User login
Physicians and their women patients have faced a continuous, confusing mix of information about the risks and benefits of hormone therapy (HT) for perimenopausal and postmenopausal women, most of it without respect to age or the timing of HT relative to menopause. Initial data from the Women’s Health Initiative (WHI) estrogen + progestin (E+P) trial, a prevention study conducted predominantly in older postmenopausal women without menopausal symptoms,1 resulted in questioning of the role of HT (unfortunately and inappropriately in younger symptomatic women). Cumulative trial data and further analyses of the WHI have refined and added to our understanding of the effects of HT, particularly with regard to cardiovascular health. This review will update physicians on the latest data on the risks and benefits of HT, with a particular focus on the heart, and will put the risks of HT into appropriate clinical context.
HORMONE THERAPY AND CARDIOVASCULAR DISEASE: A HISTORICAL PERSPECTIVE
Observational studies conducted prior to the WHI found consistently that women who self-selected to use HT had a reduction in mortality and in the incidence of cardiovascular disease relative to women who did not choose to use HT.2–8 This reduction in risk was apparent whether the HT users had taken ET (estrogen therapy) or EPT (estrogen-progestogen therapy). In contrast, randomized controlled trials failed to confirm these findings from observational studies. However, the findings from randomized controlled trials were derived from older postmenopausal women who were many years past menopause. Often overlooked is the WHI observational study of ET and EPT,9,10 in which women who chose to use HT had a reduction in the risk of coronary heart disease (CHD) similar to that observed among the HT users in other observational studies.
RECENT REPORTS FROM THE WHI
Since the original publication of the WHI E+P trial in 2002,1 an extensive collection of data have been published in piecemeal fashion, contributing to the confusion and misperception of the effects of HT on risks and benefits. It is important to note that the WHI consists of both randomized and observational components, as detailed below, and that data have come from both. Together, these data help clarify the misperceptions generated from the first WHI report of 2002,1 particularly misperceptions regarding the timing of HT initiation relative to menopause and the effect of HT duration on cardiovascular disease outcomes. In most instances, the conclusions drawn from this recent research run counter to the inaccurate but prevalent perception that HT use at any time and at any age is associated with cardiovascular harm, a perception that has unfortunately prevailed since the initial publication of the WHI E+P trial findings in 2002.
The WHI randomized trials and combined analysis
The WHI trials enrolled 27,347 postmenopausal women aged 50 to 79 years at baseline; almost two-thirds of the women enrolled were 60 years of age or older, and the majority of women were more than 10 years past menopause. WHI actually comprised two parallel randomized trials:
- One among 16,608 women who had not undergone hysterectomy (ie, with uterus intact), who were randomized to EPT or placebo (ie, WHI E+P trial) 1
- One among 10,739 women who had undergone hysterectomy, who were randomized to ET or placebo.11
Recent analyses from the WHI, published in 2007, assessed the cardiovascular effects of ET and EPT independently and combined, both overall and according to subject age and years since menopause when randomized.12 Other analyses following the initial WHI E+P trial publication have analyzed the effects of HT according to duration of HT use and according to secondary end points. Many of these analyses have presented risks and benefits in terms of both nominal and adjusted confidence intervals (CIs). Nominal 95% CIs describe the variability in risk estimates that would arise from a simple trial for a single end point. Although nominal CIs are traditionally used, they do not take into account the multiple statistical testing issues (across time and across outcome categories) that occur in a trial. In contrast, adjusted 95% CIs correct for these stastical testing issues. From a clinical perspective, it is most appropriate to look at the adjusted CIs.
WHI: EPT vs placebo
CHD. Although the point estimate for CHD is increased, the 95% CI indicates that EPT has a non-significant effect on CHD outcome relative to placebo among all women randomized in the WHI E+P trial (mean age, 63 years).12 This is a very important point for cardiologists and primary care physicians to note.
In a 2008 analysis of the WHI E+P trial that included a 2.4-year open-label follow-up subsequent to the randomized trial,19 the randomized trial data reported were again different than in previous reports, but remained nonsignificant. The hazard ratio (HR) reported for CHD in the 2008 analysis for the randomized portion of the trial was 1.22 (95% CI, 0.99 to 1.51), as compared with 1.23 (95% CI, 0.99 to 1.53) reported in 200712 (Table 1), 1.24 (95% CI, 1.00 to 1.54) reported in 2003,20 and 1.29 (95% CI, 1.02 to 1.63) reported in 2002.1 In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens (EPT or placebo), the HR for CHD between the original randomized treatment groups was nonsignificant at 0.95 (95% CI, 0.73 to 1.26) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Stroke. The risk of stroke was increased significantly (by an additional 8 events per 10,000 women treated per year) in the EPT arm versus the placebo arm in the nominal analysis,12 but this difference was nonsignificant after adjustment.13
In the 2008 WHI E+P analysis, the HR reported for stroke during the randomized trial phase was different than in previous reports—1.34 (95% CI, 1.05 to 1.71)19 versus 1.31 (95% CI, 1.03 to 1.68) reported in 200712 (Table 1)—and the adjusted analysis was not reported. In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens, the HR for stroke between the original randomized treatment groups was nonsignifi-cant at 1.16 (95% CI, 0.83 to 1.61) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Breast cancer. Breast cancer risk was originally reported to be increased significantly (by an additional 8 cases per 10,000 women per year) in the EPT arm versus the placebo arm with the nominal statistic, but this increase was nonsignificant after adjustment.1 This risk estimate was revised in a follow-up publication 1 year after the original data were reported, and the increase in risk in the EPT arm was still no longer significant in the adjusted analysis.21 Importantly, another subsequent analysis that adjusted for baseline risk factors for breast cancer resulted in a further revision of the risk estimate, which again showed a nonsignificant increase in the EPT arm relative to the placebo arm.14 This is very important since it is commonly accepted that EPT increases the risk of breast cancer when this has not been definitively proven in any randomized controlled trial.
Unfortunately, the most recent breast cancer data14 (Table 1) were not used in the 2008 WHI E+P analysis.19 However, even using the unadjusted data in the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR for breast cancer between the original randomized treatment groups was nonsignificant and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Venous thromboembolism (VTE). EPT was associated with a doubling of the risk of VTE (ie, deep vein thrombosis and pulmonary embolism) compared with placebo, resulting in an excess of 18 VTE events per 10,000 women per year of therapy.15 The risk of VTE was significant across the entire cohort of women (mean age, 63 years).
In the 2008 WHI E+P analysis, the HR reported for VTE during the randomized phase was different than in previous reports—1.98 (95% CI, 1.52 to 2.59)19 versus 2.06 (95% CI, 1.57 to 2.70) reported in 200415 (Table 1)—and the HR during the 2.4-year open-label follow-up, in which women were no longer on their randomized regimens, was no longer significant (HR = 0.95; 95% CI, 0.63 to 1.44). This change in the HR over time from the randomized phase to the open-label phase was statistically significant.19
Fracture. The risk of hip fracture was reduced by 33% with EPT relative to placebo, which was statistically significant in the nominal analysis but not in the adjusted analysis.17 The risk of any fracture was reduced by 24% in women randomized to EPT compared with placebo, which was statistically significant and translated to 47 fewer fractures per 10,000 women per year of therapy.17 Clinically, these results are most impressive given that women randomized in the WHI were not selected on the basis of risk for osteoporosis or fracture. This claim cannot be made for any other therapy.
In the 2008 WHI E+P analysis of the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR between the original randomized treatment groups was nonsignificant for hip fracture and any fracture—0.92 (95% CI, 0.64 to 1.34) and 0.91 (95% CI, 0.78 to 1.06), respectively—and the change in the HR over time from the randomized phase to the open-label phase was not significant for either fracture outcome.19
Diabetes. The risk of new-onset diabetes was reduced by a statistically significant 21% in women randomized to EPT compared with placebo.18
WHI: ET vs placebo
CHD. Importantly, no significant difference was found between the ET and placebo arms with respect to CHD events in the overall cohort of women, whose average age was 64 years.12
Stroke. The risk of stroke was greater with ET than with placebo in the nominal analysis, but importantly, the difference in event rates (11 per 10,000 women per year of therapy) failed to reach significance in the adjusted analysis.11,12
Breast cancer. A strong but nonsignificant trend toward a reduction in breast cancer risk was apparent in the ET arm (8 fewer breast cancer cases per 10,000 women per year of therapy). Among women who actually were adherent to their study regimen (ie, consuming ≥ 80% of their study medication), there was a statistically significant 33% reduction in breast cancer risk with ET relative to placebo.22 Importantly, the reduction in breast cancer risk relative to placebo was found across all the age ranges studied.11
VTE. The excess risk of VTE with ET versus placebo (32%) was less than the excess risk of VTE associated with EPT (Table 1). Importantly, the risk of VTE associated with ET was not statistically significant.11,23
Fracture. The risk of any fracture (hip or vertebral) was reduced significantly in the ET arm compared with the placebo arm.11
Diabetes. In a nominal analysis, there was a trend toward a reduction in the risk of new-onset diabetes in women randomized to ET relative to placebo, which nearly achieved statistical significance.24
WHAT EXPLAINS THE DISCORDANCE BETWEEN OBSERVATIONAL AND RANDOMIZED TRIALS OF HT?
How can the perceived discordance between the results from observational studies and those from randomized controlled trials be explained? There are currently three hypotheses:
- The populations differ in the two types of study designs (observational studies and randomized controlled trials)
- The duration of HT use differs
- The timing of HT initiation differs in relation to age, time since menopause, and stage of atherosclerosis.
Population characteristics
One obvious difference between randomized trials and observational studies of HT is the presence of menopausal symptoms. To maintain blinding, women with hot flashes were predominantly excluded from randomized trials of HT, whereas the presence of hot flashes is the predominant menopausal symptom of women included in observational studies and the main reason women seek HT from their providers.
Other consistent and possibly explanatory differences between clinical trials and observational studies of HT are patient age at enrollment, years since menopause, and body mass index (BMI). Comparing randomized controlled trials with observational studies, age at enrollment was much higher in the clinical trials (mean age ≥ 63 years) than in the observational studies (range of 30 to 55 years). Similarly, women enrolled in randomized trials were more than 10 years beyond menopause, whereas those in observational studies were less than 5 years beyond menopause. In fact, more than 80% of HT users in observational studies initiated HT within 1 or 2 years of menopause.
Additionally, women in randomized trials of HT tend to have higher BMIs than their counterparts in observational studies. For example, mean BMI was considerably higher in the WHI randomized trials (28.5 kg/m2 and 30.1 kg/m2)1,11 than in the observational Nurses’ Health Study (25.8 kg/m2),25 and a full third (34%) of women in the WHI randomized trials were severely obese (BMI ≥ 30 kg/m2).
This point about BMI is noteworthy in light of findings on the effect of BMI on breast cancer risk from an analysis of the WHI observational study among 85,917 women aged 50 to 79 years old at enrollment.26 This analysis found that BMI was unrelated to breast cancer risk among women who had used HT; however, among nonusers of HT, a baseline BMI greater than 31.1 kg/m2 was associated with a 2.52 relative risk of breast cancer compared with a baseline BMI less than 22.6 kg/m2. The risk of breast cancer with increasing BMI was most pronounced in younger postmenopausal women. One interpretation is that high endogenous estrogen levels in postmenopausal women with an elevated BMI serve to increase breast cancer risk to a level beyond which HT adds no further risk. Alternatively, conjugated equine estrogens may act through a selective estrogen receptor modulator mechanism to block any potential adverse breast tissue effects of elevated endogenous estrone and estradiol levels.
Duration of HT
Another hypothesis for the discordant findings between observational and randomized studies of HT focuses on differences in duration of HT use between the two types of studies. Duration of HT use has been substantially longer in observational studies, ranging from 10 years to 40 years, compared with no more than 7 or 8 years in randomized trials to date. Moreover, the results from observational studies have suggested that the longer the duration of HT use, the greater the benefit in terms of CHD risk.
For example, a case-control study by Chilvers et al showed that HT protected against nonfatal myocardial infarction only when used for more than 60 months.27 Analysis of data on EPT use from the Heart and Estrogen/progestin Replacement Study (HERS),28 the randomized WHI E+P trial,20 and the WHI observational study9 reveals an interesting and consistent trend: rates of CHD events increased during the first year of EPT therapy (compared with placebo or nonuse) but then declined over time, ending up below the rates of CHD events in placebo recipients or nonusers of HT after approximately 5 years of therapy. This trend was not as pronounced with ET use in either the WHI randomized trial29 or the WHI observational study,10 but the incidence of CHD events did decline over time with ET use in both studies, and in the WHI observational study, greater than 5 years of ET use was associated with a significant 27% reduction (HR, 0.73; 95% CI, 0.61 to 0.84) in CHD events compared with nonuse.10
Timing of HT initiation
A third hypothesis for the discordance between randomized and observational studies of HT concerns the timing of HT initiation relative to patient age, time since menopause, and stage of atherosclerosis. As noted above, women enrolled in randomized trials have tended to be considerably older and further from menopause compared with their counterparts in observational studies. The effects of differences in timing of HT initiation on specific clinical end points, particularly in relation to cardiovascular health, are reviewed below.
Early and continued HT use may interrupt the pathogenic sequence of vascular aging, potentially preventing progression of atherosclerosis to the late stage at which plaque rupture and clinical events occur. In contrast, late intervention with HT may have little effect on established plaque and, in the case of EPT, may actually predispose to plaque rupture. This concept has been demonstrated in a pair of sister studies, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELLHART)31 and the Estrogen Prevention Atherosclerosis Trial (EPAT).32
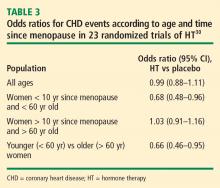
Mortality. The same Stanford University researchers who performed the above meta-analysis of CHD events also assessed odd ratios for overall mortality in a meta-analysis of 30 randomized trials of HT versus placebo that included a total of 26,708 postmenopausal women (representing 119,118 patient-years).33 They found the timing of HT initiation to have an effect on mortality similar to its effect on CHD events. In the overall cohort of women, the odds of death were not different between the HT and placebo groups, but among women younger than 60 years (mean, 54 years), those randomized to HT had a significant 39% reduction in the risk of death (HR = 0.61; 95% CI, 0.39 to 0.95) compared with those randomized to placebo. HT had no effect on mortality among women older than 60 years (mean, 66 years) in this analysis.
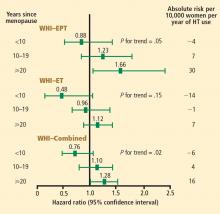
These mortality data are consistent with those from the WHI randomized trials, in which both EPT and ET were associated with a 30% reduction in overall mortality relative to placebo among women 50 to 59 years old.12 When both the EPT and ET portions of the WHI were combined to increase the sample size, HT was associated with a significant 30% reduction in mortality (HR = 0.70; 95% CI, 0.51 to 0.96) compared with placebo among women 50 to 59 years old.12
Stroke. The most recent WHI data indicate that stroke is not increased with ET in women 50 to 59 years of age, as there were 2 fewer events per 10,000 women per year of ET relative to placebo.12 In this same age group, the risk of stroke from EPT was increased by 5 events per 10,000 women per year of therapy relative to placebo.12 Among women randomized within 5 years of menopause, stroke risk was increased by 3 events per 10,000 women per year of EPT relative to placebo.13
VTE. Although age was not a significant contributing factor to the risk of VTE from HT in the WHI, the absolute risk of VTE was lower in younger versus older women. The additional absolute risk for VTE events per 10,000 women per year of EPT use was 11 events for women 50 to 59 years old at randomization, 16 events for women 60 to 69 years old at randomization, and 35 events for women 70 to 79 years old at randomization.15 The additional absolute risk for VTE events per 10,000 women per year of ET use was 4 events for women 50 to 59 years old at randomization, 7 events for women 60 to 69 years old at randomization, and 11 events for women 70 to 79 years old at randomization.23 A history of a prior thromboembolic event increases the risk of VTE with postmenopausal HT use, which should be considered before HT is initiated.
HORMONE THERAPY IN CLINICAL PERSPECTIVE
Comparative effects of lipid-lowering therapy and HT
Examination of the evidence regarding lipid-lowering therapy for prevention of CHD, as well as its effects on breast cancer risk and coronary artery calcium scores in women, can add some much-needed perspective and context to the HT data reviewed above.
CHD prevention. Walsh and Pignone examined six randomized controlled trials (N = 11,435) of primary prevention with lipid-lowering medication in women and found no significant effect on CHD events, nonfatal myocardial infarction, CHD mortality, or total mortality.34 In eight randomized controlled trials of secondary prevention (N = 8,272), lipid-lowering therapy in women resulted in significant reductions in CHD end points but had no effect on total mortality.34
Atherosclerosis progression. In three randomized controlled trials, 1 to 4 years of statin therapy had no effect on the progression of coronary artery calcium compared with placebo.41–43 Among 1,064 women 50 to 59 years old who participated in a WHI substudy called the WHI Coronary Artery Calcium Study (WHI-CACS), those who were randomized to ET had significantly less coronary artery calcium at year 7 compared with those assigned to placebo.44 The mean Agatston coronary artery calcium scores were 83.1 with ET versus 123.1 with placebo (P = .02).
Comparing risk of HT with risk of other therapies
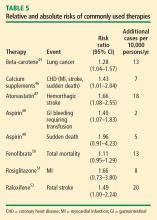
Other comparisons yield similar conclusions.35 For instance, a comparison of the ET arm of the WHI randomized trial with raloxifene in the Raloxifene Use for the Heart (RUTH) trial52 reveals similar effects on CHD, stroke, VTE, pulmonary embolism, deep vein thrombosis, and breast cancer, with the only large difference being a greater reduction in bone fracture risk with ET compared with raloxifene.35 Finally, in putting the risk of VTE with HT into perspective, consider that the risk of thromboembolic events with selective estrogen receptor modulators52 and with the fibric acid derivative fenofibrate in diabetics50 is of similar magnitude as the risk with HT.
Risk must be viewed in light of age at HT initiation
CONCLUSIONS FROM RANDOMIZED TRIALS
HT’s effects on CHD and mortality: Timing is everything
Cumulative data from randomized trials indicate that in the overall population of women studied, HT, aspirin, and lipid-lowering therapy each have a null effect on the incidence of CHD and mortality. However, within this overall null effect, early initiation of HT (in terms of time from menopause [< 10 years] and age at initiation [< 60 years]) is associated with reductions in total mortality and CHD incidence. Additionally, a duration of HT use beyond 5 years is associated with a reduction in the incidence of CHD.9,10,20,28,29 These effects of the timing and duration of therapy are unique to HT. Unopposed ET may have an advantageous profile relative to EPT for reducing the incidence of CHD and mortality in postmenopausal women.
Gaining perspective on risks
The risks of stroke and VTE associated with HT are low for women overall and lower still for women who are within 10 years of menopause or younger than age 60 when they start HT. With respect to stroke, fewer cases of stroke developed in users of ET compared with placebo in women who started HT before age 60. The risks of EPT are comparable to those of other medications commonly used in this population of women. More broadly, the magnitude and types of risk associated with HT are similar to those associated with other commonly used therapies.
In addition, the underappreciated benefits of HT, such as potential prevention of diabetes mellitus (15 fewer cases of incident diabetes per 10,000 women per year with EPT and 14 fewer cases with ET), need to be recognized and discussed with our patients. These data are consistent in both observational studies and randomized trials.
The bottom line
As data from randomized trials of HT accumulate, the results are clearly similar to those from the more than 20 observational studies indicating that young, symptomatic postmenopausal women who use HT for long periods have lower rates of CHD and total mortality compared with postmenopausal women who do not use HT. Consistent with these data, the American Association of Clinical Endocrinologists issued a position statement in 2008 concluding that for symptomatic menopausal women under the age of 60, the benefits of HT exceed the risks.53
Nevertheless, the bottom line remains that the estrogen-cardioprotective hypothesis has yet to be studied, since randomized trials have not been conducted in the same population of women from which the hypothesis was generated. This hypothesis will be directly evaluated, however, in the ongoing Early versus Late Intervention Trial with Estradiol (ELITE). This randomized trial, funded by the National Institute on Aging, is designed to determine the effects of 17β-estradiol on the progression of atherosclerosis, cognition, and other postmenopausal health issues in recently menopausal (< 6 years) and remotely menopausal (≥ 10 years) women with no history of cardiovascular disease or diabetes. Until data from trials like ELITE emerge, guidelines such as those from the North American Menopause Society54 (reviewed in the next article in this supplement) are reasonable for clinical practice.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Rosenberg L, Palmer JR, Shapiro S. A case-control study of myocardial infarction in relation to use of estrogen supplements. Am J Epidemiol 1993; 137:54–63.
- Mann RD, Lis Y, Chukwujindu J, Chanter DO. A study of the association between hormone replacement therapy, smoking and the occurrence of myocardial infarction in women. J Clin Epidemiol 1994; 47:307–312.
- Psaty B, Heckbert SR, Atkins D, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med 1994; 154:1333–1339.
- Sidney S, Petitti DB, Quisenberry CP Jr. Myocardial infarction and the use of estrogen and estrogen-progestogen in postmenopausal women. Ann Intern Med 1997; 127:501–508.
- Grodstein F, Stampfer MJ, Falkeborn M, Naessen T, Persson I. Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology 1999; 10:476–480.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Varas-Lorenzo C, García-Rodríguez LA, Perez-Gutthann S, Duque-Oliart A. Hormone replacement therapy and incidence of acute myocardial infarction. A population-based nested case-control study. Circulation 2000; 101:2572–2578.
- Prentice RL, Langer R, Stefanick ML, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol 2005; 162:404–414.
- Prentice RL, Langer RD, Stefanick ML, et al. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol 2006; 163:589–599.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 2003; 289:2673–2684.
- Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006; 55:103–115.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292:1573–1580.
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004; 350:991–1004.
- Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004; 47:1175–1187.
- Heiss G, Wallace R, Anderson GL, et al, for the WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008; 299:1036–1045.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA 2003; 289:3243–3253.
- Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in post-menopausal women with hysterectomy. JAMA 2006; 295:1647–1657.
- Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med 2006; 166:772–780.
- Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 2006; 49:459–468.
- Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 1996; 335:453–461.
- Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 2002; 13:741–751.
- Chilvers CE, Knibb RC, Armstrong SJ, Woods KL, Logan RF. Postmenopausal hormone replacement therapy and risk of acute myocardial infarction—a case control study of women in the East Midlands, UK. Eur Heart J 2003; 24:2197–2205.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med 2006; 166:357–365.
- Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Hodis HN, Mack WJ, Lobo RA, et al. Hormone therapy and progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med 2003; 349:535–545.
- Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.
- Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004; 291:2243–2252.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006; 295:74–80.
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005; 23:8606–8612.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–1278.
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006; 113:427–437.
- Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005; 112:563–571.
- Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005; 46:166–172.
- Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007; 356:2591–2602.
- Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996; 334:1150–1155.
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336:262–266.
- Amarenco P, Bogousslavsky J, Callahan A III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglita-zone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: randomised controlled trial. Lancet 2006; 368:1096–1105.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- American Association of Clinical Endocrinologists (AACE) position statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause 2007; 14:168–182.
Physicians and their women patients have faced a continuous, confusing mix of information about the risks and benefits of hormone therapy (HT) for perimenopausal and postmenopausal women, most of it without respect to age or the timing of HT relative to menopause. Initial data from the Women’s Health Initiative (WHI) estrogen + progestin (E+P) trial, a prevention study conducted predominantly in older postmenopausal women without menopausal symptoms,1 resulted in questioning of the role of HT (unfortunately and inappropriately in younger symptomatic women). Cumulative trial data and further analyses of the WHI have refined and added to our understanding of the effects of HT, particularly with regard to cardiovascular health. This review will update physicians on the latest data on the risks and benefits of HT, with a particular focus on the heart, and will put the risks of HT into appropriate clinical context.
HORMONE THERAPY AND CARDIOVASCULAR DISEASE: A HISTORICAL PERSPECTIVE
Observational studies conducted prior to the WHI found consistently that women who self-selected to use HT had a reduction in mortality and in the incidence of cardiovascular disease relative to women who did not choose to use HT.2–8 This reduction in risk was apparent whether the HT users had taken ET (estrogen therapy) or EPT (estrogen-progestogen therapy). In contrast, randomized controlled trials failed to confirm these findings from observational studies. However, the findings from randomized controlled trials were derived from older postmenopausal women who were many years past menopause. Often overlooked is the WHI observational study of ET and EPT,9,10 in which women who chose to use HT had a reduction in the risk of coronary heart disease (CHD) similar to that observed among the HT users in other observational studies.
RECENT REPORTS FROM THE WHI
Since the original publication of the WHI E+P trial in 2002,1 an extensive collection of data have been published in piecemeal fashion, contributing to the confusion and misperception of the effects of HT on risks and benefits. It is important to note that the WHI consists of both randomized and observational components, as detailed below, and that data have come from both. Together, these data help clarify the misperceptions generated from the first WHI report of 2002,1 particularly misperceptions regarding the timing of HT initiation relative to menopause and the effect of HT duration on cardiovascular disease outcomes. In most instances, the conclusions drawn from this recent research run counter to the inaccurate but prevalent perception that HT use at any time and at any age is associated with cardiovascular harm, a perception that has unfortunately prevailed since the initial publication of the WHI E+P trial findings in 2002.
The WHI randomized trials and combined analysis
The WHI trials enrolled 27,347 postmenopausal women aged 50 to 79 years at baseline; almost two-thirds of the women enrolled were 60 years of age or older, and the majority of women were more than 10 years past menopause. WHI actually comprised two parallel randomized trials:
- One among 16,608 women who had not undergone hysterectomy (ie, with uterus intact), who were randomized to EPT or placebo (ie, WHI E+P trial) 1
- One among 10,739 women who had undergone hysterectomy, who were randomized to ET or placebo.11
Recent analyses from the WHI, published in 2007, assessed the cardiovascular effects of ET and EPT independently and combined, both overall and according to subject age and years since menopause when randomized.12 Other analyses following the initial WHI E+P trial publication have analyzed the effects of HT according to duration of HT use and according to secondary end points. Many of these analyses have presented risks and benefits in terms of both nominal and adjusted confidence intervals (CIs). Nominal 95% CIs describe the variability in risk estimates that would arise from a simple trial for a single end point. Although nominal CIs are traditionally used, they do not take into account the multiple statistical testing issues (across time and across outcome categories) that occur in a trial. In contrast, adjusted 95% CIs correct for these stastical testing issues. From a clinical perspective, it is most appropriate to look at the adjusted CIs.
WHI: EPT vs placebo
CHD. Although the point estimate for CHD is increased, the 95% CI indicates that EPT has a non-significant effect on CHD outcome relative to placebo among all women randomized in the WHI E+P trial (mean age, 63 years).12 This is a very important point for cardiologists and primary care physicians to note.
In a 2008 analysis of the WHI E+P trial that included a 2.4-year open-label follow-up subsequent to the randomized trial,19 the randomized trial data reported were again different than in previous reports, but remained nonsignificant. The hazard ratio (HR) reported for CHD in the 2008 analysis for the randomized portion of the trial was 1.22 (95% CI, 0.99 to 1.51), as compared with 1.23 (95% CI, 0.99 to 1.53) reported in 200712 (Table 1), 1.24 (95% CI, 1.00 to 1.54) reported in 2003,20 and 1.29 (95% CI, 1.02 to 1.63) reported in 2002.1 In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens (EPT or placebo), the HR for CHD between the original randomized treatment groups was nonsignificant at 0.95 (95% CI, 0.73 to 1.26) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Stroke. The risk of stroke was increased significantly (by an additional 8 events per 10,000 women treated per year) in the EPT arm versus the placebo arm in the nominal analysis,12 but this difference was nonsignificant after adjustment.13
In the 2008 WHI E+P analysis, the HR reported for stroke during the randomized trial phase was different than in previous reports—1.34 (95% CI, 1.05 to 1.71)19 versus 1.31 (95% CI, 1.03 to 1.68) reported in 200712 (Table 1)—and the adjusted analysis was not reported. In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens, the HR for stroke between the original randomized treatment groups was nonsignifi-cant at 1.16 (95% CI, 0.83 to 1.61) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Breast cancer. Breast cancer risk was originally reported to be increased significantly (by an additional 8 cases per 10,000 women per year) in the EPT arm versus the placebo arm with the nominal statistic, but this increase was nonsignificant after adjustment.1 This risk estimate was revised in a follow-up publication 1 year after the original data were reported, and the increase in risk in the EPT arm was still no longer significant in the adjusted analysis.21 Importantly, another subsequent analysis that adjusted for baseline risk factors for breast cancer resulted in a further revision of the risk estimate, which again showed a nonsignificant increase in the EPT arm relative to the placebo arm.14 This is very important since it is commonly accepted that EPT increases the risk of breast cancer when this has not been definitively proven in any randomized controlled trial.
Unfortunately, the most recent breast cancer data14 (Table 1) were not used in the 2008 WHI E+P analysis.19 However, even using the unadjusted data in the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR for breast cancer between the original randomized treatment groups was nonsignificant and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Venous thromboembolism (VTE). EPT was associated with a doubling of the risk of VTE (ie, deep vein thrombosis and pulmonary embolism) compared with placebo, resulting in an excess of 18 VTE events per 10,000 women per year of therapy.15 The risk of VTE was significant across the entire cohort of women (mean age, 63 years).
In the 2008 WHI E+P analysis, the HR reported for VTE during the randomized phase was different than in previous reports—1.98 (95% CI, 1.52 to 2.59)19 versus 2.06 (95% CI, 1.57 to 2.70) reported in 200415 (Table 1)—and the HR during the 2.4-year open-label follow-up, in which women were no longer on their randomized regimens, was no longer significant (HR = 0.95; 95% CI, 0.63 to 1.44). This change in the HR over time from the randomized phase to the open-label phase was statistically significant.19
Fracture. The risk of hip fracture was reduced by 33% with EPT relative to placebo, which was statistically significant in the nominal analysis but not in the adjusted analysis.17 The risk of any fracture was reduced by 24% in women randomized to EPT compared with placebo, which was statistically significant and translated to 47 fewer fractures per 10,000 women per year of therapy.17 Clinically, these results are most impressive given that women randomized in the WHI were not selected on the basis of risk for osteoporosis or fracture. This claim cannot be made for any other therapy.
In the 2008 WHI E+P analysis of the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR between the original randomized treatment groups was nonsignificant for hip fracture and any fracture—0.92 (95% CI, 0.64 to 1.34) and 0.91 (95% CI, 0.78 to 1.06), respectively—and the change in the HR over time from the randomized phase to the open-label phase was not significant for either fracture outcome.19
Diabetes. The risk of new-onset diabetes was reduced by a statistically significant 21% in women randomized to EPT compared with placebo.18
WHI: ET vs placebo
CHD. Importantly, no significant difference was found between the ET and placebo arms with respect to CHD events in the overall cohort of women, whose average age was 64 years.12
Stroke. The risk of stroke was greater with ET than with placebo in the nominal analysis, but importantly, the difference in event rates (11 per 10,000 women per year of therapy) failed to reach significance in the adjusted analysis.11,12
Breast cancer. A strong but nonsignificant trend toward a reduction in breast cancer risk was apparent in the ET arm (8 fewer breast cancer cases per 10,000 women per year of therapy). Among women who actually were adherent to their study regimen (ie, consuming ≥ 80% of their study medication), there was a statistically significant 33% reduction in breast cancer risk with ET relative to placebo.22 Importantly, the reduction in breast cancer risk relative to placebo was found across all the age ranges studied.11
VTE. The excess risk of VTE with ET versus placebo (32%) was less than the excess risk of VTE associated with EPT (Table 1). Importantly, the risk of VTE associated with ET was not statistically significant.11,23
Fracture. The risk of any fracture (hip or vertebral) was reduced significantly in the ET arm compared with the placebo arm.11
Diabetes. In a nominal analysis, there was a trend toward a reduction in the risk of new-onset diabetes in women randomized to ET relative to placebo, which nearly achieved statistical significance.24
WHAT EXPLAINS THE DISCORDANCE BETWEEN OBSERVATIONAL AND RANDOMIZED TRIALS OF HT?
How can the perceived discordance between the results from observational studies and those from randomized controlled trials be explained? There are currently three hypotheses:
- The populations differ in the two types of study designs (observational studies and randomized controlled trials)
- The duration of HT use differs
- The timing of HT initiation differs in relation to age, time since menopause, and stage of atherosclerosis.
Population characteristics
One obvious difference between randomized trials and observational studies of HT is the presence of menopausal symptoms. To maintain blinding, women with hot flashes were predominantly excluded from randomized trials of HT, whereas the presence of hot flashes is the predominant menopausal symptom of women included in observational studies and the main reason women seek HT from their providers.
Other consistent and possibly explanatory differences between clinical trials and observational studies of HT are patient age at enrollment, years since menopause, and body mass index (BMI). Comparing randomized controlled trials with observational studies, age at enrollment was much higher in the clinical trials (mean age ≥ 63 years) than in the observational studies (range of 30 to 55 years). Similarly, women enrolled in randomized trials were more than 10 years beyond menopause, whereas those in observational studies were less than 5 years beyond menopause. In fact, more than 80% of HT users in observational studies initiated HT within 1 or 2 years of menopause.
Additionally, women in randomized trials of HT tend to have higher BMIs than their counterparts in observational studies. For example, mean BMI was considerably higher in the WHI randomized trials (28.5 kg/m2 and 30.1 kg/m2)1,11 than in the observational Nurses’ Health Study (25.8 kg/m2),25 and a full third (34%) of women in the WHI randomized trials were severely obese (BMI ≥ 30 kg/m2).
This point about BMI is noteworthy in light of findings on the effect of BMI on breast cancer risk from an analysis of the WHI observational study among 85,917 women aged 50 to 79 years old at enrollment.26 This analysis found that BMI was unrelated to breast cancer risk among women who had used HT; however, among nonusers of HT, a baseline BMI greater than 31.1 kg/m2 was associated with a 2.52 relative risk of breast cancer compared with a baseline BMI less than 22.6 kg/m2. The risk of breast cancer with increasing BMI was most pronounced in younger postmenopausal women. One interpretation is that high endogenous estrogen levels in postmenopausal women with an elevated BMI serve to increase breast cancer risk to a level beyond which HT adds no further risk. Alternatively, conjugated equine estrogens may act through a selective estrogen receptor modulator mechanism to block any potential adverse breast tissue effects of elevated endogenous estrone and estradiol levels.
Duration of HT
Another hypothesis for the discordant findings between observational and randomized studies of HT focuses on differences in duration of HT use between the two types of studies. Duration of HT use has been substantially longer in observational studies, ranging from 10 years to 40 years, compared with no more than 7 or 8 years in randomized trials to date. Moreover, the results from observational studies have suggested that the longer the duration of HT use, the greater the benefit in terms of CHD risk.
For example, a case-control study by Chilvers et al showed that HT protected against nonfatal myocardial infarction only when used for more than 60 months.27 Analysis of data on EPT use from the Heart and Estrogen/progestin Replacement Study (HERS),28 the randomized WHI E+P trial,20 and the WHI observational study9 reveals an interesting and consistent trend: rates of CHD events increased during the first year of EPT therapy (compared with placebo or nonuse) but then declined over time, ending up below the rates of CHD events in placebo recipients or nonusers of HT after approximately 5 years of therapy. This trend was not as pronounced with ET use in either the WHI randomized trial29 or the WHI observational study,10 but the incidence of CHD events did decline over time with ET use in both studies, and in the WHI observational study, greater than 5 years of ET use was associated with a significant 27% reduction (HR, 0.73; 95% CI, 0.61 to 0.84) in CHD events compared with nonuse.10
Timing of HT initiation
A third hypothesis for the discordance between randomized and observational studies of HT concerns the timing of HT initiation relative to patient age, time since menopause, and stage of atherosclerosis. As noted above, women enrolled in randomized trials have tended to be considerably older and further from menopause compared with their counterparts in observational studies. The effects of differences in timing of HT initiation on specific clinical end points, particularly in relation to cardiovascular health, are reviewed below.
Early and continued HT use may interrupt the pathogenic sequence of vascular aging, potentially preventing progression of atherosclerosis to the late stage at which plaque rupture and clinical events occur. In contrast, late intervention with HT may have little effect on established plaque and, in the case of EPT, may actually predispose to plaque rupture. This concept has been demonstrated in a pair of sister studies, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELLHART)31 and the Estrogen Prevention Atherosclerosis Trial (EPAT).32
Mortality. The same Stanford University researchers who performed the above meta-analysis of CHD events also assessed odd ratios for overall mortality in a meta-analysis of 30 randomized trials of HT versus placebo that included a total of 26,708 postmenopausal women (representing 119,118 patient-years).33 They found the timing of HT initiation to have an effect on mortality similar to its effect on CHD events. In the overall cohort of women, the odds of death were not different between the HT and placebo groups, but among women younger than 60 years (mean, 54 years), those randomized to HT had a significant 39% reduction in the risk of death (HR = 0.61; 95% CI, 0.39 to 0.95) compared with those randomized to placebo. HT had no effect on mortality among women older than 60 years (mean, 66 years) in this analysis.
These mortality data are consistent with those from the WHI randomized trials, in which both EPT and ET were associated with a 30% reduction in overall mortality relative to placebo among women 50 to 59 years old.12 When both the EPT and ET portions of the WHI were combined to increase the sample size, HT was associated with a significant 30% reduction in mortality (HR = 0.70; 95% CI, 0.51 to 0.96) compared with placebo among women 50 to 59 years old.12
Stroke. The most recent WHI data indicate that stroke is not increased with ET in women 50 to 59 years of age, as there were 2 fewer events per 10,000 women per year of ET relative to placebo.12 In this same age group, the risk of stroke from EPT was increased by 5 events per 10,000 women per year of therapy relative to placebo.12 Among women randomized within 5 years of menopause, stroke risk was increased by 3 events per 10,000 women per year of EPT relative to placebo.13
VTE. Although age was not a significant contributing factor to the risk of VTE from HT in the WHI, the absolute risk of VTE was lower in younger versus older women. The additional absolute risk for VTE events per 10,000 women per year of EPT use was 11 events for women 50 to 59 years old at randomization, 16 events for women 60 to 69 years old at randomization, and 35 events for women 70 to 79 years old at randomization.15 The additional absolute risk for VTE events per 10,000 women per year of ET use was 4 events for women 50 to 59 years old at randomization, 7 events for women 60 to 69 years old at randomization, and 11 events for women 70 to 79 years old at randomization.23 A history of a prior thromboembolic event increases the risk of VTE with postmenopausal HT use, which should be considered before HT is initiated.
HORMONE THERAPY IN CLINICAL PERSPECTIVE
Comparative effects of lipid-lowering therapy and HT
Examination of the evidence regarding lipid-lowering therapy for prevention of CHD, as well as its effects on breast cancer risk and coronary artery calcium scores in women, can add some much-needed perspective and context to the HT data reviewed above.
CHD prevention. Walsh and Pignone examined six randomized controlled trials (N = 11,435) of primary prevention with lipid-lowering medication in women and found no significant effect on CHD events, nonfatal myocardial infarction, CHD mortality, or total mortality.34 In eight randomized controlled trials of secondary prevention (N = 8,272), lipid-lowering therapy in women resulted in significant reductions in CHD end points but had no effect on total mortality.34
Atherosclerosis progression. In three randomized controlled trials, 1 to 4 years of statin therapy had no effect on the progression of coronary artery calcium compared with placebo.41–43 Among 1,064 women 50 to 59 years old who participated in a WHI substudy called the WHI Coronary Artery Calcium Study (WHI-CACS), those who were randomized to ET had significantly less coronary artery calcium at year 7 compared with those assigned to placebo.44 The mean Agatston coronary artery calcium scores were 83.1 with ET versus 123.1 with placebo (P = .02).
Comparing risk of HT with risk of other therapies
Other comparisons yield similar conclusions.35 For instance, a comparison of the ET arm of the WHI randomized trial with raloxifene in the Raloxifene Use for the Heart (RUTH) trial52 reveals similar effects on CHD, stroke, VTE, pulmonary embolism, deep vein thrombosis, and breast cancer, with the only large difference being a greater reduction in bone fracture risk with ET compared with raloxifene.35 Finally, in putting the risk of VTE with HT into perspective, consider that the risk of thromboembolic events with selective estrogen receptor modulators52 and with the fibric acid derivative fenofibrate in diabetics50 is of similar magnitude as the risk with HT.
Risk must be viewed in light of age at HT initiation
CONCLUSIONS FROM RANDOMIZED TRIALS
HT’s effects on CHD and mortality: Timing is everything
Cumulative data from randomized trials indicate that in the overall population of women studied, HT, aspirin, and lipid-lowering therapy each have a null effect on the incidence of CHD and mortality. However, within this overall null effect, early initiation of HT (in terms of time from menopause [< 10 years] and age at initiation [< 60 years]) is associated with reductions in total mortality and CHD incidence. Additionally, a duration of HT use beyond 5 years is associated with a reduction in the incidence of CHD.9,10,20,28,29 These effects of the timing and duration of therapy are unique to HT. Unopposed ET may have an advantageous profile relative to EPT for reducing the incidence of CHD and mortality in postmenopausal women.
Gaining perspective on risks
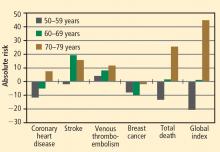
The risks of stroke and VTE associated with HT are low for women overall and lower still for women who are within 10 years of menopause or younger than age 60 when they start HT. With respect to stroke, fewer cases of stroke developed in users of ET compared with placebo in women who started HT before age 60. The risks of EPT are comparable to those of other medications commonly used in this population of women. More broadly, the magnitude and types of risk associated with HT are similar to those associated with other commonly used therapies.
In addition, the underappreciated benefits of HT, such as potential prevention of diabetes mellitus (15 fewer cases of incident diabetes per 10,000 women per year with EPT and 14 fewer cases with ET), need to be recognized and discussed with our patients. These data are consistent in both observational studies and randomized trials.
The bottom line
As data from randomized trials of HT accumulate, the results are clearly similar to those from the more than 20 observational studies indicating that young, symptomatic postmenopausal women who use HT for long periods have lower rates of CHD and total mortality compared with postmenopausal women who do not use HT. Consistent with these data, the American Association of Clinical Endocrinologists issued a position statement in 2008 concluding that for symptomatic menopausal women under the age of 60, the benefits of HT exceed the risks.53
Nevertheless, the bottom line remains that the estrogen-cardioprotective hypothesis has yet to be studied, since randomized trials have not been conducted in the same population of women from which the hypothesis was generated. This hypothesis will be directly evaluated, however, in the ongoing Early versus Late Intervention Trial with Estradiol (ELITE). This randomized trial, funded by the National Institute on Aging, is designed to determine the effects of 17β-estradiol on the progression of atherosclerosis, cognition, and other postmenopausal health issues in recently menopausal (< 6 years) and remotely menopausal (≥ 10 years) women with no history of cardiovascular disease or diabetes. Until data from trials like ELITE emerge, guidelines such as those from the North American Menopause Society54 (reviewed in the next article in this supplement) are reasonable for clinical practice.
Physicians and their women patients have faced a continuous, confusing mix of information about the risks and benefits of hormone therapy (HT) for perimenopausal and postmenopausal women, most of it without respect to age or the timing of HT relative to menopause. Initial data from the Women’s Health Initiative (WHI) estrogen + progestin (E+P) trial, a prevention study conducted predominantly in older postmenopausal women without menopausal symptoms,1 resulted in questioning of the role of HT (unfortunately and inappropriately in younger symptomatic women). Cumulative trial data and further analyses of the WHI have refined and added to our understanding of the effects of HT, particularly with regard to cardiovascular health. This review will update physicians on the latest data on the risks and benefits of HT, with a particular focus on the heart, and will put the risks of HT into appropriate clinical context.
HORMONE THERAPY AND CARDIOVASCULAR DISEASE: A HISTORICAL PERSPECTIVE
Observational studies conducted prior to the WHI found consistently that women who self-selected to use HT had a reduction in mortality and in the incidence of cardiovascular disease relative to women who did not choose to use HT.2–8 This reduction in risk was apparent whether the HT users had taken ET (estrogen therapy) or EPT (estrogen-progestogen therapy). In contrast, randomized controlled trials failed to confirm these findings from observational studies. However, the findings from randomized controlled trials were derived from older postmenopausal women who were many years past menopause. Often overlooked is the WHI observational study of ET and EPT,9,10 in which women who chose to use HT had a reduction in the risk of coronary heart disease (CHD) similar to that observed among the HT users in other observational studies.
RECENT REPORTS FROM THE WHI
Since the original publication of the WHI E+P trial in 2002,1 an extensive collection of data have been published in piecemeal fashion, contributing to the confusion and misperception of the effects of HT on risks and benefits. It is important to note that the WHI consists of both randomized and observational components, as detailed below, and that data have come from both. Together, these data help clarify the misperceptions generated from the first WHI report of 2002,1 particularly misperceptions regarding the timing of HT initiation relative to menopause and the effect of HT duration on cardiovascular disease outcomes. In most instances, the conclusions drawn from this recent research run counter to the inaccurate but prevalent perception that HT use at any time and at any age is associated with cardiovascular harm, a perception that has unfortunately prevailed since the initial publication of the WHI E+P trial findings in 2002.
The WHI randomized trials and combined analysis
The WHI trials enrolled 27,347 postmenopausal women aged 50 to 79 years at baseline; almost two-thirds of the women enrolled were 60 years of age or older, and the majority of women were more than 10 years past menopause. WHI actually comprised two parallel randomized trials:
- One among 16,608 women who had not undergone hysterectomy (ie, with uterus intact), who were randomized to EPT or placebo (ie, WHI E+P trial) 1
- One among 10,739 women who had undergone hysterectomy, who were randomized to ET or placebo.11
Recent analyses from the WHI, published in 2007, assessed the cardiovascular effects of ET and EPT independently and combined, both overall and according to subject age and years since menopause when randomized.12 Other analyses following the initial WHI E+P trial publication have analyzed the effects of HT according to duration of HT use and according to secondary end points. Many of these analyses have presented risks and benefits in terms of both nominal and adjusted confidence intervals (CIs). Nominal 95% CIs describe the variability in risk estimates that would arise from a simple trial for a single end point. Although nominal CIs are traditionally used, they do not take into account the multiple statistical testing issues (across time and across outcome categories) that occur in a trial. In contrast, adjusted 95% CIs correct for these stastical testing issues. From a clinical perspective, it is most appropriate to look at the adjusted CIs.
WHI: EPT vs placebo
CHD. Although the point estimate for CHD is increased, the 95% CI indicates that EPT has a non-significant effect on CHD outcome relative to placebo among all women randomized in the WHI E+P trial (mean age, 63 years).12 This is a very important point for cardiologists and primary care physicians to note.
In a 2008 analysis of the WHI E+P trial that included a 2.4-year open-label follow-up subsequent to the randomized trial,19 the randomized trial data reported were again different than in previous reports, but remained nonsignificant. The hazard ratio (HR) reported for CHD in the 2008 analysis for the randomized portion of the trial was 1.22 (95% CI, 0.99 to 1.51), as compared with 1.23 (95% CI, 0.99 to 1.53) reported in 200712 (Table 1), 1.24 (95% CI, 1.00 to 1.54) reported in 2003,20 and 1.29 (95% CI, 1.02 to 1.63) reported in 2002.1 In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens (EPT or placebo), the HR for CHD between the original randomized treatment groups was nonsignificant at 0.95 (95% CI, 0.73 to 1.26) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Stroke. The risk of stroke was increased significantly (by an additional 8 events per 10,000 women treated per year) in the EPT arm versus the placebo arm in the nominal analysis,12 but this difference was nonsignificant after adjustment.13
In the 2008 WHI E+P analysis, the HR reported for stroke during the randomized trial phase was different than in previous reports—1.34 (95% CI, 1.05 to 1.71)19 versus 1.31 (95% CI, 1.03 to 1.68) reported in 200712 (Table 1)—and the adjusted analysis was not reported. In the 2.4-year open-label follow-up period in which women were no longer on their randomized regimens, the HR for stroke between the original randomized treatment groups was nonsignifi-cant at 1.16 (95% CI, 0.83 to 1.61) and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Breast cancer. Breast cancer risk was originally reported to be increased significantly (by an additional 8 cases per 10,000 women per year) in the EPT arm versus the placebo arm with the nominal statistic, but this increase was nonsignificant after adjustment.1 This risk estimate was revised in a follow-up publication 1 year after the original data were reported, and the increase in risk in the EPT arm was still no longer significant in the adjusted analysis.21 Importantly, another subsequent analysis that adjusted for baseline risk factors for breast cancer resulted in a further revision of the risk estimate, which again showed a nonsignificant increase in the EPT arm relative to the placebo arm.14 This is very important since it is commonly accepted that EPT increases the risk of breast cancer when this has not been definitively proven in any randomized controlled trial.
Unfortunately, the most recent breast cancer data14 (Table 1) were not used in the 2008 WHI E+P analysis.19 However, even using the unadjusted data in the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR for breast cancer between the original randomized treatment groups was nonsignificant and the change in the HR over time from the randomized phase to the open-label phase was not significant.19
Venous thromboembolism (VTE). EPT was associated with a doubling of the risk of VTE (ie, deep vein thrombosis and pulmonary embolism) compared with placebo, resulting in an excess of 18 VTE events per 10,000 women per year of therapy.15 The risk of VTE was significant across the entire cohort of women (mean age, 63 years).
In the 2008 WHI E+P analysis, the HR reported for VTE during the randomized phase was different than in previous reports—1.98 (95% CI, 1.52 to 2.59)19 versus 2.06 (95% CI, 1.57 to 2.70) reported in 200415 (Table 1)—and the HR during the 2.4-year open-label follow-up, in which women were no longer on their randomized regimens, was no longer significant (HR = 0.95; 95% CI, 0.63 to 1.44). This change in the HR over time from the randomized phase to the open-label phase was statistically significant.19
Fracture. The risk of hip fracture was reduced by 33% with EPT relative to placebo, which was statistically significant in the nominal analysis but not in the adjusted analysis.17 The risk of any fracture was reduced by 24% in women randomized to EPT compared with placebo, which was statistically significant and translated to 47 fewer fractures per 10,000 women per year of therapy.17 Clinically, these results are most impressive given that women randomized in the WHI were not selected on the basis of risk for osteoporosis or fracture. This claim cannot be made for any other therapy.
In the 2008 WHI E+P analysis of the 2.4-year open-label follow-up in which women were no longer on their randomized regimens, the HR between the original randomized treatment groups was nonsignificant for hip fracture and any fracture—0.92 (95% CI, 0.64 to 1.34) and 0.91 (95% CI, 0.78 to 1.06), respectively—and the change in the HR over time from the randomized phase to the open-label phase was not significant for either fracture outcome.19
Diabetes. The risk of new-onset diabetes was reduced by a statistically significant 21% in women randomized to EPT compared with placebo.18
WHI: ET vs placebo
CHD. Importantly, no significant difference was found between the ET and placebo arms with respect to CHD events in the overall cohort of women, whose average age was 64 years.12
Stroke. The risk of stroke was greater with ET than with placebo in the nominal analysis, but importantly, the difference in event rates (11 per 10,000 women per year of therapy) failed to reach significance in the adjusted analysis.11,12
Breast cancer. A strong but nonsignificant trend toward a reduction in breast cancer risk was apparent in the ET arm (8 fewer breast cancer cases per 10,000 women per year of therapy). Among women who actually were adherent to their study regimen (ie, consuming ≥ 80% of their study medication), there was a statistically significant 33% reduction in breast cancer risk with ET relative to placebo.22 Importantly, the reduction in breast cancer risk relative to placebo was found across all the age ranges studied.11
VTE. The excess risk of VTE with ET versus placebo (32%) was less than the excess risk of VTE associated with EPT (Table 1). Importantly, the risk of VTE associated with ET was not statistically significant.11,23
Fracture. The risk of any fracture (hip or vertebral) was reduced significantly in the ET arm compared with the placebo arm.11
Diabetes. In a nominal analysis, there was a trend toward a reduction in the risk of new-onset diabetes in women randomized to ET relative to placebo, which nearly achieved statistical significance.24
WHAT EXPLAINS THE DISCORDANCE BETWEEN OBSERVATIONAL AND RANDOMIZED TRIALS OF HT?
How can the perceived discordance between the results from observational studies and those from randomized controlled trials be explained? There are currently three hypotheses:
- The populations differ in the two types of study designs (observational studies and randomized controlled trials)
- The duration of HT use differs
- The timing of HT initiation differs in relation to age, time since menopause, and stage of atherosclerosis.
Population characteristics
One obvious difference between randomized trials and observational studies of HT is the presence of menopausal symptoms. To maintain blinding, women with hot flashes were predominantly excluded from randomized trials of HT, whereas the presence of hot flashes is the predominant menopausal symptom of women included in observational studies and the main reason women seek HT from their providers.
Other consistent and possibly explanatory differences between clinical trials and observational studies of HT are patient age at enrollment, years since menopause, and body mass index (BMI). Comparing randomized controlled trials with observational studies, age at enrollment was much higher in the clinical trials (mean age ≥ 63 years) than in the observational studies (range of 30 to 55 years). Similarly, women enrolled in randomized trials were more than 10 years beyond menopause, whereas those in observational studies were less than 5 years beyond menopause. In fact, more than 80% of HT users in observational studies initiated HT within 1 or 2 years of menopause.
Additionally, women in randomized trials of HT tend to have higher BMIs than their counterparts in observational studies. For example, mean BMI was considerably higher in the WHI randomized trials (28.5 kg/m2 and 30.1 kg/m2)1,11 than in the observational Nurses’ Health Study (25.8 kg/m2),25 and a full third (34%) of women in the WHI randomized trials were severely obese (BMI ≥ 30 kg/m2).
This point about BMI is noteworthy in light of findings on the effect of BMI on breast cancer risk from an analysis of the WHI observational study among 85,917 women aged 50 to 79 years old at enrollment.26 This analysis found that BMI was unrelated to breast cancer risk among women who had used HT; however, among nonusers of HT, a baseline BMI greater than 31.1 kg/m2 was associated with a 2.52 relative risk of breast cancer compared with a baseline BMI less than 22.6 kg/m2. The risk of breast cancer with increasing BMI was most pronounced in younger postmenopausal women. One interpretation is that high endogenous estrogen levels in postmenopausal women with an elevated BMI serve to increase breast cancer risk to a level beyond which HT adds no further risk. Alternatively, conjugated equine estrogens may act through a selective estrogen receptor modulator mechanism to block any potential adverse breast tissue effects of elevated endogenous estrone and estradiol levels.
Duration of HT
Another hypothesis for the discordant findings between observational and randomized studies of HT focuses on differences in duration of HT use between the two types of studies. Duration of HT use has been substantially longer in observational studies, ranging from 10 years to 40 years, compared with no more than 7 or 8 years in randomized trials to date. Moreover, the results from observational studies have suggested that the longer the duration of HT use, the greater the benefit in terms of CHD risk.
For example, a case-control study by Chilvers et al showed that HT protected against nonfatal myocardial infarction only when used for more than 60 months.27 Analysis of data on EPT use from the Heart and Estrogen/progestin Replacement Study (HERS),28 the randomized WHI E+P trial,20 and the WHI observational study9 reveals an interesting and consistent trend: rates of CHD events increased during the first year of EPT therapy (compared with placebo or nonuse) but then declined over time, ending up below the rates of CHD events in placebo recipients or nonusers of HT after approximately 5 years of therapy. This trend was not as pronounced with ET use in either the WHI randomized trial29 or the WHI observational study,10 but the incidence of CHD events did decline over time with ET use in both studies, and in the WHI observational study, greater than 5 years of ET use was associated with a significant 27% reduction (HR, 0.73; 95% CI, 0.61 to 0.84) in CHD events compared with nonuse.10
Timing of HT initiation
A third hypothesis for the discordance between randomized and observational studies of HT concerns the timing of HT initiation relative to patient age, time since menopause, and stage of atherosclerosis. As noted above, women enrolled in randomized trials have tended to be considerably older and further from menopause compared with their counterparts in observational studies. The effects of differences in timing of HT initiation on specific clinical end points, particularly in relation to cardiovascular health, are reviewed below.
Early and continued HT use may interrupt the pathogenic sequence of vascular aging, potentially preventing progression of atherosclerosis to the late stage at which plaque rupture and clinical events occur. In contrast, late intervention with HT may have little effect on established plaque and, in the case of EPT, may actually predispose to plaque rupture. This concept has been demonstrated in a pair of sister studies, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELLHART)31 and the Estrogen Prevention Atherosclerosis Trial (EPAT).32
Mortality. The same Stanford University researchers who performed the above meta-analysis of CHD events also assessed odd ratios for overall mortality in a meta-analysis of 30 randomized trials of HT versus placebo that included a total of 26,708 postmenopausal women (representing 119,118 patient-years).33 They found the timing of HT initiation to have an effect on mortality similar to its effect on CHD events. In the overall cohort of women, the odds of death were not different between the HT and placebo groups, but among women younger than 60 years (mean, 54 years), those randomized to HT had a significant 39% reduction in the risk of death (HR = 0.61; 95% CI, 0.39 to 0.95) compared with those randomized to placebo. HT had no effect on mortality among women older than 60 years (mean, 66 years) in this analysis.
These mortality data are consistent with those from the WHI randomized trials, in which both EPT and ET were associated with a 30% reduction in overall mortality relative to placebo among women 50 to 59 years old.12 When both the EPT and ET portions of the WHI were combined to increase the sample size, HT was associated with a significant 30% reduction in mortality (HR = 0.70; 95% CI, 0.51 to 0.96) compared with placebo among women 50 to 59 years old.12
Stroke. The most recent WHI data indicate that stroke is not increased with ET in women 50 to 59 years of age, as there were 2 fewer events per 10,000 women per year of ET relative to placebo.12 In this same age group, the risk of stroke from EPT was increased by 5 events per 10,000 women per year of therapy relative to placebo.12 Among women randomized within 5 years of menopause, stroke risk was increased by 3 events per 10,000 women per year of EPT relative to placebo.13
VTE. Although age was not a significant contributing factor to the risk of VTE from HT in the WHI, the absolute risk of VTE was lower in younger versus older women. The additional absolute risk for VTE events per 10,000 women per year of EPT use was 11 events for women 50 to 59 years old at randomization, 16 events for women 60 to 69 years old at randomization, and 35 events for women 70 to 79 years old at randomization.15 The additional absolute risk for VTE events per 10,000 women per year of ET use was 4 events for women 50 to 59 years old at randomization, 7 events for women 60 to 69 years old at randomization, and 11 events for women 70 to 79 years old at randomization.23 A history of a prior thromboembolic event increases the risk of VTE with postmenopausal HT use, which should be considered before HT is initiated.
HORMONE THERAPY IN CLINICAL PERSPECTIVE
Comparative effects of lipid-lowering therapy and HT
Examination of the evidence regarding lipid-lowering therapy for prevention of CHD, as well as its effects on breast cancer risk and coronary artery calcium scores in women, can add some much-needed perspective and context to the HT data reviewed above.
CHD prevention. Walsh and Pignone examined six randomized controlled trials (N = 11,435) of primary prevention with lipid-lowering medication in women and found no significant effect on CHD events, nonfatal myocardial infarction, CHD mortality, or total mortality.34 In eight randomized controlled trials of secondary prevention (N = 8,272), lipid-lowering therapy in women resulted in significant reductions in CHD end points but had no effect on total mortality.34
Atherosclerosis progression. In three randomized controlled trials, 1 to 4 years of statin therapy had no effect on the progression of coronary artery calcium compared with placebo.41–43 Among 1,064 women 50 to 59 years old who participated in a WHI substudy called the WHI Coronary Artery Calcium Study (WHI-CACS), those who were randomized to ET had significantly less coronary artery calcium at year 7 compared with those assigned to placebo.44 The mean Agatston coronary artery calcium scores were 83.1 with ET versus 123.1 with placebo (P = .02).
Comparing risk of HT with risk of other therapies
Other comparisons yield similar conclusions.35 For instance, a comparison of the ET arm of the WHI randomized trial with raloxifene in the Raloxifene Use for the Heart (RUTH) trial52 reveals similar effects on CHD, stroke, VTE, pulmonary embolism, deep vein thrombosis, and breast cancer, with the only large difference being a greater reduction in bone fracture risk with ET compared with raloxifene.35 Finally, in putting the risk of VTE with HT into perspective, consider that the risk of thromboembolic events with selective estrogen receptor modulators52 and with the fibric acid derivative fenofibrate in diabetics50 is of similar magnitude as the risk with HT.
Risk must be viewed in light of age at HT initiation
CONCLUSIONS FROM RANDOMIZED TRIALS
HT’s effects on CHD and mortality: Timing is everything
Cumulative data from randomized trials indicate that in the overall population of women studied, HT, aspirin, and lipid-lowering therapy each have a null effect on the incidence of CHD and mortality. However, within this overall null effect, early initiation of HT (in terms of time from menopause [< 10 years] and age at initiation [< 60 years]) is associated with reductions in total mortality and CHD incidence. Additionally, a duration of HT use beyond 5 years is associated with a reduction in the incidence of CHD.9,10,20,28,29 These effects of the timing and duration of therapy are unique to HT. Unopposed ET may have an advantageous profile relative to EPT for reducing the incidence of CHD and mortality in postmenopausal women.
Gaining perspective on risks
The risks of stroke and VTE associated with HT are low for women overall and lower still for women who are within 10 years of menopause or younger than age 60 when they start HT. With respect to stroke, fewer cases of stroke developed in users of ET compared with placebo in women who started HT before age 60. The risks of EPT are comparable to those of other medications commonly used in this population of women. More broadly, the magnitude and types of risk associated with HT are similar to those associated with other commonly used therapies.
In addition, the underappreciated benefits of HT, such as potential prevention of diabetes mellitus (15 fewer cases of incident diabetes per 10,000 women per year with EPT and 14 fewer cases with ET), need to be recognized and discussed with our patients. These data are consistent in both observational studies and randomized trials.
The bottom line
As data from randomized trials of HT accumulate, the results are clearly similar to those from the more than 20 observational studies indicating that young, symptomatic postmenopausal women who use HT for long periods have lower rates of CHD and total mortality compared with postmenopausal women who do not use HT. Consistent with these data, the American Association of Clinical Endocrinologists issued a position statement in 2008 concluding that for symptomatic menopausal women under the age of 60, the benefits of HT exceed the risks.53
Nevertheless, the bottom line remains that the estrogen-cardioprotective hypothesis has yet to be studied, since randomized trials have not been conducted in the same population of women from which the hypothesis was generated. This hypothesis will be directly evaluated, however, in the ongoing Early versus Late Intervention Trial with Estradiol (ELITE). This randomized trial, funded by the National Institute on Aging, is designed to determine the effects of 17β-estradiol on the progression of atherosclerosis, cognition, and other postmenopausal health issues in recently menopausal (< 6 years) and remotely menopausal (≥ 10 years) women with no history of cardiovascular disease or diabetes. Until data from trials like ELITE emerge, guidelines such as those from the North American Menopause Society54 (reviewed in the next article in this supplement) are reasonable for clinical practice.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Rosenberg L, Palmer JR, Shapiro S. A case-control study of myocardial infarction in relation to use of estrogen supplements. Am J Epidemiol 1993; 137:54–63.
- Mann RD, Lis Y, Chukwujindu J, Chanter DO. A study of the association between hormone replacement therapy, smoking and the occurrence of myocardial infarction in women. J Clin Epidemiol 1994; 47:307–312.
- Psaty B, Heckbert SR, Atkins D, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med 1994; 154:1333–1339.
- Sidney S, Petitti DB, Quisenberry CP Jr. Myocardial infarction and the use of estrogen and estrogen-progestogen in postmenopausal women. Ann Intern Med 1997; 127:501–508.
- Grodstein F, Stampfer MJ, Falkeborn M, Naessen T, Persson I. Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology 1999; 10:476–480.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Varas-Lorenzo C, García-Rodríguez LA, Perez-Gutthann S, Duque-Oliart A. Hormone replacement therapy and incidence of acute myocardial infarction. A population-based nested case-control study. Circulation 2000; 101:2572–2578.
- Prentice RL, Langer R, Stefanick ML, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol 2005; 162:404–414.
- Prentice RL, Langer RD, Stefanick ML, et al. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol 2006; 163:589–599.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 2003; 289:2673–2684.
- Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006; 55:103–115.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292:1573–1580.
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004; 350:991–1004.
- Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004; 47:1175–1187.
- Heiss G, Wallace R, Anderson GL, et al, for the WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008; 299:1036–1045.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA 2003; 289:3243–3253.
- Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in post-menopausal women with hysterectomy. JAMA 2006; 295:1647–1657.
- Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med 2006; 166:772–780.
- Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 2006; 49:459–468.
- Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 1996; 335:453–461.
- Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 2002; 13:741–751.
- Chilvers CE, Knibb RC, Armstrong SJ, Woods KL, Logan RF. Postmenopausal hormone replacement therapy and risk of acute myocardial infarction—a case control study of women in the East Midlands, UK. Eur Heart J 2003; 24:2197–2205.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med 2006; 166:357–365.
- Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Hodis HN, Mack WJ, Lobo RA, et al. Hormone therapy and progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med 2003; 349:535–545.
- Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.
- Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004; 291:2243–2252.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006; 295:74–80.
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005; 23:8606–8612.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–1278.
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006; 113:427–437.
- Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005; 112:563–571.
- Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005; 46:166–172.
- Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007; 356:2591–2602.
- Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996; 334:1150–1155.
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336:262–266.
- Amarenco P, Bogousslavsky J, Callahan A III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglita-zone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: randomised controlled trial. Lancet 2006; 368:1096–1105.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- American Association of Clinical Endocrinologists (AACE) position statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause 2007; 14:168–182.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- Rosenberg L, Palmer JR, Shapiro S. A case-control study of myocardial infarction in relation to use of estrogen supplements. Am J Epidemiol 1993; 137:54–63.
- Mann RD, Lis Y, Chukwujindu J, Chanter DO. A study of the association between hormone replacement therapy, smoking and the occurrence of myocardial infarction in women. J Clin Epidemiol 1994; 47:307–312.
- Psaty B, Heckbert SR, Atkins D, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med 1994; 154:1333–1339.
- Sidney S, Petitti DB, Quisenberry CP Jr. Myocardial infarction and the use of estrogen and estrogen-progestogen in postmenopausal women. Ann Intern Med 1997; 127:501–508.
- Grodstein F, Stampfer MJ, Falkeborn M, Naessen T, Persson I. Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology 1999; 10:476–480.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Varas-Lorenzo C, García-Rodríguez LA, Perez-Gutthann S, Duque-Oliart A. Hormone replacement therapy and incidence of acute myocardial infarction. A population-based nested case-control study. Circulation 2000; 101:2572–2578.
- Prentice RL, Langer R, Stefanick ML, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol 2005; 162:404–414.
- Prentice RL, Langer RD, Stefanick ML, et al. Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol 2006; 163:589–599.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291:1701–1712.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 2003; 289:2673–2684.
- Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006; 55:103–115.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292:1573–1580.
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004; 350:991–1004.
- Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004; 47:1175–1187.
- Heiss G, Wallace R, Anderson GL, et al, for the WHI investigators. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 2008; 299:1036–1045.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA 2003; 289:3243–3253.
- Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in post-menopausal women with hysterectomy. JAMA 2006; 295:1647–1657.
- Curb JD, Prentice RL, Bray PF, et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch Intern Med 2006; 166:772–780.
- Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 2006; 49:459–468.
- Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 1996; 335:453–461.
- Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 2002; 13:741–751.
- Chilvers CE, Knibb RC, Armstrong SJ, Woods KL, Logan RF. Postmenopausal hormone replacement therapy and risk of acute myocardial infarction—a case control study of women in the East Midlands, UK. Eur Heart J 2003; 24:2197–2205.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med 2006; 166:357–365.
- Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Hodis HN, Mack WJ, Lobo RA, et al. Hormone therapy and progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med 2003; 349:535–545.
- Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med 2004; 19:791–804.
- Walsh JM, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004; 291:2243–2252.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006; 295:74–80.
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005; 23:8606–8612.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–1278.
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006; 113:427–437.
- Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005; 112:563–571.
- Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005; 46:166–172.
- Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med 2007; 356:2591–2602.
- Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996; 334:1150–1155.
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336:262–266.
- Amarenco P, Bogousslavsky J, Callahan A III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglita-zone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: randomised controlled trial. Lancet 2006; 368:1096–1105.
- Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- American Association of Clinical Endocrinologists (AACE) position statement on hormone replacement therapy (HRT) and cardiovascular risk. American Association of Clinical Endocrinologists Web site. www.aace.com/pub/pdf/guidelines/HRTCVRISKposition_ statement.pdf. Accessed March 5, 2008.
- Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause 2007; 14:168–182.