recommended the type of reevaluation used with any other treatment.
It seems as if practitioners are less willing to tolerate the risks of HT in older women than to tolerate the risks of hormonal contraceptives in younger women. Perhaps that is because hormonal contraceptives prevent pregnancy and the risks associated with it, yet this same value is not afforded to the symptoms and other effects suffered by postmenopausal women. I think we did afford HT similar value as hormonal contraceptives in the 1980s and 1990s, when we were trying to promote the potential health benefits of HT before we had bisphosphonates and statins and before we realized the risks of VTE with HT, risks that were identified much sooner with hormonal contraceptives. Since then the medical community has overcorrected by too often dismissing HT, overemphasizing the risks and ignoring very important quality-of-life issues, including sex and sleep disturbances, in the process.
Dr. Jenkins: Also, too many people associate menopause only with hot flashes, without taking into account the increased risk of serious diseases that may occur at this time, such as osteoporosis and heart disease.
Dr. Thacker: That may be because menopause is a normal event. It can be a great time of life for many women; in fact, it is associated with lower rates of depression, unless there is a prolonged symptomatic perimenopause. Menopause is certainly not a disease, and NAMS has been very good at recognizing and promoting it as a normal phase of life. But to neglect treating a woman going through menopausal symptoms just because menopause is a normal life event would be akin to withholding assistance for women during childbirth, which is another natural event.
We fail from a medical perspective if we do not take care of symptomatic women, because they will then turn to people who are not physicians and who offer unregulated therapies. These people may deliver the right message—that menopausal women deserve to feel well and look good—but the way they tell women to treat menopausal symptoms is not science-based.
At one time, we were overtreating women and not individualizing therapy, but to me it is even more worrisome to withhold therapies unless women are so highly symptomatic that they consider ending their life. We are continuing to discover the risks and benefits of HT and how to further tailor it. We have many newer, lower-dose HT options, and we are expecting the first nonhormonal therapy for menopausal vasomotor symptoms, desvenlafaxine. We are fortunate to have bone agents and local vaginal therapies for women without vasomotor symptoms. With both hormonal and nonhormonal options, we must keep the risks and benefits of any therapy in perspective.

CONCLUSIONS
Dr. Thacker: This has been a great discussion, and although we do not all agree on every point, I would like to conclude by summarizing some key points on which I think we do all agree (see sidebar above). Menopause is a normal life event, but for some women who are symptomatic, and for a smaller percentage who will be symptomatic for the rest of their lives, HT is the gold standard, although it does not treat all symptoms and has some well-defined risks. We do have other options on the horizon for relief of vasomotor symptoms, for bone health, and for urogenital atrophy.
Following the data on the effects of HT on cardiovascular health will be particularly interesting. Although there is not support for using HT specifically for cardiovascular prevention, there are provocative data that in the symptomatic woman who has self-selected it, HT has cardiovascular benefit and reduces the risk of diabetes.
A woman on HT who has not had “early harm” does not need to arbitrarily discontinue therapy based on any time limit, as long as she is being periodically reevaluated and is offered individualized options.
Dr. Holly Thacker: In light of the updates that Drs. Hodis and Gass have presented on hormone therapy (HT) for menopausal women and that Drs. Jenkins and Sikon have presented on nonhormonal options for menopausal management, let’s start our roundtable by considering a couple of case studies that will give us the chance to apply the latest data in a practical way.
CASE 1: A SYMPTOMATIC 67-YEAR-OLD IN WHOM HORMONE THERAPY WAS ABRUPTLY STOPPED
Dr. Margaret McKenzie: A 67-year-old menopausal woman presents for the evaluation of hot flashes, vaginal dryness causing dyspareunia, and decreased libido. She was previously on estrogen-progestogen therapy (EPT), consisting of daily conjugated equine estrogens and medroxyprogesterone acetate, for 15 years starting at the time of natural menopause. Her gynecologist discontinued this HT abruptly at the time of the initial release of data from the Women’s Health Initiative trial, and the patient is now seeking another opinion about resuming HT. When she presents, she has been off HT for less than 6 months. Would you restart HT in this patient?
Dr. Andrea Sikon: The abrupt discontinuation certainly contributes to her symptoms. The short duration of time off HT is important, and would lead me to restart HT after an updated review of risk factors. She had been on it for 15 years and has done fine, so she appears to be an ideal candidate to restart.
Dr. McKenzie: What specific questions would you ask when doing your risk assessment? How would you evaluate this patient to determine whether she is a good candidate to continue HT?
Dr. Thacker: I would obtain a family history. Using a population-based risk assessment such as the Gail model, I would calculate her absolute risk of breast cancer based on her duration of EPT use. I might offer her a lower-dose regimen. A conjugated equine estrogen dosage of 0.3 mg/day may be as effective in a 67-year-old woman as 0.625 mg/day is in a younger woman in terms of relieving vasomotor symptoms, depending on individual metabolism. We do have evidence from the HOPE trial that 0.3 mg/day is effective for relief of vasomotor symptoms.1 In addition, data from the Nurses’ Health Study show no increased risk of stroke with 0.3 mg/day, as opposed to the increased risk with 0.625 mg/day, and there are other data showing that the risk of stroke is possibly related to dosage.2
At the same time, we do not yet have long-term data to show that the lower dose is necessarily safer and we do not have data on bone fracture risk with the lower dose, so I would want to know this patient’s bone mineral density (BMD). I would also want to know about her cardiovascular risk profile, including her lipid profile, and I would want more details about her sexual function.
Dr. McKenzie: I will supply a few more case details. This patient’s body mass index (BMI) was 24 kg/m2. She exercised regularly. Her BMD was normal for her age. She was taking a statin to treat hyperlipidemia. She was a nonsmoker, and her family history was unremarkable. Does any of this information change the way that you would counsel her?
Dr. Howard Hodis: Knowing her BMI and that she was on a statin, I would have even less of a problem reinitiating HT.
Case continued
Dr. McKenzie: Well, EPT was reinitiated in this patient at a lower dose (0.45/1.5 mg), and she was satisfied. At her most recent visit, several years later, the possibility of reducing her dose of HT was offered; however, the patient is happy with her quality of life and accepts whatever risk that continued HT may bring. She inquired about transdermal testosterone to restore her sex drive, and it was agreed that if it receives US marketing approval for women with decreased libido, a 24-week trial would be attempted.
Dr. Hodis: If you look at the data, this patient not only may enhance her quality of life by continuing HT but might extend it as well.
Dr. Thacker: Many patients inquire about using testosterone only, without estrogen, for treating dyspareunia and low libido. Clinicians must understand that testosterone is aromatized to estrogen. If a patient is on a high dose of oral estrogen, I would consider switching to transdermal estrogen before trying testosterone, whose use in women remains off-label in the United States. But the patient in our case has been doing well for several years on low-dose estrogen and she still has her ovaries.
Dr. Margery Gass: Some colleagues and I completed a study, which was presented at a recent Endocrine Society meeting,3 in which transdermal testosterone was just as effective without estrogen in increasing libido. But this remains moot for general clinical practice unless the transdermal testosterone patch is approved in this country (as it is already approved for use in women in Europe).
Dr. Hodis: Would any of you be worried about this patient’s fracture risk after having HT stopped following 15 years of use? Data show that the rate of bone loss after abrupt cessation of HT is just as great as when a woman is going through menopause.
Dr. Gass: Yes, and that is exactly the point. The woman should be assessed under these circumstances just as she would be at menopause, using the same risk factors.
Dr. Thacker: I think that underscores that there is risk in stopping treatment, just as there is in taking treatment or not taking treatment, and all of these risks should be considered. Many times, once a patient is off HT, some clinicians forget to check the patient’s BMD or to do a complete genital examination.
Dr. Sikon: Many providers who do not specialize in women’s health may forget that when HT is stopped, it is as if a newly menopausal state is being created. Providers need to think about ensuing changes in bone and genitourinary status as well as quality-of-life concerns.
Dr. McKenzie: In today’s clinical environment, there is awareness of the importance of long-term bone health because patients are living longer. The use of BMD measurements in practice is clearly expanding.
Dr. Thacker: It is worth noting that all of the drugs used to treat osteoporosis have been studied primarily in women who already have osteoporosis. The therapy with the most data to support a reduction in the risk of all types of fracture is HT. These data are very impressive, and although fracture prevention would not be the sole reason for using HT, it can make the overall risk-benefit assessment easier, particularly if it can be determined whether or not an individual patient is at high risk of venous thromboembolism (VTE).
CASE 2: A SYMPTOMATIC 47-YEAR-OLD WITH A HISTORY OF BREAST CANCER
Dr. McKenzie: A 47-year-old postmenopausal woman with a 7-year history of breast cancer presents for the management of hot flashes, irritability, and reduced sleep. In addition, recent onset of vaginal dryness is causing dyspareunia that is not alleviated by lubricants. Her breast cancer was estrogen receptor (ER)/progesterone receptor (PR)–positive, and she received tamoxifen therapy for 5 years (now completed) following the initial diagnosis and management. How would you approach the management of this patient?
Dr. Marjorie Jenkins: I would first try to determine the severity of her hot flashes and how much her symptoms are affecting her quality of life. She may say that she is still having hot flashes, but how frequent are they? Do they cause nighttime awakening and subsequent fatigue?
Dr. McKenzie: The reason this patient presented was to ask for some form of relief because her symptoms were affecting her quality of life. Her dyspareunia was getting worse. She was trying various lubricants without success. At the same time, she expressed fear because her tumor was ER/PR-positive. Most cancer survivors have recurrence in the back of their mind even though they are in remission.
Dr. Jenkins: Was she asking specifically about HT or about help to relieve her symptoms?
Dr. McKenzie: She was asking for help for her symptoms.
Dr. Jenkins: I would consider low-dose vaginal estrogen to address her dyspareunia. I would also consider a serotonin-norepinephrine reuptake inhibitor, such as venlafaxine, to treat her hot flashes and irritability, along with lifestyle modifications, although the latter do not have evidence-based support. If these measures failed to offer relief, I would reconsider the risks and benefits of low-dose HT. I would call her oncologist for input if I planned to start vaginal estrogen, low-dose topical testosterone, or any type of hormonal treatment. I would make sure that the patient knew that her oncologist was working as part of the team responsible for her management.
Dr. Thacker: I concur. When I see hormonally sensitive breast cancer patients with vaginal symptoms, particularly when they are taking tamoxifen, I often talk to them about local estrogen before vaginal atrophy becomes severe. Once the atrophy becomes severe, local estrogen, even in low doses, may be absorbed systemically (increasing the risk of endometrial hyperplasia) until the the vagina becomes re-estrogenized and stratified with healthy squamous epithelium. Once this restratification takes place, there are generally no systemic hormonal effects with low-dose local vaginal estrogen, but it is best to avoid severe atrophy in the first place. I like to start local estrogen early if I know that the oncologist wants to use an aromatase inhibitor in a breast cancer survivor. I prescribe the low-dose vaginal form frequently in my practice, and order transvaginal ultrasonography liberally if there is concern about the endometrium.
Additionally, I would offer this patient venlafaxine or, more specifically, desvenlafaxine, as the literature has shown that the latter agent is associated with an improvement in sleep.4 (Currently, desvenlafaxine has been approved by the US Food and Drug Administration [FDA] solely to treat major depression; however, it has been studied in nondepressed women with hot flashes and is expected to be the first nonhormonal agent to receive FDA approval specifically to treat hot flashes.)
Dr. Gass: This patient still has her uterus in place. Data show that estrogens have a first-pass uterine effect, and this gives me pause because estrogen levels could well be higher in the uterus than in the bloodstream. Because of those data, as well as the absence of long-term safety data, the use of estrogen in this patient would cause me concern.
With breast cancer patients in particular, I try everything else to treat vaginal dryness before adding estrogen. I believe that if a patient is having dyspareunia despite the use of adequate lubricants, something else is the problem. In many cases these women have not had intercourse for months because they have been undergoing treatment for breast cancer, and they can have hallmark pain syndromes or constriction of the vagina that may require treatments besides just estrogen. If all else failed, I would prescribe vaginal estrogen on a temporary basis. Women who stay sexually active after menopause can do perfectly fine without treatment, but those who have periods of abstinence and then try to resume sexual activity typically run into problems.
Dr. Hodis: Would you have concern about breast cancer recurrence with estrogen reinitiation, based on the literature?
Dr. Gass: I have seen recurrences out to 20 years after the initial cancer. If the cancer does recur, the woman will always have a nagging doubt that it could have been avoided if she had not used estrogen.
Dr. Thacker: We have many breast cancer survivors in our practice. It is an easier decision to give estrogen to women who have had bilateral mastectomy with reconstruction for a stage I breast cancer and have already had a hysterectomy or bilateral salpingooophorectomy. But we otherwise try to avoid it unless the patient has first tried everything else and is miserable from her vaginal symptoms.
Dr. Gass: When a patient is diagnosed with breast cancer, I gently encourage her to continue sexual activity through the course of treatment, explaining that she may be far better off later. It may avoid a lot of problems if we can proactively get that message across.
Dr. Jenkins: That is a great point, and there is evidence that increased sexual activity decreases vaginal atrophy and assists in maintaining vaginal elasticity and the ability of urogenital congestion with arousal. Although lubricants and moisturizers do work well, they may require repeated application, and inelasticity is still a problem for some patients. It is somewhat of a “use it or lose it” proposition.
Dr. Thacker: Recurrent urinary tract infections (UTIs) are a problem as well. Patients see urologists, undergo multiple endoscopies, and are treated with antibiotics, sometimes chronically, yet often they are not even offered local vaginal estrogen, which reduces recurrent UTIs. Local estrogen should be considered in any woman with vaginal atrophy and recurrent UTIs.
Case continued
Dr. McKenzie: To return to our case, this patient’s vagina was, in fact, severely constricted because she had not been sexually active for a while. Her BMI was initially greater than 25 kg/m2, but she lost weight and became more symptomatic as she did so. She then stopped having sex because it was painful.
When she presented initially, she had a package of black cohosh with her and was willing to try it for her symptoms but was apprehensive after reading the disclaimers in the package. A 47-year-old who has already been diagnosed with ER-positive breast cancer is generally anxious about using any therapies that may be associated with an increased risk of breast cancer.
After examination of her vagina, her oncologist was consulted and suggested that her serum estradiol levels be measured; they were less than 20 pg/mL. We then agreed to a trial of estradiol vaginal tablets, vaginal dilators, and an increase in her sexual activity. She has been on vaginal estradiol for 2 years and is functioning very well. Her hot flashes improved spontaneously as her body adapted to her new weight.
BEYOND THE CASES: OTHER CHALLENGES IN MENOPAUSAL MANAGEMENT
HT discontinuation, dose reduction in real-world practice
Dr. Thacker: The first case we covered touched on both discontinuation of HT and HT dosage reduction. These are issues that come up often in clinical practice; what lessons does the panel have to share on these issues?
Dr. Gass: I find that there is a small subset of women who are highly symptomatic and are probably always going to be miserable whenever they try to go off HT. For some, if they are highly symptomatic at menopause, they tend to stay that way. They may try to go off, but a year later, they come back and say, “I am just too miserable.”
Dr. McKenzie: In my practice, I have noticed patients who end up staying on higher doses of HT for a long period because they do not tolerate weaning. If you take them down to 0.625 mg of estrogen, their hot flashes resume, so they seem to require 0.9 mg.
Dr. Thacker: I have a very small subset of those women too. I wonder if their metabolism is different; maybe 0.9 mg of conjugated equine estrogens is to them what 0.3 mg is to other women. As women get older, perhaps metabolic changes are one reason that many can reduce their hormone dosage. It is very challenging. I tell my students that it is much easier trying to determine how much thyroid hormone replacement to give a patient than how much estrogen.
When does transdermal estrogen make sense?
Dr. Sikon: I would be interested in the rest of the panel’s views on management of a woman in her early postmenopausal years who is symptomatic and has been on oral HT and is also a smoker. Would you switch her to transdermal estrogen or continue her oral HT and continue aggressive smoking cessation counseling?
Dr. Thacker: Many practitioners think that a smoker cannot take any type of HT because they equate it to oral/hormonal contraceptives, which increase the risk of heart attack in smokers over age 35, but hormonal contraceptives are different in that they involve a much higher dose of hormone. In my practice, the cases when I will offer transdermal estrogen are generally when a patient has gastrointestinal upset, known gallbladder disease, elevated triglycerides, or a prior deep vein thrombosis (DVT), even though I will tell the patient that the HT-associated risks are still possible with transdermal therapies. Many women are inappropriately and inaccurately told that compounded transdermal therapies are “risk free.”
Dr. McKenzie: Transdermal estrogen is also an option for patients who are poor pill takers or are already taking too many pills. Many of my patients are on transdermal estrogen for the convenience that it offers. It is unfortunate that transdermal progesterone cream does not protect the endometrium in all patients; for women with a uterus, an oral progestogen needs to be prescribed. However, two transdermal patches containing progestogens have been shown to be efficacious in protecting the endometrium.
How should a history of DVT affect decision-making?
Dr. McKenzie: What do you do when a patient has a history of postoperative DVT and is already on HT? How many of the panel would discontinue the HT as opposed to continuing it?
Dr. Hodis: If it were a history of spontaneous DVT, I would feel uncomfortable continuing HT. A few years ago, a clinical trial was stopped early because women with a prior spontaneous DVT who were randomized to HT had a substantial increase in DVT incidence relative to those randomized to placebo.5 In the case of provoked or postoperative DVT, it may be a tougher call.
Dr. Thacker: I think that DVT is the greatest risk with HT, even though the media are more focused on breast cancer risk. The risk of breast cancer with estrogen alone is debatable, at least with oral conjugated estrogen, which was associated with a decreased risk in women who had undergone hysterectomy in the Women’s Health Initiative (WHI).6
When I see a woman with a history of DVT in my practice, I check her homocysteine levels and check for factor V Leiden and prothrombin gene mutation. If I find an inherited hypercoagulability disorder, I tell the patient that her risk of DVT with any type of hormone product is not just multiplicative, it is logarithmic. If the patient already requires lifelong anticoagulation, then I am a bit more comfortable with prescribing HT and I usually will try the transdermal route first; however, I always consider nonhormonal treatment alternatives first.
Dr. Gass: The WHI was supposed to have excluded women with a history of DVT, but a few such women were enrolled, and it was demonstrated that they were at higher risk of DVT recurrence if randomized to HT. The majority of DVT episodes in the WHI were spontaneous, not related to surgical procedures.
Dr. Jenkins: I have a patient who had been on low-dose HT for 30 years and underwent lumbar spine surgery. She had a somewhat prolonged recovery, so her lack of mobility and her age clearly increased her risk of DVT. So she was taken off HT and became miserable from the resulting hot flashes and sleep disturbance. We thoroughly discussed the risks and benefits of restarting HT, and because she was taking warfarin, we felt comfortable restarting the HT.
Women with spontaneous DVT are a different case, however, and I have an issue with restarting oral or transdermal HT in those cases. However, if we discuss the data with these patients and document the significant risks of HT in their cases, some may want to accept the increased risk in order to improve their quality of life, and that may be reasonable if they are truly fully informed.
Dr. Thacker: What about a woman who has been on oral contraceptives for several years and has not had a DVT? Is the safe use of oral/ hormonal contraceptives something you take into account, Dr. Gass, in your decision whether to prescribe HT?
Dr. Gass: Yes, that can be reassuring. Twenty-seven percent of EPT participants and 49% of estrogen therapy (ET) participants in the WHI randomized trial had used hormones in the past, so it was as if they were already tested for an early risk of blood clots.7
What role for SERMs (estrogen agonists/antagonists)?
Dr. Thacker: I would like to discuss the use of estrogen agonists/estogen antagonists, formerly known as selective estrogen receptor modulators (SERMs), such as raloxifene. Raloxifene now has an indication for breast cancer prevention as well as for reduction of vertebral fractures. I don’t know if there is adequate recognition among practitioners that SERMs appear to be associated with the same risk of DVT that estrogen is, and a greater risk of fatal stroke.
Dr. Jenkins: I find the lack of hip fracture data with raloxifene concerning, because hip fracture carries the highest 5-year mortality of any type of fracture. Raloxifene therefore is not a first-line agent for bone loss in my practice. But we also have to consider the patient’s risk of breast cancer and whether or not she has been on tamoxifen and now needs an agent to protect her against fracture. The question is whether we should consider starting these patients on raloxifene versus a bisphosphonate.
Dr. Thacker: Dr. Gass, do you think that raloxifene is safe for the endometrium? For years, we did not know the full effects of tamoxifen on the endometrium; it took experience with millions of patients to find out that tamoxifen increases the risk of endometrial cancer.
Dr. Gass: I do think that raloxifene is safer. I use it in my practice primarily for women younger than age 65 who are not yet at high risk for hip fracture but are still concerned about breast cancer. Although this concern diminishes as women age without having developed breast cancer, for younger women, who may see their friends getting breast cancer, it is a major concern. So if a patient is a good candidate for a bone loss agent and also has concern about breast cancer, raloxifene can be a good option, especially since we do not know what the implications are of taking bisphosphonates for 30 years. Questions about that are starting to be raised, so I think it is good to consider a sequential approach for some of these patients. A sequential approach might involve use of HT in a symptomatic menopausal woman, followed by use of raloxifene after the woman no longer has menopausal symptoms but is concerned about spine fracture protection and breast cancer risk reduction, followed by bisphosphonate use as she gets older and is at increased risk for stroke/VTE and for hip fracture.
The challenge of educating younger doctors about HT
Dr. Thacker: I think we need to find ways to translate the data on HT to younger generations of physicians, because the closer one is to graduating from medical school, the less likely he or she is to offer HT to an otherwise healthy, severely symptomatic woman younger than age 60.
Dr. McKenzie: Absolutely, and I think the real challenge is to reach younger physicians who go into private practice, who generally have the fewest opportunities to stay on top of the latest evidence. We must offer evidence-based education programs on this topic to physicians in the community to ensure that they are equipped to understand and explain the real risks and benefits of HT in order to individualize treatment decisions.
As a physician at a tertiary care center, I am surprised at the number of women referred to me who should have already been on HT for menopausal symptoms, but their physicians were unduly influenced by the initial WHI publication. They need to thoroughly evaluate their patients, assess their risks, assess any new medical problems, try to educate them, and then tailor therapy to improve their patients’ quality of life.
Correcting misperceptions: WHI was not a treatment trial
Dr. Thacker: I believe that many practitioners and especially students do not realize that the WHI was a trial designed to assess prevention of chronic diseases. It was not a menopausal treatment trial, and often its data are being misapplied to women who are different from the ones enrolled in the WHI, in that they are younger and more symptomatic.
Dr. Gass: It is correct that the WHI was not a treatment trial, but that was how HT was being used by some physicians and patients prior to the WHI. Physicians in this country were giving some 65-year-old women HT for osteoporosis and dementia. These practices needed to be supported with data, and that was the impetus behind the trial. Along the same lines, it is important how we present the risks to patients. If HT is being used as a therapy for a woman suffering from menopausal symptoms, she might be willing to accept more risk than if it is being used like a vitamin pill, to promote general health, in which case the risks should be virtually nil because the woman is healthy and without complaints.
Dr. Thacker: Yes, and that is why I think the earlier discussion of comparable risks of breast cancer, stroke, and VTE with aspirin, SERMs, fibric acid derivatives, and statins helps to put the risks of HT in perspective. It appears that physicians and patients tolerate very similar risks with commonly used nonhormonal medicines in women but do not tolerate any risks with HT, even in symptomatic women. In my opinion, this is a medical travesty. It is important to recognize that there are few absolutes in medicine that apply to all patients. The only universal recommendations I make to all patients are to wear seatbelts and not to smoke.
Dr. Hodis: What I find notable is that with HT we see a reduction in mortality regardless of the risks that we have described. As the observational data show, if we start HT and continue it, there is a reduction in mortality of 30% or even greater, and the clinical trial data tend to support this benefit. So why do we shy away from HT? Because we are worried about a small increase in breast cancer diagnoses or a small increase in DVT? That is an issue I am grappling with.
Dr. Thacker: Similarly, how do you reconcile the observational data with aspirin? In the Nurses’ Health Study, the aspirin users had lower mortality, but in all of the randomized controlled trials in midlife women, we do not see a reduction in cardiovascular risk with aspirin, let alone a reduction in mortality. So, the people who self-select for treatment are obviously different from those enrolled in randomized trials. The randomized controlled trial may be our gold standard, but it is not necessarily the only evidence to consider.
Dr. Hodis: But there is a concordance between observational studies and randomized trials with respect to overall mortality and HT. The data from a meta-analysis of 30 randomized trials8 are consistent with the data from observational studies, even though they do identify risks. I wonder how many more women would select HT if we told them about the 30% reduction in mortality despite the possibility of breast cancer diagnosis and DVT risks.
Dr. Gass: Women will select according to their own agenda.
Dr. Hodis: Yes, in the end, it is all individualized.
Dr. Gass: Indeed, because women have specific concerns, such as breast cancer, fracture risk, or Alzheimer disease, and they base their personal decisions on these specific concerns. I educate them about the risks and benefits, and they pretty much decide for themselves. They know their priorities.
Age and the risk-benefit assessment with HT
Dr. Thacker: Does the panel have any comments on the recent position statement from the American Association of Clinical Endocrinologists concluding that the benefits of HT exceed the risks in symptomatic women younger than age 60?
Dr. Hodis: My only comment is to ask why it took them so long to come to that conclusion.
Dr. Thacker: We could say the same for the North American Menopause Society (NAMS). It was not until its 2007 position statement on the use of ET and EPT in perimenopausal and postmenopausal women that NAMS moved beyond the issue of a time limit for HT—the “lowest dose for the shortest amount of time” mantra—and recommended the type of reevaluation used with any other treatment.
It seems as if practitioners are less willing to tolerate the risks of HT in older women than to tolerate the risks of hormonal contraceptives in younger women. Perhaps that is because hormonal contraceptives prevent pregnancy and the risks associated with it, yet this same value is not afforded to the symptoms and other effects suffered by postmenopausal women. I think we did afford HT similar value as hormonal contraceptives in the 1980s and 1990s, when we were trying to promote the potential health benefits of HT before we had bisphosphonates and statins and before we realized the risks of VTE with HT, risks that were identified much sooner with hormonal contraceptives. Since then the medical community has overcorrected by too often dismissing HT, overemphasizing the risks and ignoring very important quality-of-life issues, including sex and sleep disturbances, in the process.
Dr. Jenkins: Also, too many people associate menopause only with hot flashes, without taking into account the increased risk of serious diseases that may occur at this time, such as osteoporosis and heart disease.
Dr. Thacker: That may be because menopause is a normal event. It can be a great time of life for many women; in fact, it is associated with lower rates of depression, unless there is a prolonged symptomatic perimenopause. Menopause is certainly not a disease, and NAMS has been very good at recognizing and promoting it as a normal phase of life. But to neglect treating a woman going through menopausal symptoms just because menopause is a normal life event would be akin to withholding assistance for women during childbirth, which is another natural event.
We fail from a medical perspective if we do not take care of symptomatic women, because they will then turn to people who are not physicians and who offer unregulated therapies. These people may deliver the right message—that menopausal women deserve to feel well and look good—but the way they tell women to treat menopausal symptoms is not science-based.
At one time, we were overtreating women and not individualizing therapy, but to me it is even more worrisome to withhold therapies unless women are so highly symptomatic that they consider ending their life. We are continuing to discover the risks and benefits of HT and how to further tailor it. We have many newer, lower-dose HT options, and we are expecting the first nonhormonal therapy for menopausal vasomotor symptoms, desvenlafaxine. We are fortunate to have bone agents and local vaginal therapies for women without vasomotor symptoms. With both hormonal and nonhormonal options, we must keep the risks and benefits of any therapy in perspective.

CONCLUSIONS
Dr. Thacker: This has been a great discussion, and although we do not all agree on every point, I would like to conclude by summarizing some key points on which I think we do all agree (see sidebar above). Menopause is a normal life event, but for some women who are symptomatic, and for a smaller percentage who will be symptomatic for the rest of their lives, HT is the gold standard, although it does not treat all symptoms and has some well-defined risks. We do have other options on the horizon for relief of vasomotor symptoms, for bone health, and for urogenital atrophy.
Following the data on the effects of HT on cardiovascular health will be particularly interesting. Although there is not support for using HT specifically for cardiovascular prevention, there are provocative data that in the symptomatic woman who has self-selected it, HT has cardiovascular benefit and reduces the risk of diabetes.
A woman on HT who has not had “early harm” does not need to arbitrarily discontinue therapy based on any time limit, as long as she is being periodically reevaluated and is offered individualized options.
- Utian WH, Shoupe D, Backmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 2001; 75:1065–1079.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Davis S, Studd J, Bouchard C, Kroll R, Moufarege A, Von Schoultz B. The effect of a testosterone transdermal system on hypoactive sexual desire disorder in postmenopausal women not receiving systemic estrogen therapy, the APHRODITE study. Abstract presented at: 86th Annual Meeting of the Endocrine Society; June 16–19, 2004; New Orleans, LA.
- Speroff L, Gass M, Constantine G, Olivier S; Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Høibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrøm E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy: results of the randomized, double-blind, placebo-controlled Estrogen in Venous Thromboembolism Trial (EVTET). Thromb Haemost 2000; 84:961–967.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003; 13:S78–S86.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.
- Utian WH, Shoupe D, Backmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 2001; 75:1065–1079.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Davis S, Studd J, Bouchard C, Kroll R, Moufarege A, Von Schoultz B. The effect of a testosterone transdermal system on hypoactive sexual desire disorder in postmenopausal women not receiving systemic estrogen therapy, the APHRODITE study. Abstract presented at: 86th Annual Meeting of the Endocrine Society; June 16–19, 2004; New Orleans, LA.
- Speroff L, Gass M, Constantine G, Olivier S; Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2008; 111:77–87.
- Høibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrøm E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy: results of the randomized, double-blind, placebo-controlled Estrogen in Venous Thromboembolism Trial (EVTET). Thromb Haemost 2000; 84:961–967.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003; 13:S78–S86.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.