User login
Mitral replacement may grow with infant
NEW YORK – Physicians at Boston Children’s Hospital replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani. The results were presented at the 2013 Mitral Valve Conclave earlier this year.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
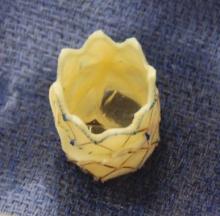
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani did a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
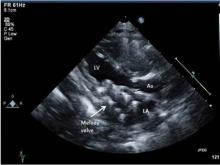
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
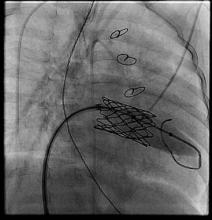
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," he said at the meeting, which was sponsored by the AATS.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
He reported that friction of the stent against the annulus prevents leakage. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs."
Dr. Emani reported no disclosures.
NEW YORK – Physicians at Boston Children’s Hospital replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani. The results were presented at the 2013 Mitral Valve Conclave earlier this year.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani did a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," he said at the meeting, which was sponsored by the AATS.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
He reported that friction of the stent against the annulus prevents leakage. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs."
Dr. Emani reported no disclosures.
NEW YORK – Physicians at Boston Children’s Hospital replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani. The results were presented at the 2013 Mitral Valve Conclave earlier this year.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani did a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," he said at the meeting, which was sponsored by the AATS.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
He reported that friction of the stent against the annulus prevents leakage. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs."
Dr. Emani reported no disclosures.
Neurologist calls for shift in stroke paradigm
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
SAN DIEGO – Many of the guideline recommendations for intra-arterial catheter-directed treatment of acute ischemic stroke that were recently endorsed by neuroradiology and interventional neurology groups should be "revisited" to hasten time to revascularization to salvage more brain tissue following stroke, according to Dr. Jeffrey L. Saver.
While the multisociety guidelines endorsed by the American Society of Neuroradiology, the Society for Neurointerventional Surgery, and other professional groups recommend a door-to-revascularization time of 210 minutes (J. Vasc. Int. Radiol. 2013;24:151-63), Dr. Saver calls for 130 minutes and says this is an attainable goal in the age of stent retrievers.
"If you wait 3 hours and 30 minutes to get arteries unclogged, most of the affected brain is dead," Dr. Saver said at the annual meeting of the American Society of Neuroradiology. "That is playing Little League, and the ischemic brain is playing Major League."
Lessons from IV TPA show the dramatic effect of time in stroke outcomes, said Dr. Saver, director of the UCLA Comprehensive Stroke Center at the University of California, Los Angeles. In a study of more than 58,000 patients seen at 1,400 Get With the Guidelines stroke hospitals, he and his colleagues found that faster onset-to-treatment, in 15-minute increments, was associated with reduced in-hospital mortality (odds ratio, 0.96, P less than .001), reduced symptomatic intracranial hemorrhage (OR 0.96, P less than .001), increased achievement of independent ambulation at discharge (OR 1.04, P less than .001), and increased discharge to home (OR 1.03, P less than .001) (JAMA 2013;309:2480-8).
"In other words, for each 1,000 patients, accelerating TPA treatment by 15 minutes resulted in 18 more patients with improved ambulation at discharge, including 8 more who will ambulate fully independently, 13 more who will be discharged to a more independent environment, and 4 fewer patient deaths prior to discharge," he said.
Dr. Saver suggests paring down the endovascular time targets of the guidelines. For example, door-to-puncture time goals should be reduced from 120 minutes to 90 minutes, picture-to-puncture times from 95 to 65 minutes, puncture to first-pass from 45 to 20 minutes, and puncture to revascularization from 90 to 40 minutes.
One way to cut down time is to begin treatment before the patient arrives at the hospital. Dr. Saver described a German study comparing outcomes of initiating lytic therapy in a mobile stroke unit (with a neurologist and a CT scanner on board the ambulance) to lytic therapy given when the patient reached the emergency department. The average time from alarm to treatment was twice as long for those given hospital lysis, compared with those treated by the mobile units (73 minutes vs. 38 minutes), although no differences were found in efficacy or safety outcomes. (Lancet Neurol. 2012;11:397-404).
"This is a remarkable acceleration of care," Dr. Saver said. By putting head-only CT scanners into ambulances, the German groups have the capability of performing not only noncontrast CT, but also CT angiography or perfusion CT on site. Dr. Saver’s team is also investigating whether other treatments given in the field, such as neuroprotective agents or antihypertensives, might be beneficial.
Other strategies to shave time include emergency medical services prenotification, having a stroke tool kit in place, and rapid triage and stroke team notification. He also recommends having a single-call activation system of the stroke team. TPA can be premixed and should be easily accessible and stored in the emergency department and radiology suite. At UCLA, a visual decision aid outlining the pros and cons of TPA allows families to make rapid informed consent.
Rapid treatment times also depend upon access to specialized stroke care centers. In 2010, 47% of the U.S. population, or 147 million Americans, lived in jurisdictions that routed stroke patients to the nearest hospital, not a primary stroke center (PSC). "But we have reached a tipping point now that more than half of the U.S. population resides in a stroke-center domain," Dr. Saver said. Since the early 2000s, there has been a shift from an acute ischemic stroke treatment model that relied primarily on transport of patients to PSCs or stroke-ready hospitals where they could receive TPA and other acute therapies safely and efficiently. Nowadays, favor is given to a two-tier system of spokes (PSCs) and hubs (comprehensive stroke centers that offer advanced treatments for complex patients, staffed by stroke specialists). More than ever, rapid identification and evaluation of stroke patients and effective differential diversion assessments are critical in reducing door-to-needle or door-to-recanalization times.
According to Dr. Saver, in 2005, only one-quarter of patients were being treated based on best practice standards of door-to-IV TPA of 60 minutes or less, and that level remained virtually unchanged through 2009. More recently, that number has begun to inch up. "Nationally, we are moving the curve, but the best centers are not going for 60 minutes door-to-needle, they are going for 30 minutes," he said, noting that nationally the fastest door-to-needle times in the United States are being reported by Washington University in St. Louis (39 minutes). The world’s record is 20 minutes in Helsinki.
For a best practice example of rapid catheter-based reperfusion, Dr. Saver cited a recent report from the University of Calgary (J. Neurointervent. Surg. 2013;5[Suppl. 1]:i58-61) in which imaging to reperfusion time averaged 47 minutes. More than 80% of the 11 patients left the hospital with modified Rankin Scale scores of 1 or less.
Dr. Saver reported that UCLA receives compensation for his service as a consultant to Covidien, Grifols, and Lundbeck on the design and conduct of trials.
EXPERT ANALYSIS FROM THE ASNR ANNUAL MEETING
Percent insula infarction may help predict stroke treatment response
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
SAN DIEGO – The percentage of anterior insula that is infarcted at the time of admission in patients with acute middle cerebral artery occlusive stroke predicted infarct growth without significant revascularization in 74 consecutive patients who underwent intra-arterial therapy.
"We believe the insula is an overlooked biomarker. ... Although we have not proven it yet, we think it may do a better job than perfusion mismatch in stratifying patients who are likely to benefit from intra-arterial therapy [IAT] from patients unlikely to benefit," said Dr. Michael H. Lev, coauthor of the study and director of emergency neuroradiology and radiology at Massachusetts General Hospital’s Institute for Heart, Vascular, and Stroke Care in Boston.
Two neuroradiologists rated the admission diffusion-weighted imaging (DWI) scans of the patients according to percent anterior and posterior insula infarction using a 4-point scale (normal, less than 50%, greater than 50%, and 100%). (The anterior insula is approximately 25% of the whole insula.) Admission DWI and follow-up MRI core infarct volumes were segmented, and infarct growth was determined. Patients were stratified into those with good (TICI [thrombolysis in cerebral infarction] grade 2-3) vs. poor (TICI grade 0-1) recanalization.
No or poor recanalization occurred in 23 (31%) patients, according to Dr. Livia Morais, who presented the findings at the annual meeting of the American Society of Neuroradiology. In this group, the percent anterior insula infarct was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04). Predictors of final infarct volume were age, anterior/posterior/total insula percent infarct, and DWI lesion volume at admission (all P less than .05).
For the 69% with good recanalization, National Institutes of Health Stroke Scale score at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.34, P = .02). Predictors of final infarct volume were percent anterior/posterior/total insula infarct and DWI lesion volume at admission (all P less than .05).
Patients who had less than 50% anterior insula infarct had significantly lower infarct volume (P less than .0001) and infarct growth (P less than .03), compared with those who had greater than 50% anterior insula infarct involvement.
One reason the investigators are focusing on the insula is that it is seen as a region of high ischemic vulnerability. "We speculate that anterior insula infarction may be a stronger surrogate for overall stroke severity than is DWI lesion volume because the unique vascular supply of this location reflects the combined effects of not only the degree of superior division MCA occlusion, but also the quality of collateral flow from both the anterior and inferior MCA divisions, as well as other pial collateral sources," said Dr. Morais, a neuroimaging fellow at Massachusetts General Hospital’s neurovascular laboratory.
This work is part of a body of research looking to identify treatment-relevant acute imaging targets, Dr. Lev said. "We are trying to break patients up into groups with high risk-to-benefit ratios and low risk-to-benefit ratios. Those who fall in-between, if they have no other exclusion criteria, can be treated." The results of this study indicate that patients who are IAT candidates with diffusion-weighted imaging lesion volume of less than 70 mL may be stratified into those with a high likelihood of benefitting from IAT based on the percent anterior insula involvement, he said.
In previous work, Dr. Lev showed that the insula was a very good predictor of aphasia recovery after infarction (Am. J. Neuroradiol. 2010;31:1661-8). The insula was also one of several predictors of motor improvement after stroke (Neurology 2012;78:1853-9). In another study, Dr. Lev said that infarction of the right insula and peri-insular regions was associated with the development of hospital-acquired pneumonia, which he attributed to the insula’s involvement with swallowing and immune modulation.
Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
AT THE ASNR ANNUAL MEETING
Major finding: The percent anterior insula infarct at admission was the only predictor of infarct growth in both univariate and multivariate analyses (Spearman Rho = 0.43, P = .04) in 23 patients who had no or poor recanalization following intra-arterial therapy for acute infarction of the middle cerebral artery.
Data source: A prospective study of 74 consecutive patients with acute middle cerebral artery occlusive stroke who underwent intra-arterial therapy.
Disclosures: Dr. Morais said he had no relevant financial disclosures. Dr. Lev said he has received research funding from GE Healthcare.
Design flaws hamper lupus nephritis biologic trials
NEW YORK – Evidence for the use of biologics such as rituximab and abatacept to treat refractory lupus nephritis has been hampered by varying definitions of the condition and design differences in clinical trials, according to Dr. Brad H. Rovin.
Evidence of the benefit to using rituximab 1 g every 2 weeks for refractory lupus nephritis, as suggested by the American College of Rheumatology’s guidelines (Arthritis Care Res. 2012;64:797-808), is hard to confirm with the available data, Dr. Rovin said at a meeting sponsored by New York University.
"The rituximab studies are very difficult to interpret due to small sample size, aggressive concomitant therapies, variable dosing regimens, and nonuniform definitions of refractory," said Dr. Rovin, professor of nephrology and pathology and director of the division of nephrology at Ohio State University in Columbus. "Nonetheless, this collection of data has prompted the idea that rituximab is effective for refractory lupus nephritis."
Although pooled data suggest that rituximab might be helpful in refractory lupus nephritis (Autoimmun. Rev. 2012:11:357-64), only a true randomized controlled trial with sufficient power that is designed to overcome these criticisms will settle the issue, he noted. The European RING (Rituximab for Lupus Nephritis With Remission as a Goal) trial, which started in November 2012, will initially ask whether rituximab can be a useful salvage therapy once a patient still has active disease after 6 months of standard-of-care treatment. However, in Dr. Rovin’s opinion, the patients being recruited for the trial are not truly refractory because the failure of two inductiontype regimens before beginning rituximab is not required.
Examining the LUNAR trial failure
The randomized, double-blind, placebo-controlled phase III LUNAR (Lupus Nephritis Assessment With Rituximab) trial – one of the most anticipated clinical trials of lupus nephritis treatment – failed to reach its primary endpoint (Arthritis Rheum. 2012;64:1215-26), said Dr. Rovin, who was the lead author of the report. In fact, in the last few years, none of the trials of novel therapeutics for lupus nephritis have achieved their primary endpoints.
The primary endpoint of renal response (complete and partial) at week 52 was similar in patients who received rituximab or placebo (56.0% vs. 45.8%, respectively), although improvements were seen with rituximab in serum C3 and C4 and anti–double-stranded DNA antibodies. The trial involved 144 patients with class III or IV lupus nephritis treated with rituximab (1,000 mg) or placebo added on to mycophenolate mofetil and corticosteroids. Patients received rituximab or placebo on days 1, 15, 168, and 182.
Did LUNAR fail because it was added on to an already effective therapy? Dr. Rovin asked. He pointed to data from studies of other current therapies, including intravenous cyclophosphamide, which showed relatively high partial responses (63%) but low complete responses (12%-31%). "Current therapies are only modestly effective, and there is plenty of room for improvement. Evaluating add-on therapies to the standard of care is an acceptable trial design for what we can accomplish now," Dr. Rovin said.
The LUNAR trial was powered to detect complete renal remissions, and its failure to detect a difference between treatment groups does not mean that the trial should have been powered to favor partial remissions, Dr. Rovin said. In a study in which he participated that was presented last year at the American Society of Nephrology, Dr. Rovin and his colleagues found that over a period of 4.5 years, patients who have acute kidney injury as evidenced by renal flares were at heightened risk of developing new chronic kidney disease (CKD) or progression of established kidney disease. Patients with new CKD had a significantly greater frequency of new renal flares than did those without CKD (0.72 per year vs. 0.14 per year; P = .001) as well as a greater duration of time spent in flare (20 months vs. 0 months; P = .0003). These results show that "we should not be satisfied with partial remissions as an endpoint, because unresolved kidney inflammation and injury predispose these patients to CKD," he said.
The issue has also been raised as to whether LUNAR failed because its endpoint of 1 year was too short. There may have been better separation between rituximab and placebo with a 2-year endpoint, but Dr. Rovin’s own findings with repeat biopsies following good clinical responses after the first 6 months of conventional therapy (mycophenolate mofetil or intravenous cyclophosphamide for 6 months) have shown evidence of progressive renal scarring despite significant reductions in creatinine and proteinuria. These data suggest that the goal of therapy should be to decrease the time to response as much as possible and thereby perhaps decrease kidney scarring because scarring and damage to the kidney tubulointerstitium are what determine the renal prognosis. "We should not be satisfied with taking a year or more to achieve remissions," he said.
Failure of abatacept lupus nephritis trial
The failure of another recent trial to show any benefit of abatacept in treating refractory lupus nephritis could also be related to the problem of inconsistent trial endpoints making it difficult to compare therapies (Arthritis Rheum. 2012;64:3660-5). For instance, urine protein-creatinine ratios (UPCRs) are subject to individual variability but the criteria for UPCRs have varied from 0.2 to 0.5 in different lupus nephritis trials, including this abatacept trial, the LUNAR trial, the Aspreva Lupus Management Study (ALMS), and the Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis (ACCESS) trial, as well as in the ACR’s recommendations for lupus nephritis.
Estimated glomerular filtration rate (eGFR) criteria also are inconsistent, and endpoints that refer to "normal" eGFRs are hard to interpret, Dr. Rovin said, because it is unclear what is normal for creatine "unless the patient’s true serum creatinine before SLE is known."
"We need uniform, reasonable and consistent response criteria for lupus nephritis clinical trials, and these responses should be associated with long-term outcomes," said Dr. Rovin, who noted that he is involved in preparing a white paper on clinical trial design for lupus nephritis.
Dr. Rovin has served as a consultant to Bristol-Myers Squibb, which markets abatacept, as well as Biogen Idec and Genentech, which comarket rituximab in the United States. He has also received honoraria from Biogen Idec and Genentech and research funding from Roche, Genentech’s parent company.
NEW YORK – Evidence for the use of biologics such as rituximab and abatacept to treat refractory lupus nephritis has been hampered by varying definitions of the condition and design differences in clinical trials, according to Dr. Brad H. Rovin.
Evidence of the benefit to using rituximab 1 g every 2 weeks for refractory lupus nephritis, as suggested by the American College of Rheumatology’s guidelines (Arthritis Care Res. 2012;64:797-808), is hard to confirm with the available data, Dr. Rovin said at a meeting sponsored by New York University.
"The rituximab studies are very difficult to interpret due to small sample size, aggressive concomitant therapies, variable dosing regimens, and nonuniform definitions of refractory," said Dr. Rovin, professor of nephrology and pathology and director of the division of nephrology at Ohio State University in Columbus. "Nonetheless, this collection of data has prompted the idea that rituximab is effective for refractory lupus nephritis."
Although pooled data suggest that rituximab might be helpful in refractory lupus nephritis (Autoimmun. Rev. 2012:11:357-64), only a true randomized controlled trial with sufficient power that is designed to overcome these criticisms will settle the issue, he noted. The European RING (Rituximab for Lupus Nephritis With Remission as a Goal) trial, which started in November 2012, will initially ask whether rituximab can be a useful salvage therapy once a patient still has active disease after 6 months of standard-of-care treatment. However, in Dr. Rovin’s opinion, the patients being recruited for the trial are not truly refractory because the failure of two inductiontype regimens before beginning rituximab is not required.
Examining the LUNAR trial failure
The randomized, double-blind, placebo-controlled phase III LUNAR (Lupus Nephritis Assessment With Rituximab) trial – one of the most anticipated clinical trials of lupus nephritis treatment – failed to reach its primary endpoint (Arthritis Rheum. 2012;64:1215-26), said Dr. Rovin, who was the lead author of the report. In fact, in the last few years, none of the trials of novel therapeutics for lupus nephritis have achieved their primary endpoints.
The primary endpoint of renal response (complete and partial) at week 52 was similar in patients who received rituximab or placebo (56.0% vs. 45.8%, respectively), although improvements were seen with rituximab in serum C3 and C4 and anti–double-stranded DNA antibodies. The trial involved 144 patients with class III or IV lupus nephritis treated with rituximab (1,000 mg) or placebo added on to mycophenolate mofetil and corticosteroids. Patients received rituximab or placebo on days 1, 15, 168, and 182.
Did LUNAR fail because it was added on to an already effective therapy? Dr. Rovin asked. He pointed to data from studies of other current therapies, including intravenous cyclophosphamide, which showed relatively high partial responses (63%) but low complete responses (12%-31%). "Current therapies are only modestly effective, and there is plenty of room for improvement. Evaluating add-on therapies to the standard of care is an acceptable trial design for what we can accomplish now," Dr. Rovin said.
The LUNAR trial was powered to detect complete renal remissions, and its failure to detect a difference between treatment groups does not mean that the trial should have been powered to favor partial remissions, Dr. Rovin said. In a study in which he participated that was presented last year at the American Society of Nephrology, Dr. Rovin and his colleagues found that over a period of 4.5 years, patients who have acute kidney injury as evidenced by renal flares were at heightened risk of developing new chronic kidney disease (CKD) or progression of established kidney disease. Patients with new CKD had a significantly greater frequency of new renal flares than did those without CKD (0.72 per year vs. 0.14 per year; P = .001) as well as a greater duration of time spent in flare (20 months vs. 0 months; P = .0003). These results show that "we should not be satisfied with partial remissions as an endpoint, because unresolved kidney inflammation and injury predispose these patients to CKD," he said.
The issue has also been raised as to whether LUNAR failed because its endpoint of 1 year was too short. There may have been better separation between rituximab and placebo with a 2-year endpoint, but Dr. Rovin’s own findings with repeat biopsies following good clinical responses after the first 6 months of conventional therapy (mycophenolate mofetil or intravenous cyclophosphamide for 6 months) have shown evidence of progressive renal scarring despite significant reductions in creatinine and proteinuria. These data suggest that the goal of therapy should be to decrease the time to response as much as possible and thereby perhaps decrease kidney scarring because scarring and damage to the kidney tubulointerstitium are what determine the renal prognosis. "We should not be satisfied with taking a year or more to achieve remissions," he said.
Failure of abatacept lupus nephritis trial
The failure of another recent trial to show any benefit of abatacept in treating refractory lupus nephritis could also be related to the problem of inconsistent trial endpoints making it difficult to compare therapies (Arthritis Rheum. 2012;64:3660-5). For instance, urine protein-creatinine ratios (UPCRs) are subject to individual variability but the criteria for UPCRs have varied from 0.2 to 0.5 in different lupus nephritis trials, including this abatacept trial, the LUNAR trial, the Aspreva Lupus Management Study (ALMS), and the Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis (ACCESS) trial, as well as in the ACR’s recommendations for lupus nephritis.
Estimated glomerular filtration rate (eGFR) criteria also are inconsistent, and endpoints that refer to "normal" eGFRs are hard to interpret, Dr. Rovin said, because it is unclear what is normal for creatine "unless the patient’s true serum creatinine before SLE is known."
"We need uniform, reasonable and consistent response criteria for lupus nephritis clinical trials, and these responses should be associated with long-term outcomes," said Dr. Rovin, who noted that he is involved in preparing a white paper on clinical trial design for lupus nephritis.
Dr. Rovin has served as a consultant to Bristol-Myers Squibb, which markets abatacept, as well as Biogen Idec and Genentech, which comarket rituximab in the United States. He has also received honoraria from Biogen Idec and Genentech and research funding from Roche, Genentech’s parent company.
NEW YORK – Evidence for the use of biologics such as rituximab and abatacept to treat refractory lupus nephritis has been hampered by varying definitions of the condition and design differences in clinical trials, according to Dr. Brad H. Rovin.
Evidence of the benefit to using rituximab 1 g every 2 weeks for refractory lupus nephritis, as suggested by the American College of Rheumatology’s guidelines (Arthritis Care Res. 2012;64:797-808), is hard to confirm with the available data, Dr. Rovin said at a meeting sponsored by New York University.
"The rituximab studies are very difficult to interpret due to small sample size, aggressive concomitant therapies, variable dosing regimens, and nonuniform definitions of refractory," said Dr. Rovin, professor of nephrology and pathology and director of the division of nephrology at Ohio State University in Columbus. "Nonetheless, this collection of data has prompted the idea that rituximab is effective for refractory lupus nephritis."
Although pooled data suggest that rituximab might be helpful in refractory lupus nephritis (Autoimmun. Rev. 2012:11:357-64), only a true randomized controlled trial with sufficient power that is designed to overcome these criticisms will settle the issue, he noted. The European RING (Rituximab for Lupus Nephritis With Remission as a Goal) trial, which started in November 2012, will initially ask whether rituximab can be a useful salvage therapy once a patient still has active disease after 6 months of standard-of-care treatment. However, in Dr. Rovin’s opinion, the patients being recruited for the trial are not truly refractory because the failure of two inductiontype regimens before beginning rituximab is not required.
Examining the LUNAR trial failure
The randomized, double-blind, placebo-controlled phase III LUNAR (Lupus Nephritis Assessment With Rituximab) trial – one of the most anticipated clinical trials of lupus nephritis treatment – failed to reach its primary endpoint (Arthritis Rheum. 2012;64:1215-26), said Dr. Rovin, who was the lead author of the report. In fact, in the last few years, none of the trials of novel therapeutics for lupus nephritis have achieved their primary endpoints.
The primary endpoint of renal response (complete and partial) at week 52 was similar in patients who received rituximab or placebo (56.0% vs. 45.8%, respectively), although improvements were seen with rituximab in serum C3 and C4 and anti–double-stranded DNA antibodies. The trial involved 144 patients with class III or IV lupus nephritis treated with rituximab (1,000 mg) or placebo added on to mycophenolate mofetil and corticosteroids. Patients received rituximab or placebo on days 1, 15, 168, and 182.
Did LUNAR fail because it was added on to an already effective therapy? Dr. Rovin asked. He pointed to data from studies of other current therapies, including intravenous cyclophosphamide, which showed relatively high partial responses (63%) but low complete responses (12%-31%). "Current therapies are only modestly effective, and there is plenty of room for improvement. Evaluating add-on therapies to the standard of care is an acceptable trial design for what we can accomplish now," Dr. Rovin said.
The LUNAR trial was powered to detect complete renal remissions, and its failure to detect a difference between treatment groups does not mean that the trial should have been powered to favor partial remissions, Dr. Rovin said. In a study in which he participated that was presented last year at the American Society of Nephrology, Dr. Rovin and his colleagues found that over a period of 4.5 years, patients who have acute kidney injury as evidenced by renal flares were at heightened risk of developing new chronic kidney disease (CKD) or progression of established kidney disease. Patients with new CKD had a significantly greater frequency of new renal flares than did those without CKD (0.72 per year vs. 0.14 per year; P = .001) as well as a greater duration of time spent in flare (20 months vs. 0 months; P = .0003). These results show that "we should not be satisfied with partial remissions as an endpoint, because unresolved kidney inflammation and injury predispose these patients to CKD," he said.
The issue has also been raised as to whether LUNAR failed because its endpoint of 1 year was too short. There may have been better separation between rituximab and placebo with a 2-year endpoint, but Dr. Rovin’s own findings with repeat biopsies following good clinical responses after the first 6 months of conventional therapy (mycophenolate mofetil or intravenous cyclophosphamide for 6 months) have shown evidence of progressive renal scarring despite significant reductions in creatinine and proteinuria. These data suggest that the goal of therapy should be to decrease the time to response as much as possible and thereby perhaps decrease kidney scarring because scarring and damage to the kidney tubulointerstitium are what determine the renal prognosis. "We should not be satisfied with taking a year or more to achieve remissions," he said.
Failure of abatacept lupus nephritis trial
The failure of another recent trial to show any benefit of abatacept in treating refractory lupus nephritis could also be related to the problem of inconsistent trial endpoints making it difficult to compare therapies (Arthritis Rheum. 2012;64:3660-5). For instance, urine protein-creatinine ratios (UPCRs) are subject to individual variability but the criteria for UPCRs have varied from 0.2 to 0.5 in different lupus nephritis trials, including this abatacept trial, the LUNAR trial, the Aspreva Lupus Management Study (ALMS), and the Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis (ACCESS) trial, as well as in the ACR’s recommendations for lupus nephritis.
Estimated glomerular filtration rate (eGFR) criteria also are inconsistent, and endpoints that refer to "normal" eGFRs are hard to interpret, Dr. Rovin said, because it is unclear what is normal for creatine "unless the patient’s true serum creatinine before SLE is known."
"We need uniform, reasonable and consistent response criteria for lupus nephritis clinical trials, and these responses should be associated with long-term outcomes," said Dr. Rovin, who noted that he is involved in preparing a white paper on clinical trial design for lupus nephritis.
Dr. Rovin has served as a consultant to Bristol-Myers Squibb, which markets abatacept, as well as Biogen Idec and Genentech, which comarket rituximab in the United States. He has also received honoraria from Biogen Idec and Genentech and research funding from Roche, Genentech’s parent company.
EXPERT ANALYSIS FROM THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Stroke outcomes poorer when criteria precluded endovascular therapy
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
SAN DIEGO – Acute ischemic stroke patients who didn’t meet criteria for endovascular intervention were significantly less likely than treated patients to be discharged to home and half as likely to achieve good functional outcomes at 90 days, according to a retrospective study.
More than one-third of the patients excluded from endovascular intervention, however, went on to achieve functional independence at 3 months.
The results suggest that endovascular intervention selection strategies may need to be revamped and made more inclusive, Dr. Ali Shaibani said at the annual meeting of the American Society of Neuroradiology.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention," said Dr. Shaibani, associate professor in radiology and neurological surgery at Northwestern University, Chicago.
In the retrospective study, investigators reviewed the charts of all 110 acute ischemic stroke (AIS) patients who underwent perfusion imaging from February 2010 to August 2012. The inclusion criteria were symptom onset-to-presentation time of 8 hours or less, anterior circulation large-vessel occlusion (either of the middle cerebral artery (MCA) or internal carotid artery (ICA)) as determined by CT/MR angiography, and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 8.
Patients who were selected for endovascular treatment had the following perfusion imaging profiles: cerebral blood volume/diffusion weighted imaging (CBV/DWI) infarct core less than 1/3 MCA territory and mismatch of the ischemic penumbra more than 20% of the infarct core.
"While a good number of studies have been dedicated to investigating the outcomes of AIS patients who undergo endovascular intervention, outcomes have not been well studied in those who are deemed ineligible for endovascular intervention."
Less than half (43.6%) of the patients were found to be eligible for endovascular treatment. Patients who were not selected were significantly older (81 years vs. 74 years, P = .04) and had more risk factors (53.2% vs. 29.2%, P = .04), such as hyperlipidemia (83.9% vs. 25.0%, P = .03), than those selected for treatment. Patients not selected for treatment were less likely to be hypertensive (3.2% vs. 14.6%, P = .05). No significant differences between groups were found for atrial fibrillation or diabetes mellitus.
Patients not selected for endovascular therapy also were more likely to have received intravenous tissue plasminogen activator (TPA) than those selected (64.5% vs. 41.7%, P = .04), and they presented to the hospital significantly earlier (166 min. vs. 250 min., P = .03). That may reflect the finding that 20% of those selected for endovascular therapy transferred from other medical facilities, while none of the nonselected patients were transfers (P = .03).
Despite earlier time to presentation and greater receipt of intravenous TPA, patients who weren’t selected for endovascular intervention were discharged to home at almost one-sixth the rate of selected patients (3.2% vs. 18.8%, P = .001). There were no significant differences in baseline NIHSS scores between nonselected and selected groups (19.35 vs. 18.67), but selected patients had better NIHSS scores at discharge (13.43 vs. 9.8, P = .02).
Although selected patients were also twice as likely (66.7% vs. 35.7%) to have good functional outcomes at 90 days (as defined by modified Rankin Scale scores of 0-2), Dr. Shaibani pointed out that nearly one-third of nonintervention patients did achieve functional independence.
"This suggests the need to refine patient selection strategies for [intra-arterial] intervention and to be more inclusive," said Dr. Shaibani. He suggested reconsidering the importance of other factors besides perfusion imaging criteria, such as the location of the occlusion in the MCA and pre-existing modified Rankin Scale scores.
Dr. Shaibani had no relevant financial relationships.
AT THE ASNR ANNUAL MEETING
Major finding: Acute ischemic stroke patients excluded from endovascular intervention because they failed to meet perfusion imaging criteria were discharged home at nearly one-sixth the rate of treated patients, and they were half as likely to have good functional outcomes at 90 days. However, one-third of those excluded from intervention were able to achieve functional independence at 3 months.
Data source: Retrospective study of 110 patients.
Disclosures: Dr. Shaibani had no relevant financial relationships.
A mitral valve replacement that may grow with the child
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
NEW YORK – Physicians at Boston Children’s Hospital have replaced the mitral valves of eight infants with irreparable mitral valve disease with a valve that offers the opportunity of sequential expansion as the child grows, according to Dr. Sitaram M. Emani, who described the results at the 2013 Mitral Valve Conclave.
"The Melody valve retains its competence if you expand it before putting it in. We asked whether the valve retains the ability to maintain competence even if expansion is performed after implantation as the patient grows," said Dr. Emani, a pediatric cardiac surgeon at Boston Children’s Hospital.
According to Dr. Emani, the current options for infants with damaged mitral valves that are beyond repair are replacement with mechanical or bioprosthetic valves or the Ross mitral procedure. Perhaps the main disadvantage of these options is the lack of a prosthetic valve small enough for an infant, one that is less than 12 mm in diameter. Another problem is the possibility of stenosis developing as the child grows, since the diameters of the prosthetics are fixed. Other drawbacks are that supra-annular fixation is generally associated with poor outcomes and that annular fixation limits the ability to upsize at reoperation.
The Melody valve is an externally stented bovine jugular vein graft that was designed for transcatheter pulmonary valve replacement. In this study, the valve was inserted surgically. The valve maintains competence over a range of sizes up to 22 mm. Although this valve is not approved for use for mitral valve replacement, the hope of using such a prosthetic is that it can be enlarged in the catheterization laboratory as the child grows.
Dr. Emani conducted a retrospective study of his experience with the Melody valve for mitral valve replacement in eight infants less than 12 months of age. The median age at implantation was 6 months (range, 1-9 months). Four infants had an atrioventricular canal (AVC) defect and four had congenital mitral valve stenosis. Most of the children had two prior operations for mitral valve repair. The longest follow-up to date has been 2 years.
At a median follow-up of 8 months, regurgitation on the echocardiogram was considered to be mild or less in all patients. The median gradient was 3 mm Hg (range, 2-7 mm Hg) on the immediate postoperative echocardiogram. Three patients developed a mild paravalvular leak; one of these patients had undergone aggressive stent resection, a modification Dr. Emani does not recommend. One patient developed left ventricular outflow tract obstruction (LVOTO), which Dr. Emani attributed to the lack of distal stent fixation in this patient. Another patient with an AVC defect developed complete heart block.
One patient who died 3 days postoperatively had heterotaxy, severe mitral regurgitation, and prior ventricular failure on extracorporeal membrane oxygenation support. That patient had undergone valve implantation as a last resort.
Three patients underwent sequential expansion about 6 months after implantation. After valve expansion, the median balloon size was 12 mm, ranging from 12 to 16 mm. None of the patients developed worsening valvular function and all had relief of obstruction. Transcatheter intervention was used to correct a paravalvular leak in one patient and to treat a left ventricular outflow tract problem in another. None of the patients developed endocarditis or a strut fracture, "although I worry about strut fracture if aggressive stent resection and manipulation is performed," Dr. Emani said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Dr. Emani offered some procedural tips. First, the Melody valve must be optimized for surgical implantation in infants. The length of the valve must be reduced by trimming it to reduce the chance of LVOTO or pulmonary vein obstruction. He recommends sizing the valves by echocardiogram and fixating the distal stent to the inferior free wall of the ventricle.
Although he has used both circumferential and four-point fixation, Dr. Emani has learned all that is really needed to prevent leakage is friction of the stent against the annulus. Early on he used a pericardial cuff to anchor to the annulus, particularly in patients who had undergone failed AVC repair. He tries to preserve at least part of the anterior leaflet to facilitate suture placement and create a "stand-off" from the LVOTO.
Dr. Emani also advised limiting intraoperative dilation to no more than 1 mm greater than the measured annulus. "Try not to overdilate at implantation to avoid heart block, LVOTO, and coronary compression. The nice thing is you don’t have to decide then and there what size you want. You can go back to the cath lab and, under direct visualization with the coronary view, you can dilate it under more controlled circumstances.
"The hope is that we will be able to dilate these valves as the patients grow into adolescence. If we can dilate them up to 22 mm, hopefully we will decrease the number of repeat replacements, delay the time to reoperation, and perhaps modify our thresholds for tolerating significant disease after unsuccessful repairs," he said. The investigators continue to accumulate experience with this device and hope to design a multicenter trial evaluating its safety and efficacy for this indication.
Dr. Emani had no relevant financial disclosures.
AT THE 2013 MITRAL VALVE CONCLAVE
Major finding: Eight infants with irreparable mitral valve disease underwent mitral valve replacement with the Melody valve, which offers the opportunity of sequential expansion as the child grows.
Data source: Small case series.
Disclosures: Dr. Emani had no relevant financial disclosures.
ICU stay may be unnecessary after elective endovascular aneurysm treatment
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Only 4% of 687 patients undergoing routine endovascular aneurysm treatment experienced postoperative complications and almost three-quarters of these complications occurred within 4 hours of the procedure.
Data source: Retrospective review.
Disclosures: Dr. Patel has no relevant financial disclosures.
EVEREST II: Tight MR control improves MitraClip patients' survival
NEW YORK – In a group of 351 high-risk surgical patients with severe mitral regurgitation (grade 3 or 4) at baseline, more than 80% were alive 1 year after transcatheter treatment with the MitraClip device if their MR was well controlled (MR grade 1+ or 2+), compared with 58% survival for those with persistent severe MR.
These results from the EVEREST II High Surgical Risk cohort were presented by Dr. Paul A. Grayburn at the 2013 Mitral Valve Conclave.
The presentation was chosen as one of the Plenary "Top 10" Abstract Presentations at the meeting.
"Many high-risk surgical patients do not undergo mitral valve surgery and therefore have no therapeutic option for MR. Percutaneous repair with MitraClip is an option for selected patients with suitable anatomy who are too high of a risk for surgery. In this study, we found that the extent of clinical benefit observed corresponds with the degree of MR reduction achieved," said Dr. Grayburn, a cardiologist at the Baylor Heart and Vascular Institute in Dallas. "MR reduction to 1+ or 2+ results in significant clinical and symptomatic improvement."
The MitraClip is a catheter-based valve repair system intended to reduce MR. The device is inserted via the femoral vein using a guide catheter, and the MitraClip implant is attached directly to the mitral valve. On March 20, 2013, the FDA’s Circulatory System Devices Advisory Panel voted by a narrow margin (5-3) in favor of the device, finding that the benefits outweighed the risks.
Data were pooled from the EVEREST II High Risk Study and the EVEREST II REALISM Continued Access Study. The mean age of the 351 patients was 76 years, with 58% of the group over age 75. At baseline, patients had significant comorbidities, including 82% with coronary artery disease, 51% with a prior myocardial infarction, 69% with a history of atrial fibrillation, and 60% with previous cardiovascular surgery. Of the 98% with heart failure, 85% were New York Heart Association (NYHA) functional class III/IV. Aside from cardiac problems, 31% had moderate to severe renal disease, and 29% had chronic obstructive pulmonary disease. Functional MR was present in 70% of the group.
With these health problems in mind, the patients were considered to be at high risk for mitral valve surgery as indicated by a Society of Thoracic Surgeons (STS) risk calculator operative mortality of 12% or more.
Mortality at 1 year was 22.8%. Looking at freedom from mortality at 1 year after treatment, 80.9% of those discharged with MR greater than or equal to 1+ and 80.3% of those discharged with an MR grade of 2+ were alive. For those discharged with a grade of 3+ or 4+, 58.4% were alive (P = .0003).
"As you can see, it is not good for you to leave the operating room after MitraClip with an MR grade of 3+ or 4+," Dr. Grayburn said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Residual MR grade also influenced other measures. For instance, patients with low levels of MR showed better left ventricular remodeling, as measured by larger changes from baseline in left ventricular end diastolic volume (LVEDV) and left ventricular systolic volume. For LVEDV, there was a 15% reduction from baseline in the group with MR greater than or equal to 1+ and a 10% reduction in the 2+ group, but no statistically significant reduction in the 3+/4+ groups.
After treatment, significantly fewer patients with an MR grade greater than or equal to 1+ were considered NYHA functional class III/IV, compared with those with MR 3+ or 4+.
Other benefits of treatment included a 54% reduction in the annual rate of hospitalization due to congestive heart failure in the 12 months after the MitraClip procedure vs. the 12 months pre-MitraClip in patients with an MR grade of 2+ at discharge, compared with no change in hospitalization rate in those discharged with MR greater than 2+. Trends were noted between better MR control and scores on the Short Form-36 (SF-36) quality of life score and the mental component score.
Dr. Grayburn emphasized that the MitraClip procedure is not for everybody but offers an option for those considered at high risk. "If you can operate on these patients, you should; then you can get their MR to null. This device is really a palliative offering for those turned down by a mitral valve surgeon at an experienced mitral valve center. At our center, many patients referred to us for MitraClip actually end up getting surgery because they turn out to be surgical candidates." At his center, Dr. Grayburn says that for every patient who gets a MitraClip, two undergo surgery.
Nevertheless, Dr. Grayburn believes that some high-risk patients who might benefit from MitraClip are unaware of this alternative. "We haven’t figured out how to capture patients with severe symptomatic MR in the general internist’s or even cardiologist’s office."
Dr. Grayburn received research support from Abbott Vascular.
NEW YORK – In a group of 351 high-risk surgical patients with severe mitral regurgitation (grade 3 or 4) at baseline, more than 80% were alive 1 year after transcatheter treatment with the MitraClip device if their MR was well controlled (MR grade 1+ or 2+), compared with 58% survival for those with persistent severe MR.
These results from the EVEREST II High Surgical Risk cohort were presented by Dr. Paul A. Grayburn at the 2013 Mitral Valve Conclave.
The presentation was chosen as one of the Plenary "Top 10" Abstract Presentations at the meeting.
"Many high-risk surgical patients do not undergo mitral valve surgery and therefore have no therapeutic option for MR. Percutaneous repair with MitraClip is an option for selected patients with suitable anatomy who are too high of a risk for surgery. In this study, we found that the extent of clinical benefit observed corresponds with the degree of MR reduction achieved," said Dr. Grayburn, a cardiologist at the Baylor Heart and Vascular Institute in Dallas. "MR reduction to 1+ or 2+ results in significant clinical and symptomatic improvement."
The MitraClip is a catheter-based valve repair system intended to reduce MR. The device is inserted via the femoral vein using a guide catheter, and the MitraClip implant is attached directly to the mitral valve. On March 20, 2013, the FDA’s Circulatory System Devices Advisory Panel voted by a narrow margin (5-3) in favor of the device, finding that the benefits outweighed the risks.
Data were pooled from the EVEREST II High Risk Study and the EVEREST II REALISM Continued Access Study. The mean age of the 351 patients was 76 years, with 58% of the group over age 75. At baseline, patients had significant comorbidities, including 82% with coronary artery disease, 51% with a prior myocardial infarction, 69% with a history of atrial fibrillation, and 60% with previous cardiovascular surgery. Of the 98% with heart failure, 85% were New York Heart Association (NYHA) functional class III/IV. Aside from cardiac problems, 31% had moderate to severe renal disease, and 29% had chronic obstructive pulmonary disease. Functional MR was present in 70% of the group.
With these health problems in mind, the patients were considered to be at high risk for mitral valve surgery as indicated by a Society of Thoracic Surgeons (STS) risk calculator operative mortality of 12% or more.
Mortality at 1 year was 22.8%. Looking at freedom from mortality at 1 year after treatment, 80.9% of those discharged with MR greater than or equal to 1+ and 80.3% of those discharged with an MR grade of 2+ were alive. For those discharged with a grade of 3+ or 4+, 58.4% were alive (P = .0003).
"As you can see, it is not good for you to leave the operating room after MitraClip with an MR grade of 3+ or 4+," Dr. Grayburn said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Residual MR grade also influenced other measures. For instance, patients with low levels of MR showed better left ventricular remodeling, as measured by larger changes from baseline in left ventricular end diastolic volume (LVEDV) and left ventricular systolic volume. For LVEDV, there was a 15% reduction from baseline in the group with MR greater than or equal to 1+ and a 10% reduction in the 2+ group, but no statistically significant reduction in the 3+/4+ groups.
After treatment, significantly fewer patients with an MR grade greater than or equal to 1+ were considered NYHA functional class III/IV, compared with those with MR 3+ or 4+.
Other benefits of treatment included a 54% reduction in the annual rate of hospitalization due to congestive heart failure in the 12 months after the MitraClip procedure vs. the 12 months pre-MitraClip in patients with an MR grade of 2+ at discharge, compared with no change in hospitalization rate in those discharged with MR greater than 2+. Trends were noted between better MR control and scores on the Short Form-36 (SF-36) quality of life score and the mental component score.
Dr. Grayburn emphasized that the MitraClip procedure is not for everybody but offers an option for those considered at high risk. "If you can operate on these patients, you should; then you can get their MR to null. This device is really a palliative offering for those turned down by a mitral valve surgeon at an experienced mitral valve center. At our center, many patients referred to us for MitraClip actually end up getting surgery because they turn out to be surgical candidates." At his center, Dr. Grayburn says that for every patient who gets a MitraClip, two undergo surgery.
Nevertheless, Dr. Grayburn believes that some high-risk patients who might benefit from MitraClip are unaware of this alternative. "We haven’t figured out how to capture patients with severe symptomatic MR in the general internist’s or even cardiologist’s office."
Dr. Grayburn received research support from Abbott Vascular.
NEW YORK – In a group of 351 high-risk surgical patients with severe mitral regurgitation (grade 3 or 4) at baseline, more than 80% were alive 1 year after transcatheter treatment with the MitraClip device if their MR was well controlled (MR grade 1+ or 2+), compared with 58% survival for those with persistent severe MR.
These results from the EVEREST II High Surgical Risk cohort were presented by Dr. Paul A. Grayburn at the 2013 Mitral Valve Conclave.
The presentation was chosen as one of the Plenary "Top 10" Abstract Presentations at the meeting.
"Many high-risk surgical patients do not undergo mitral valve surgery and therefore have no therapeutic option for MR. Percutaneous repair with MitraClip is an option for selected patients with suitable anatomy who are too high of a risk for surgery. In this study, we found that the extent of clinical benefit observed corresponds with the degree of MR reduction achieved," said Dr. Grayburn, a cardiologist at the Baylor Heart and Vascular Institute in Dallas. "MR reduction to 1+ or 2+ results in significant clinical and symptomatic improvement."
The MitraClip is a catheter-based valve repair system intended to reduce MR. The device is inserted via the femoral vein using a guide catheter, and the MitraClip implant is attached directly to the mitral valve. On March 20, 2013, the FDA’s Circulatory System Devices Advisory Panel voted by a narrow margin (5-3) in favor of the device, finding that the benefits outweighed the risks.
Data were pooled from the EVEREST II High Risk Study and the EVEREST II REALISM Continued Access Study. The mean age of the 351 patients was 76 years, with 58% of the group over age 75. At baseline, patients had significant comorbidities, including 82% with coronary artery disease, 51% with a prior myocardial infarction, 69% with a history of atrial fibrillation, and 60% with previous cardiovascular surgery. Of the 98% with heart failure, 85% were New York Heart Association (NYHA) functional class III/IV. Aside from cardiac problems, 31% had moderate to severe renal disease, and 29% had chronic obstructive pulmonary disease. Functional MR was present in 70% of the group.
With these health problems in mind, the patients were considered to be at high risk for mitral valve surgery as indicated by a Society of Thoracic Surgeons (STS) risk calculator operative mortality of 12% or more.
Mortality at 1 year was 22.8%. Looking at freedom from mortality at 1 year after treatment, 80.9% of those discharged with MR greater than or equal to 1+ and 80.3% of those discharged with an MR grade of 2+ were alive. For those discharged with a grade of 3+ or 4+, 58.4% were alive (P = .0003).
"As you can see, it is not good for you to leave the operating room after MitraClip with an MR grade of 3+ or 4+," Dr. Grayburn said at the meeting, which was sponsored by the American Association for Thoracic Surgery.
Residual MR grade also influenced other measures. For instance, patients with low levels of MR showed better left ventricular remodeling, as measured by larger changes from baseline in left ventricular end diastolic volume (LVEDV) and left ventricular systolic volume. For LVEDV, there was a 15% reduction from baseline in the group with MR greater than or equal to 1+ and a 10% reduction in the 2+ group, but no statistically significant reduction in the 3+/4+ groups.
After treatment, significantly fewer patients with an MR grade greater than or equal to 1+ were considered NYHA functional class III/IV, compared with those with MR 3+ or 4+.
Other benefits of treatment included a 54% reduction in the annual rate of hospitalization due to congestive heart failure in the 12 months after the MitraClip procedure vs. the 12 months pre-MitraClip in patients with an MR grade of 2+ at discharge, compared with no change in hospitalization rate in those discharged with MR greater than 2+. Trends were noted between better MR control and scores on the Short Form-36 (SF-36) quality of life score and the mental component score.
Dr. Grayburn emphasized that the MitraClip procedure is not for everybody but offers an option for those considered at high risk. "If you can operate on these patients, you should; then you can get their MR to null. This device is really a palliative offering for those turned down by a mitral valve surgeon at an experienced mitral valve center. At our center, many patients referred to us for MitraClip actually end up getting surgery because they turn out to be surgical candidates." At his center, Dr. Grayburn says that for every patient who gets a MitraClip, two undergo surgery.
Nevertheless, Dr. Grayburn believes that some high-risk patients who might benefit from MitraClip are unaware of this alternative. "We haven’t figured out how to capture patients with severe symptomatic MR in the general internist’s or even cardiologist’s office."
Dr. Grayburn received research support from Abbott Vascular.
AT THE 2013 MITRAL VALVE CONCLAVE
Major finding: In a study of 351 high-risk patients with severe mitral regurgitation (MR) who underwent repair with the MitraClip device, survival was more than 80% for those with well-controlled MR, compared with 58% for those with persistent severe MR.
Data source: The prospective EVEREST II High Risk Study and the EVEREST II REALISM Continued Access Study.
Disclosures: Dr. Grayburn received research support from Abbott Vascular.
Stent-retriever outcomes good in stroke patients with 'T' occlusions
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
SAN DIEGO – Stent retrievers were associated with higher rates of recanalization, shorter recanalization times, and better outcomes than multimodality recanalization strategies, based on a retrospective, single-center chart review of 60 acute stroke patients with a substantial proportion of carotid "T" occlusions.
The study, presented at the annual meeting of the American Society of Neuroradiology, included 23 patients treated primarily with stent retrievers (Solitaire AB neurovascular remodeling device) and 37 treated with other modalities.
The multimodality group received intra-arterial tissue plasminogen activator therapy (10), mechanical thromboaspiration (18), angioplasty (6), earlier-generation mechanical retriever devices (2), and stent only (1). The two groups were similar in age, gender, intravenous tissue plasminogen activator use (65% and 68%), and stroke risk factors.
Terminal carotid "T" occlusions, which generally respond poorly to thrombolysis, were present in 12 of the 23 patients in the stent-retrieval group and in 12 of the 37 patients in the multimodality cohort, said Dr. Cheemun Lum, a neurointerventional radiologist at the Ottawa Hospital, University of Ottawa.
The stent-retriever group had higher recanalization rates than the multimodality group (87% vs. 51%, P = .005). There was no significant difference in median time to recanalization between groups (68 minutes vs. 79 minutes).
Mortality was eight-fold higher in the multimodality group than in the stent-retriever group (32% vs. 4%, P = .011). At 30-day follow-up, 61% of the stent retriever group had good outcomes, defined as a Modified Rankin Scale score of 2 or less. When the outcomes definition was expanded to include a 10-point reduction in score on the National Institutes of Health Stroke Scale, 78% of the stent-retriever group had good outcomes at 30 days, compared with 43% in the multimodality group (P = .008).
Restricting the analysis to patients with terminal carotid "T" occlusions, recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) and recanalization times were shorter (69 minutes vs. 191 minutes, P = .002). Three times as many patients with terminal carotid "T" occlusions in the stent-retriever group had good outcomes as did those undergoing older techniques (67% vs. 22%, P = .04).
Dr. Lum said that he had no financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Recanalization rates were higher in the stent-retriever group (83% vs. 38%, P = .012) when the analysis was restricted to patients with terminal carotid "T" occlusions.
Data source: Single-center chart review of 60 acute stroke patients.
Disclosures: Dr. Lum said that he had no financial disclosures.
Mitral valve bioprosthetic shows durability
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the AATS Mitral Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the AATS Mitral Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
NEW YORK – After following more than 400 patients who underwent mitral valve replacement for almost 25 years, French investigators have found that the Carpentier-Edwards Perimount Pericardial prosthetic has an expected durability of more than 16 years, with a low incidence of valve-related complications. The findings were presented by Dr. Thierry Bourguignon as one of the Plenary "Top 10" abstracts at the AATS Mitral Conclave.
"This is a great study. This very-long-term data has been missing for the Carpentier-Edwards Perimount bioprosthetic," said session moderator Dr. David H. Adams of Mount Sinai Medical Center in New York.
In this prospective study, investigators followed 404 patients who underwent mitral valve replacement between August 1984 and March 2011; 46 of these patients eventually needed a second bioprosthesis. Patients were asked to complete yearly clinical questionnaires and undergo an echocardiographic study. Their mean age was 68 years, but the range was 22-89 years. Almost one-fifth of the group was aged 60 years or younger. The mean follow-up time was 7.2 years, although it ranged from 0 to 24.8 years. Ten patients were lost during follow-up, yielding almost a 98% completion rate. Fifty-seven percent were New York Heart Association (NYHA) class III or IV.
The operative mortality rate was 3.3%. A total of 188 patients had late death (5.8%/valve-year). Forty of the deaths were valve related, including 5 due to thromboembolism, 4 to hemorrhage, 4 to endocarditis, 4 to structural valve dysfunction (SVD), and 23 to sudden death. Valve-related survival was more than 60% at 20 years post surgery. Risk factors affecting late survival were age at implant (hazard ratio, 1.06; P less than .001) and preoperative NYHA class III or IV (HR, 1.86; P less than .001).
"Valve-related events, including endocarditis, thromboembolism, and bleeding, were rare," said Dr. Bourguignon, a cardiovascular surgeon at Trousseau Hospital in Chambray Les Tours, France. There were no cases of valve thrombosis.
Seventy-six patients had an SVD, which was defined by echocardiography as severe mitral regurgitation and/or having a mean gradient of more than 8 mm Hg, even if patients were asymptomatic. Of these, 63 were reoperated and 13 died before reoperation. Three-quarters of the valves failed due to calcification, while 20% had late leaflet tears and 4% had mixed problems.
For the entire group, it took an average of 16.6 years before an SVD occurred, although freedom from SVD differed according to age at surgery. Older patients fared better. After 16.6 years, 75% of those over age 70 were expected to be free from SVD, while the rates were lower for those between 60 and 70 years (52%) and those under age 60 (40%). Older patients were also less likely to need the valve removed due to SVD.
What should a surgeon tell a patient about the risk of needing another operation to replace a failing mitral valve bioprosthetic? Using competing risk analysis, the authors predict that, for example, a 60-year-old patient at time of surgery will have a 20% chance of requiring reoperation due to an SVD after 11.9 years.
"In our experience, the CE Perimount valve is a reliable choice for patients older than 60, depending on the accepted risk of reoperation," said Dr. Bourguignon. Addressing a question from the audience, Dr. Bourguignon suggested that although the 25-year data were not available as yet for aortic valve replacements, preliminary findings indicate aortic valve replacement with bioprosthetics lasts longer than mitral valve replacement. At his hospital, patients under age 60 generally receive mechanical valves.
The conclave was sponsored by the American Association for Thoracic Surgery.
Dr. Bourguignon has a financial relationship with Edwards Lifesciences.
AT THE 2013 MITRAL CONCLAVE
Major finding: The mean durability of the Carpentier-Edwards Perimount Pericardial prosthetic was 16.6 years before a structural valve dysfunction occurred.
Data source: Prospective study of 404 patients who underwent mitral valve replacement using the Perimount prosthetic.
Disclosures: Dr. Bourguignon has a financial relationship with Edwards Lifesciences.