User login
Metal Orthopedic Implants Unlikely to Trigger Allergy
NAPA, CALIF. – There is little risk that patients with orthopedic metal implants will have a skin reaction or joint pain from the device, said Dr. Joseph F. Fowler.
"I do patch test patients ... who have concerns about a metal allergy or are going to have an implant. I tell them if they have a positive patch test, they may have a skin reaction," said Dr. Fowler. However, there is a small chance they will have a cutaneous reaction, and a smaller chance they will have problems with the joint.
Metals used in orthopedic implants include stainless steel, which is composed of an iron-chromium alloy plus nickel and molybdenum, and sometimes a little chromium and titanium; nitinol (55% nickel and 45% titanium); vitallium (iron cobalt plus chromium 30% and molybdenum 5%); and titanium. Two of the four metals contain nickel, a common contact allergen, noted Dr. Fowler, a dermatologist at the University of Louisville (Ky.).
When it comes to joint pain from implants, findings from a small German study of 15 patients showed that levels of the inflammatory marker interleukin-17 (IL-17) were increased in only those patients with nickel containing replacement joints who both had a positive patch test to nickel and complaints of joint pain. IL-17 levels were not increased in patients with a positive patch test to nickel who did not complain of joint pain (Contact Dermatitis 2010;63:15-22).
In an unpublished study, Dr. James Taylor of the Cleveland Clinic found that 60% of a small group of patients (10) with a metal implant who had a positive patch to metal experienced resolution of joint pain after the metal was replaced. "There is getting to be more and more of a suggestion that metal allergies and joint problems do go hand in hand," said Dr. Fowler.
If you just had to guess what would be the best material to put in a patient who with a chance of a metal allergy, titanium is the best bet, he said. "It comes down to a medical legal question. Will doctors and patients accept a small amount of risk if there is an implant that is better than titanium?"
He said that he will patch test patients if requested by the physician who will be performing the implant surgery, but it is not normally done because there is less than a 1% chance that even if they do have a positive patch test to metal, dermatitis will occur. There have been reports, although rare, that a metal allergy may cause joint failure, but prospective studies are needed, he said.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies.
NAPA, CALIF. – There is little risk that patients with orthopedic metal implants will have a skin reaction or joint pain from the device, said Dr. Joseph F. Fowler.
"I do patch test patients ... who have concerns about a metal allergy or are going to have an implant. I tell them if they have a positive patch test, they may have a skin reaction," said Dr. Fowler. However, there is a small chance they will have a cutaneous reaction, and a smaller chance they will have problems with the joint.
Metals used in orthopedic implants include stainless steel, which is composed of an iron-chromium alloy plus nickel and molybdenum, and sometimes a little chromium and titanium; nitinol (55% nickel and 45% titanium); vitallium (iron cobalt plus chromium 30% and molybdenum 5%); and titanium. Two of the four metals contain nickel, a common contact allergen, noted Dr. Fowler, a dermatologist at the University of Louisville (Ky.).
When it comes to joint pain from implants, findings from a small German study of 15 patients showed that levels of the inflammatory marker interleukin-17 (IL-17) were increased in only those patients with nickel containing replacement joints who both had a positive patch test to nickel and complaints of joint pain. IL-17 levels were not increased in patients with a positive patch test to nickel who did not complain of joint pain (Contact Dermatitis 2010;63:15-22).
In an unpublished study, Dr. James Taylor of the Cleveland Clinic found that 60% of a small group of patients (10) with a metal implant who had a positive patch to metal experienced resolution of joint pain after the metal was replaced. "There is getting to be more and more of a suggestion that metal allergies and joint problems do go hand in hand," said Dr. Fowler.
If you just had to guess what would be the best material to put in a patient who with a chance of a metal allergy, titanium is the best bet, he said. "It comes down to a medical legal question. Will doctors and patients accept a small amount of risk if there is an implant that is better than titanium?"
He said that he will patch test patients if requested by the physician who will be performing the implant surgery, but it is not normally done because there is less than a 1% chance that even if they do have a positive patch test to metal, dermatitis will occur. There have been reports, although rare, that a metal allergy may cause joint failure, but prospective studies are needed, he said.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies.
NAPA, CALIF. – There is little risk that patients with orthopedic metal implants will have a skin reaction or joint pain from the device, said Dr. Joseph F. Fowler.
"I do patch test patients ... who have concerns about a metal allergy or are going to have an implant. I tell them if they have a positive patch test, they may have a skin reaction," said Dr. Fowler. However, there is a small chance they will have a cutaneous reaction, and a smaller chance they will have problems with the joint.
Metals used in orthopedic implants include stainless steel, which is composed of an iron-chromium alloy plus nickel and molybdenum, and sometimes a little chromium and titanium; nitinol (55% nickel and 45% titanium); vitallium (iron cobalt plus chromium 30% and molybdenum 5%); and titanium. Two of the four metals contain nickel, a common contact allergen, noted Dr. Fowler, a dermatologist at the University of Louisville (Ky.).
When it comes to joint pain from implants, findings from a small German study of 15 patients showed that levels of the inflammatory marker interleukin-17 (IL-17) were increased in only those patients with nickel containing replacement joints who both had a positive patch test to nickel and complaints of joint pain. IL-17 levels were not increased in patients with a positive patch test to nickel who did not complain of joint pain (Contact Dermatitis 2010;63:15-22).
In an unpublished study, Dr. James Taylor of the Cleveland Clinic found that 60% of a small group of patients (10) with a metal implant who had a positive patch to metal experienced resolution of joint pain after the metal was replaced. "There is getting to be more and more of a suggestion that metal allergies and joint problems do go hand in hand," said Dr. Fowler.
If you just had to guess what would be the best material to put in a patient who with a chance of a metal allergy, titanium is the best bet, he said. "It comes down to a medical legal question. Will doctors and patients accept a small amount of risk if there is an implant that is better than titanium?"
He said that he will patch test patients if requested by the physician who will be performing the implant surgery, but it is not normally done because there is less than a 1% chance that even if they do have a positive patch test to metal, dermatitis will occur. There have been reports, although rare, that a metal allergy may cause joint failure, but prospective studies are needed, he said.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Pharma-Sponsored Comparison Studies Hold Clinical Value
NAPA, CALIF. – "You are almost never going to see a study where the sponsoring company’s product wasn’t at least as good if not better," than the comparator drug, said Dr. Joseph F. Fowler. But that reality does not necessarily mean the study results don’t provide good clinical information.
"Once a drug goes generic, you’re almost never going to see any further study on it."
Most comparison studies are done by pharmaceutical companies and are limited to comparing competing drugs to the company’s branded drugs, explained Dr. Fowler, a dermatologist at the University of Louisville (Ky.). Also, "once a drug goes generic, you’re almost never going to see any further study on it."
Dr. Fowler discussed several comparison studies that run across the dermatology gamut at the meeting, and then asked the audience which drug they prefer.
Ivermectin Vs. Malathion for Lice
In a recent comparison of oral ivermectin to malathion lotion for difficult-to-treat head lice, ivermectin came out slightly on top in terms of clearance at day 15 (95% vs. 85%, respectively), he reported.
In the multicenter trial, 812 patients from 376 households were randomized to receive 400 mcg/kg of body weight of ivermectin or 0.5% malathion lotion on days 1 and 8. The study patients had lice that were not cleared with a topical insecticide 2-6 weeks before study enrollment (N. Engl. J. Med. 2010;362:896-905).
"I tend to use more ivermectin when I see these patients than I ever used to, and I tend to use less topicals," said Dr. Fowler. However, systemic drugs probably have more of a chance of causing toxicity than topicals.
Ivermectin Vs. Permethrin for Scabies
In another comparison of oral ivermectin, this time for scabies, permethrin came out slightly on top. Patients with scabies (n = 85) were randomized to treatment with 200 mcg/kg body weight of ivermectin or a single topical application of 5% permethrin cream. Clearance was seen in 95% of permethrin-treated patients, compared with 70% of ivermectin-treated patients at 8 weeks (J. Am. Acad. Dermatol. 2000;42:236-40).
Topical permethrin was also found to be more effective than topical crotamiton, topical lindane, and oral ivermectin for scabies in a Cochrane database system review (Arch. Dermatol. 2008;144:1638-40).
A show of hands in the audience found that the room was divided on their preferred treatment for head lice and scabies.
Griseofulvin Vs. Terbinafine for Tinea Capitis
A meta-analysis of seven randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis resulted in a draw. However, griseofulvin was found to be slightly better for the Microsporum species, while terbinafine was found to be slightly more effective for the Trichophyton species (J. Am. Acad. Dermatol. 2011;64:663-70).
If cost is an issue, terbinafine is the cheaper drug, noted Dr. Fowler, who said the cost is about $4 in Kentucky. Further, treatment time is shorter with terbinafine at 4 weeks as compared with griseofulvin at 8 weeks. Also, terbinafine appears to be associated with fewer adverse effects than griseofulvin.
The majority of the audience members agreed, via a show of hands, noting their preference for terbinafine to treat children and adults with tinea capitis.
Tazarotene Vs. Adapalene for Acne
In a 16-week, multicenter, randomized, investigator-blinded study, patients treated once a day with tazarotene 0.1% cream had better acne lesion clearance than patients treated with adapalene 0.3% gel. More patients treated with tazarotene had at least a 50% reduction in lesions and less disease severity as compared with adapalene-treated patients (J. Drugs Dermatol. 2010;9:549-58).
However, tazarotene was somewhat less tolerated than adapalene, reported Dr. Fowler. Erythema occurred in 6% of tazarotene patients, dryness in 7%, and peeling in 11%. In the adapalene group, peeling was the only adverse event and was reported by 1% of patients.
By a show of hands, the majority of the audience members prefer adapalene.
Calcitriol Vs. Calcipotriol for Psoriasis
Calcitriol ointment was found to be better tolerated than calcipotriol for treating mild to moderate psoriasis in sensitive areas, such as on the face, around the hairline, and behind the ears (Br. J. Dermatol. 2003;148:326-33).
In the left-to-right, randomized, multicenter, investigator-blinded study, calcitriol 3 mcg ointment was compared with calcipotriol 50 mcg ointment in 75 patients. After 6 weeks, study patients reported preferring calcitriol ointment, which demonstrated "superior" tolerability and equal efficacy, reported Dr. Fowler. Adverse events (contact or irritant dermatitis) occurred in eight patients on the calcipotriol-treated side of their face (one patient reported dermatitis on both sides).
When asked to take cost and possible systemic adverse events into consideration, most audience members noted a preference for calcitriol ointment.
Methotrexate Vs. Azathioprine for Atopy
In the treatment of severe atopic dermatitis, methotrexate and azathioprine were found to be almost equal in efficacy (42% vs. 39%, respectively). Patients (52 completed the study) were randomized to receive 10-22.5 mg of methotrexate per week or 1.5-2.2 mg/kg per day for 12 weeks (J. Allergy Clin. Immunol. 2011;128:353-9). Outcome was assessed by blinded reviewers.
"I think that the adverse event profile of methotrexate in most people is much better, so I tend to disagree with the outcome of this study," said Dr. Fowler.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies, including many of the products mentioned during his presentation.
NAPA, CALIF. – "You are almost never going to see a study where the sponsoring company’s product wasn’t at least as good if not better," than the comparator drug, said Dr. Joseph F. Fowler. But that reality does not necessarily mean the study results don’t provide good clinical information.
"Once a drug goes generic, you’re almost never going to see any further study on it."
Most comparison studies are done by pharmaceutical companies and are limited to comparing competing drugs to the company’s branded drugs, explained Dr. Fowler, a dermatologist at the University of Louisville (Ky.). Also, "once a drug goes generic, you’re almost never going to see any further study on it."
Dr. Fowler discussed several comparison studies that run across the dermatology gamut at the meeting, and then asked the audience which drug they prefer.
Ivermectin Vs. Malathion for Lice
In a recent comparison of oral ivermectin to malathion lotion for difficult-to-treat head lice, ivermectin came out slightly on top in terms of clearance at day 15 (95% vs. 85%, respectively), he reported.
In the multicenter trial, 812 patients from 376 households were randomized to receive 400 mcg/kg of body weight of ivermectin or 0.5% malathion lotion on days 1 and 8. The study patients had lice that were not cleared with a topical insecticide 2-6 weeks before study enrollment (N. Engl. J. Med. 2010;362:896-905).
"I tend to use more ivermectin when I see these patients than I ever used to, and I tend to use less topicals," said Dr. Fowler. However, systemic drugs probably have more of a chance of causing toxicity than topicals.
Ivermectin Vs. Permethrin for Scabies
In another comparison of oral ivermectin, this time for scabies, permethrin came out slightly on top. Patients with scabies (n = 85) were randomized to treatment with 200 mcg/kg body weight of ivermectin or a single topical application of 5% permethrin cream. Clearance was seen in 95% of permethrin-treated patients, compared with 70% of ivermectin-treated patients at 8 weeks (J. Am. Acad. Dermatol. 2000;42:236-40).
Topical permethrin was also found to be more effective than topical crotamiton, topical lindane, and oral ivermectin for scabies in a Cochrane database system review (Arch. Dermatol. 2008;144:1638-40).
A show of hands in the audience found that the room was divided on their preferred treatment for head lice and scabies.
Griseofulvin Vs. Terbinafine for Tinea Capitis
A meta-analysis of seven randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis resulted in a draw. However, griseofulvin was found to be slightly better for the Microsporum species, while terbinafine was found to be slightly more effective for the Trichophyton species (J. Am. Acad. Dermatol. 2011;64:663-70).
If cost is an issue, terbinafine is the cheaper drug, noted Dr. Fowler, who said the cost is about $4 in Kentucky. Further, treatment time is shorter with terbinafine at 4 weeks as compared with griseofulvin at 8 weeks. Also, terbinafine appears to be associated with fewer adverse effects than griseofulvin.
The majority of the audience members agreed, via a show of hands, noting their preference for terbinafine to treat children and adults with tinea capitis.
Tazarotene Vs. Adapalene for Acne
In a 16-week, multicenter, randomized, investigator-blinded study, patients treated once a day with tazarotene 0.1% cream had better acne lesion clearance than patients treated with adapalene 0.3% gel. More patients treated with tazarotene had at least a 50% reduction in lesions and less disease severity as compared with adapalene-treated patients (J. Drugs Dermatol. 2010;9:549-58).
However, tazarotene was somewhat less tolerated than adapalene, reported Dr. Fowler. Erythema occurred in 6% of tazarotene patients, dryness in 7%, and peeling in 11%. In the adapalene group, peeling was the only adverse event and was reported by 1% of patients.
By a show of hands, the majority of the audience members prefer adapalene.
Calcitriol Vs. Calcipotriol for Psoriasis
Calcitriol ointment was found to be better tolerated than calcipotriol for treating mild to moderate psoriasis in sensitive areas, such as on the face, around the hairline, and behind the ears (Br. J. Dermatol. 2003;148:326-33).
In the left-to-right, randomized, multicenter, investigator-blinded study, calcitriol 3 mcg ointment was compared with calcipotriol 50 mcg ointment in 75 patients. After 6 weeks, study patients reported preferring calcitriol ointment, which demonstrated "superior" tolerability and equal efficacy, reported Dr. Fowler. Adverse events (contact or irritant dermatitis) occurred in eight patients on the calcipotriol-treated side of their face (one patient reported dermatitis on both sides).
When asked to take cost and possible systemic adverse events into consideration, most audience members noted a preference for calcitriol ointment.
Methotrexate Vs. Azathioprine for Atopy
In the treatment of severe atopic dermatitis, methotrexate and azathioprine were found to be almost equal in efficacy (42% vs. 39%, respectively). Patients (52 completed the study) were randomized to receive 10-22.5 mg of methotrexate per week or 1.5-2.2 mg/kg per day for 12 weeks (J. Allergy Clin. Immunol. 2011;128:353-9). Outcome was assessed by blinded reviewers.
"I think that the adverse event profile of methotrexate in most people is much better, so I tend to disagree with the outcome of this study," said Dr. Fowler.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies, including many of the products mentioned during his presentation.
NAPA, CALIF. – "You are almost never going to see a study where the sponsoring company’s product wasn’t at least as good if not better," than the comparator drug, said Dr. Joseph F. Fowler. But that reality does not necessarily mean the study results don’t provide good clinical information.
"Once a drug goes generic, you’re almost never going to see any further study on it."
Most comparison studies are done by pharmaceutical companies and are limited to comparing competing drugs to the company’s branded drugs, explained Dr. Fowler, a dermatologist at the University of Louisville (Ky.). Also, "once a drug goes generic, you’re almost never going to see any further study on it."
Dr. Fowler discussed several comparison studies that run across the dermatology gamut at the meeting, and then asked the audience which drug they prefer.
Ivermectin Vs. Malathion for Lice
In a recent comparison of oral ivermectin to malathion lotion for difficult-to-treat head lice, ivermectin came out slightly on top in terms of clearance at day 15 (95% vs. 85%, respectively), he reported.
In the multicenter trial, 812 patients from 376 households were randomized to receive 400 mcg/kg of body weight of ivermectin or 0.5% malathion lotion on days 1 and 8. The study patients had lice that were not cleared with a topical insecticide 2-6 weeks before study enrollment (N. Engl. J. Med. 2010;362:896-905).
"I tend to use more ivermectin when I see these patients than I ever used to, and I tend to use less topicals," said Dr. Fowler. However, systemic drugs probably have more of a chance of causing toxicity than topicals.
Ivermectin Vs. Permethrin for Scabies
In another comparison of oral ivermectin, this time for scabies, permethrin came out slightly on top. Patients with scabies (n = 85) were randomized to treatment with 200 mcg/kg body weight of ivermectin or a single topical application of 5% permethrin cream. Clearance was seen in 95% of permethrin-treated patients, compared with 70% of ivermectin-treated patients at 8 weeks (J. Am. Acad. Dermatol. 2000;42:236-40).
Topical permethrin was also found to be more effective than topical crotamiton, topical lindane, and oral ivermectin for scabies in a Cochrane database system review (Arch. Dermatol. 2008;144:1638-40).
A show of hands in the audience found that the room was divided on their preferred treatment for head lice and scabies.
Griseofulvin Vs. Terbinafine for Tinea Capitis
A meta-analysis of seven randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis resulted in a draw. However, griseofulvin was found to be slightly better for the Microsporum species, while terbinafine was found to be slightly more effective for the Trichophyton species (J. Am. Acad. Dermatol. 2011;64:663-70).
If cost is an issue, terbinafine is the cheaper drug, noted Dr. Fowler, who said the cost is about $4 in Kentucky. Further, treatment time is shorter with terbinafine at 4 weeks as compared with griseofulvin at 8 weeks. Also, terbinafine appears to be associated with fewer adverse effects than griseofulvin.
The majority of the audience members agreed, via a show of hands, noting their preference for terbinafine to treat children and adults with tinea capitis.
Tazarotene Vs. Adapalene for Acne
In a 16-week, multicenter, randomized, investigator-blinded study, patients treated once a day with tazarotene 0.1% cream had better acne lesion clearance than patients treated with adapalene 0.3% gel. More patients treated with tazarotene had at least a 50% reduction in lesions and less disease severity as compared with adapalene-treated patients (J. Drugs Dermatol. 2010;9:549-58).
However, tazarotene was somewhat less tolerated than adapalene, reported Dr. Fowler. Erythema occurred in 6% of tazarotene patients, dryness in 7%, and peeling in 11%. In the adapalene group, peeling was the only adverse event and was reported by 1% of patients.
By a show of hands, the majority of the audience members prefer adapalene.
Calcitriol Vs. Calcipotriol for Psoriasis
Calcitriol ointment was found to be better tolerated than calcipotriol for treating mild to moderate psoriasis in sensitive areas, such as on the face, around the hairline, and behind the ears (Br. J. Dermatol. 2003;148:326-33).
In the left-to-right, randomized, multicenter, investigator-blinded study, calcitriol 3 mcg ointment was compared with calcipotriol 50 mcg ointment in 75 patients. After 6 weeks, study patients reported preferring calcitriol ointment, which demonstrated "superior" tolerability and equal efficacy, reported Dr. Fowler. Adverse events (contact or irritant dermatitis) occurred in eight patients on the calcipotriol-treated side of their face (one patient reported dermatitis on both sides).
When asked to take cost and possible systemic adverse events into consideration, most audience members noted a preference for calcitriol ointment.
Methotrexate Vs. Azathioprine for Atopy
In the treatment of severe atopic dermatitis, methotrexate and azathioprine were found to be almost equal in efficacy (42% vs. 39%, respectively). Patients (52 completed the study) were randomized to receive 10-22.5 mg of methotrexate per week or 1.5-2.2 mg/kg per day for 12 weeks (J. Allergy Clin. Immunol. 2011;128:353-9). Outcome was assessed by blinded reviewers.
"I think that the adverse event profile of methotrexate in most people is much better, so I tend to disagree with the outcome of this study," said Dr. Fowler.
Dr. Fowler reported financial conflicts of interest with multiple pharmaceutical companies, including many of the products mentioned during his presentation.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Natural Disaster Sites Breed Serious Infections
NAPA, CALIF. – Although the risk of a serious disease outbreak after natural disasters is rare and often exaggerated by the media, it is important to be prepared to respond to patient infections after such an event, according to Dr. Miriam S. Bettencourt of the University of Nevada in Las Vegas.
Some of the conditions patients may present with after natural disasters include bacterial and fungal infections, leptospirosis, measles, dengue fever, trench foot, arthropod bites, and radiation exposure. Dr. Bettencourt described some of the conditions in detail during her presentation at the Coastal Dermatology Symposisum.
Vibrio
After Hurricane Katrina in 2005, there were 18 reported cases of Vibrio-associated illness because of exposure of patients’ wounds to coastal flood waters. The warm seawater of the Atlantic Ocean that flooded the Gulf Coast was the perfect breeding ground for V. vulnificus and V. parahaemolyticus.
Following the rapid onset of illness, V. vulnificus was identified in 82% of the reported cases, and V. parahaemolyticus in the other 18%, said Dr. Bettencourt. The patients were aged 31-89, with the majority being male (83%); five of the patients died. Comorbidities that may have made people more susceptible to infection, such as heart disease and diabetes, were identified in 72% of the patients.
Vibrio infection starts as edema, erythema, and pain, and, if left untreated, can progress to hemorrhagic blisters, necrotizing fasciitis, and sepsis.
If patients must work in conditions such as those present after Hurricane Katrina, they should wear protective clothing to prevent wound exposure to contaminated water. They should also wash all wounds with soap and water after water exposure to reduce the risk of infection.
"Clinicians should be vigilant for Vibrio infection in hurricane evacuee populations, particularly in patients with infected wounds and especially if the patients are in a high-risk group. If V. vulnificus is suspected, antimicrobial therapy should be initiated immediately; prompt treatment can improve survival," noted the Centers for Disease Control and Prevention (MMWR 2005;54;928-31).
Recommended treatments include doxycycline, third-generation cephalosporins (such as intravenous ceftazidime), fluoroquinolones (such as levofloxacin or cyclosporine), and aminoglycosides. Limb amputation is sometimes necessary, according to Dr. Bettencourt.
MRSA
Wounds are at a particular risk of becoming infected with methicillin-resistant Staphylococcus aureus (MRSA) because of conditions that are likely to occur after a natural disaster, such as close skin-to-skin contact with other individuals, use of contaminated items to treat wounds, a lack of cleanliness, and crowded living conditions.
Dr. Bettencourt recommended looking for signs of MRSA in cuts and abrasions and in areas of a patient that are covered by hair, which could easily be missed.
She noted that new Infectious Diseases Society of America treatment guidelines recommend incision and drainage of mature MRSA infections. Antibiotic treatment should be started if the patient is very young, very old, or immunocompromised; if there are signs of severe or extensive disease; if there is a rapid progression to cellulitis; if symptoms of systemic disease are present; if areas for treatment are difficult to drain (face, hands, genitalia); or if patients are unresponsive to drainage.
Recommended antibiotics include clindamycin, trimethoprim/sulfamethoxazole, tetracycline, doxycycline, minocycline, and linezolid. She pointed out that the presence of pus suggests S. aureus infection, while cellulitis without puss suggests group A streptococcus.
For patients with recurrent MRSA, consider nasal decolonization with mupirocin twice a day for 5 days or body decolonization with bleach baths or chlorhexidine. Oral antibiotics can be started if the patient has active lesions, Dr. Bettencourt said. Family members and close contacts of the patient may need to be evaluated and treated, as well.
Leptospirosis
The floods and mud slides that devastated Rio de Janeiro in January were described as the worst weather-related natural disaster ever to hit Brazil, Dr. Bettencourt said.
Animals carrying the bacterial pathogen Leptospira urinated in the flood waters, which humans then drank or subjected their wounds to.
The bacteria causing leptospirosis can survive for several months, she noted, and the incubation period is 2-4 weeks. Symptoms in people include fever, headache, muscle ache, vomiting, jaundice, red eyes, abdominal pain, diarrhea, and rash (common on the legs). If left untreated, the infection can cause kidney and liver failure.
Severe infection requires hospitalization, and about 5% of patients die (Cochrane Database Syst. Rev. 2000;CD001306). High doses of antibiotics are used to treat patients.
The Centers for Disease Control and Prevention has estimated that there are 100-200 cases of leptospirosis each year in the United States, transmitted primarily through rats and dogs. The infection is most likely to occur in men (75%) – in Hawaii (50%), the Southern Atlantic, and in the Gulf and Pacific coastal states – in July through October.
"The largest recorded U.S. outbreak occurred in 1998, when 775 people were exposed to the disease. Of these, 110 became infected," according to the CDC. "Although the incidence in the United States is relatively low, leptospirosis is considered to be the most widespread zoonotic disease in the world."
Mycobacteria
After the tsunami in Thailand in 2004, 15 people who had crush traumatic injuries developed rapid-growing mycobacterial infections 20-105 days after the tsunami in undamaged skin near sutured wounds. Seven of the infections were from the organism Mycobacterium abscessus, six were from M. fortuitum, one was from M. peregrinum, and the last was from M. mageritense.
All of the patients were treated and free of infection after 12 months.
Mucormycosis
Mucormycosis (also known as zygomycosis) is a rare fungal infection that results from the fungi group Mucoromycotina found in the soil of decaying organic matter. In hospitals, infection is associated with the use of contaminated materials or organ transplantation and carries a mortality rate of 30%-80%, Dr. Bettencourt noted.
There was an outbreak of the rare infection in May, in Joplin, Mo., 12 days after a tornado plowed through the community causing mass destruction. The infection penetrated the open wounds of hospitalized tornado victims, resulting in five deaths. Contaminated tongue dispensers were the vector of transmission.
The first two patients diagnosed with mucormycosis presented with a necrotizing soft tissue fungal infection. Eighteen cases were suspected, with 13 confirmed. None of the patients with the infection were immunocompromised, but two had diabetes. No additional cases were reported after June 17.
Mucormycosis can take many clinical forms, including rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated (most commonly in the brain). The fungi move aggressively into the bloodstream, causing everything from sinus pain and fever to black pus drainage from the eyes and cutaneous necrosis, she reported.
Coccidiomycosis
Coccidiomycosis, also known as valley fever, is a fungus found in the soil of arid areas. Inhalation of conidia from dust stirred up by human activity or a natural disaster can cause infection, said Dr. Bettencourt.
California, Arizona, Nevada, New Mexico, Texas, and Utah are U.S. endemic areas, where 10%-50% of the population will have evidence of exposure to Coccidioides species, according to the CDC.
An earthquake was the cause of the 1994 outbreak of coccidiomycosis in Northridge, Calif., where 203 cases of infection and 3 deaths were reported (JAMA 1997;277:904-8).
Symptoms of acute infection are flulike, occurring 1-3 weeks after exposure, with fever, cough, headache, rash, and muscle ache being the most common events. However, 60% of infected individuals will have no symptoms. Severe infection can cause lung problems, meningitis, skin ulcers, and bone and joint infection, but most patients make a full recovery, Dr. Bettencourt noted. Chronic pulmonary coccidioidomycosis may develop 20 years or more after infection.
Disseminated infection can spread to the skin, brain, bones, and heart. Some of the symptoms of disseminated skin infection are nodules, papules, plaques, furuncles, abscesses, and ulcers. Erythema nodosum is the most common skin presentation, she said.
Other natural disaster–related disease outbreaks include measles. Thirty-five cases were reported after the 2004 tsunami in Indonesia, and 400 cases were reported after the 2005 earthquake in Pakistan. "Malaria outbreaks are directly associated with flooding, while dengue transmission is influenced by rainfall and humidity, but is not directly associated with flooding," reported Dr. Bettencourt. Tetanus, hepatitis, and diarrhea from cholera and salmonella also can affect people after natural disasters.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Although the risk of a serious disease outbreak after natural disasters is rare and often exaggerated by the media, it is important to be prepared to respond to patient infections after such an event, according to Dr. Miriam S. Bettencourt of the University of Nevada in Las Vegas.
Some of the conditions patients may present with after natural disasters include bacterial and fungal infections, leptospirosis, measles, dengue fever, trench foot, arthropod bites, and radiation exposure. Dr. Bettencourt described some of the conditions in detail during her presentation at the Coastal Dermatology Symposisum.
Vibrio
After Hurricane Katrina in 2005, there were 18 reported cases of Vibrio-associated illness because of exposure of patients’ wounds to coastal flood waters. The warm seawater of the Atlantic Ocean that flooded the Gulf Coast was the perfect breeding ground for V. vulnificus and V. parahaemolyticus.
Following the rapid onset of illness, V. vulnificus was identified in 82% of the reported cases, and V. parahaemolyticus in the other 18%, said Dr. Bettencourt. The patients were aged 31-89, with the majority being male (83%); five of the patients died. Comorbidities that may have made people more susceptible to infection, such as heart disease and diabetes, were identified in 72% of the patients.
Vibrio infection starts as edema, erythema, and pain, and, if left untreated, can progress to hemorrhagic blisters, necrotizing fasciitis, and sepsis.
If patients must work in conditions such as those present after Hurricane Katrina, they should wear protective clothing to prevent wound exposure to contaminated water. They should also wash all wounds with soap and water after water exposure to reduce the risk of infection.
"Clinicians should be vigilant for Vibrio infection in hurricane evacuee populations, particularly in patients with infected wounds and especially if the patients are in a high-risk group. If V. vulnificus is suspected, antimicrobial therapy should be initiated immediately; prompt treatment can improve survival," noted the Centers for Disease Control and Prevention (MMWR 2005;54;928-31).
Recommended treatments include doxycycline, third-generation cephalosporins (such as intravenous ceftazidime), fluoroquinolones (such as levofloxacin or cyclosporine), and aminoglycosides. Limb amputation is sometimes necessary, according to Dr. Bettencourt.
MRSA
Wounds are at a particular risk of becoming infected with methicillin-resistant Staphylococcus aureus (MRSA) because of conditions that are likely to occur after a natural disaster, such as close skin-to-skin contact with other individuals, use of contaminated items to treat wounds, a lack of cleanliness, and crowded living conditions.
Dr. Bettencourt recommended looking for signs of MRSA in cuts and abrasions and in areas of a patient that are covered by hair, which could easily be missed.
She noted that new Infectious Diseases Society of America treatment guidelines recommend incision and drainage of mature MRSA infections. Antibiotic treatment should be started if the patient is very young, very old, or immunocompromised; if there are signs of severe or extensive disease; if there is a rapid progression to cellulitis; if symptoms of systemic disease are present; if areas for treatment are difficult to drain (face, hands, genitalia); or if patients are unresponsive to drainage.
Recommended antibiotics include clindamycin, trimethoprim/sulfamethoxazole, tetracycline, doxycycline, minocycline, and linezolid. She pointed out that the presence of pus suggests S. aureus infection, while cellulitis without puss suggests group A streptococcus.
For patients with recurrent MRSA, consider nasal decolonization with mupirocin twice a day for 5 days or body decolonization with bleach baths or chlorhexidine. Oral antibiotics can be started if the patient has active lesions, Dr. Bettencourt said. Family members and close contacts of the patient may need to be evaluated and treated, as well.
Leptospirosis
The floods and mud slides that devastated Rio de Janeiro in January were described as the worst weather-related natural disaster ever to hit Brazil, Dr. Bettencourt said.
Animals carrying the bacterial pathogen Leptospira urinated in the flood waters, which humans then drank or subjected their wounds to.
The bacteria causing leptospirosis can survive for several months, she noted, and the incubation period is 2-4 weeks. Symptoms in people include fever, headache, muscle ache, vomiting, jaundice, red eyes, abdominal pain, diarrhea, and rash (common on the legs). If left untreated, the infection can cause kidney and liver failure.
Severe infection requires hospitalization, and about 5% of patients die (Cochrane Database Syst. Rev. 2000;CD001306). High doses of antibiotics are used to treat patients.
The Centers for Disease Control and Prevention has estimated that there are 100-200 cases of leptospirosis each year in the United States, transmitted primarily through rats and dogs. The infection is most likely to occur in men (75%) – in Hawaii (50%), the Southern Atlantic, and in the Gulf and Pacific coastal states – in July through October.
"The largest recorded U.S. outbreak occurred in 1998, when 775 people were exposed to the disease. Of these, 110 became infected," according to the CDC. "Although the incidence in the United States is relatively low, leptospirosis is considered to be the most widespread zoonotic disease in the world."
Mycobacteria
After the tsunami in Thailand in 2004, 15 people who had crush traumatic injuries developed rapid-growing mycobacterial infections 20-105 days after the tsunami in undamaged skin near sutured wounds. Seven of the infections were from the organism Mycobacterium abscessus, six were from M. fortuitum, one was from M. peregrinum, and the last was from M. mageritense.
All of the patients were treated and free of infection after 12 months.
Mucormycosis
Mucormycosis (also known as zygomycosis) is a rare fungal infection that results from the fungi group Mucoromycotina found in the soil of decaying organic matter. In hospitals, infection is associated with the use of contaminated materials or organ transplantation and carries a mortality rate of 30%-80%, Dr. Bettencourt noted.
There was an outbreak of the rare infection in May, in Joplin, Mo., 12 days after a tornado plowed through the community causing mass destruction. The infection penetrated the open wounds of hospitalized tornado victims, resulting in five deaths. Contaminated tongue dispensers were the vector of transmission.
The first two patients diagnosed with mucormycosis presented with a necrotizing soft tissue fungal infection. Eighteen cases were suspected, with 13 confirmed. None of the patients with the infection were immunocompromised, but two had diabetes. No additional cases were reported after June 17.
Mucormycosis can take many clinical forms, including rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated (most commonly in the brain). The fungi move aggressively into the bloodstream, causing everything from sinus pain and fever to black pus drainage from the eyes and cutaneous necrosis, she reported.
Coccidiomycosis
Coccidiomycosis, also known as valley fever, is a fungus found in the soil of arid areas. Inhalation of conidia from dust stirred up by human activity or a natural disaster can cause infection, said Dr. Bettencourt.
California, Arizona, Nevada, New Mexico, Texas, and Utah are U.S. endemic areas, where 10%-50% of the population will have evidence of exposure to Coccidioides species, according to the CDC.
An earthquake was the cause of the 1994 outbreak of coccidiomycosis in Northridge, Calif., where 203 cases of infection and 3 deaths were reported (JAMA 1997;277:904-8).
Symptoms of acute infection are flulike, occurring 1-3 weeks after exposure, with fever, cough, headache, rash, and muscle ache being the most common events. However, 60% of infected individuals will have no symptoms. Severe infection can cause lung problems, meningitis, skin ulcers, and bone and joint infection, but most patients make a full recovery, Dr. Bettencourt noted. Chronic pulmonary coccidioidomycosis may develop 20 years or more after infection.
Disseminated infection can spread to the skin, brain, bones, and heart. Some of the symptoms of disseminated skin infection are nodules, papules, plaques, furuncles, abscesses, and ulcers. Erythema nodosum is the most common skin presentation, she said.
Other natural disaster–related disease outbreaks include measles. Thirty-five cases were reported after the 2004 tsunami in Indonesia, and 400 cases were reported after the 2005 earthquake in Pakistan. "Malaria outbreaks are directly associated with flooding, while dengue transmission is influenced by rainfall and humidity, but is not directly associated with flooding," reported Dr. Bettencourt. Tetanus, hepatitis, and diarrhea from cholera and salmonella also can affect people after natural disasters.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Although the risk of a serious disease outbreak after natural disasters is rare and often exaggerated by the media, it is important to be prepared to respond to patient infections after such an event, according to Dr. Miriam S. Bettencourt of the University of Nevada in Las Vegas.
Some of the conditions patients may present with after natural disasters include bacterial and fungal infections, leptospirosis, measles, dengue fever, trench foot, arthropod bites, and radiation exposure. Dr. Bettencourt described some of the conditions in detail during her presentation at the Coastal Dermatology Symposisum.
Vibrio
After Hurricane Katrina in 2005, there were 18 reported cases of Vibrio-associated illness because of exposure of patients’ wounds to coastal flood waters. The warm seawater of the Atlantic Ocean that flooded the Gulf Coast was the perfect breeding ground for V. vulnificus and V. parahaemolyticus.
Following the rapid onset of illness, V. vulnificus was identified in 82% of the reported cases, and V. parahaemolyticus in the other 18%, said Dr. Bettencourt. The patients were aged 31-89, with the majority being male (83%); five of the patients died. Comorbidities that may have made people more susceptible to infection, such as heart disease and diabetes, were identified in 72% of the patients.
Vibrio infection starts as edema, erythema, and pain, and, if left untreated, can progress to hemorrhagic blisters, necrotizing fasciitis, and sepsis.
If patients must work in conditions such as those present after Hurricane Katrina, they should wear protective clothing to prevent wound exposure to contaminated water. They should also wash all wounds with soap and water after water exposure to reduce the risk of infection.
"Clinicians should be vigilant for Vibrio infection in hurricane evacuee populations, particularly in patients with infected wounds and especially if the patients are in a high-risk group. If V. vulnificus is suspected, antimicrobial therapy should be initiated immediately; prompt treatment can improve survival," noted the Centers for Disease Control and Prevention (MMWR 2005;54;928-31).
Recommended treatments include doxycycline, third-generation cephalosporins (such as intravenous ceftazidime), fluoroquinolones (such as levofloxacin or cyclosporine), and aminoglycosides. Limb amputation is sometimes necessary, according to Dr. Bettencourt.
MRSA
Wounds are at a particular risk of becoming infected with methicillin-resistant Staphylococcus aureus (MRSA) because of conditions that are likely to occur after a natural disaster, such as close skin-to-skin contact with other individuals, use of contaminated items to treat wounds, a lack of cleanliness, and crowded living conditions.
Dr. Bettencourt recommended looking for signs of MRSA in cuts and abrasions and in areas of a patient that are covered by hair, which could easily be missed.
She noted that new Infectious Diseases Society of America treatment guidelines recommend incision and drainage of mature MRSA infections. Antibiotic treatment should be started if the patient is very young, very old, or immunocompromised; if there are signs of severe or extensive disease; if there is a rapid progression to cellulitis; if symptoms of systemic disease are present; if areas for treatment are difficult to drain (face, hands, genitalia); or if patients are unresponsive to drainage.
Recommended antibiotics include clindamycin, trimethoprim/sulfamethoxazole, tetracycline, doxycycline, minocycline, and linezolid. She pointed out that the presence of pus suggests S. aureus infection, while cellulitis without puss suggests group A streptococcus.
For patients with recurrent MRSA, consider nasal decolonization with mupirocin twice a day for 5 days or body decolonization with bleach baths or chlorhexidine. Oral antibiotics can be started if the patient has active lesions, Dr. Bettencourt said. Family members and close contacts of the patient may need to be evaluated and treated, as well.
Leptospirosis
The floods and mud slides that devastated Rio de Janeiro in January were described as the worst weather-related natural disaster ever to hit Brazil, Dr. Bettencourt said.
Animals carrying the bacterial pathogen Leptospira urinated in the flood waters, which humans then drank or subjected their wounds to.
The bacteria causing leptospirosis can survive for several months, she noted, and the incubation period is 2-4 weeks. Symptoms in people include fever, headache, muscle ache, vomiting, jaundice, red eyes, abdominal pain, diarrhea, and rash (common on the legs). If left untreated, the infection can cause kidney and liver failure.
Severe infection requires hospitalization, and about 5% of patients die (Cochrane Database Syst. Rev. 2000;CD001306). High doses of antibiotics are used to treat patients.
The Centers for Disease Control and Prevention has estimated that there are 100-200 cases of leptospirosis each year in the United States, transmitted primarily through rats and dogs. The infection is most likely to occur in men (75%) – in Hawaii (50%), the Southern Atlantic, and in the Gulf and Pacific coastal states – in July through October.
"The largest recorded U.S. outbreak occurred in 1998, when 775 people were exposed to the disease. Of these, 110 became infected," according to the CDC. "Although the incidence in the United States is relatively low, leptospirosis is considered to be the most widespread zoonotic disease in the world."
Mycobacteria
After the tsunami in Thailand in 2004, 15 people who had crush traumatic injuries developed rapid-growing mycobacterial infections 20-105 days after the tsunami in undamaged skin near sutured wounds. Seven of the infections were from the organism Mycobacterium abscessus, six were from M. fortuitum, one was from M. peregrinum, and the last was from M. mageritense.
All of the patients were treated and free of infection after 12 months.
Mucormycosis
Mucormycosis (also known as zygomycosis) is a rare fungal infection that results from the fungi group Mucoromycotina found in the soil of decaying organic matter. In hospitals, infection is associated with the use of contaminated materials or organ transplantation and carries a mortality rate of 30%-80%, Dr. Bettencourt noted.
There was an outbreak of the rare infection in May, in Joplin, Mo., 12 days after a tornado plowed through the community causing mass destruction. The infection penetrated the open wounds of hospitalized tornado victims, resulting in five deaths. Contaminated tongue dispensers were the vector of transmission.
The first two patients diagnosed with mucormycosis presented with a necrotizing soft tissue fungal infection. Eighteen cases were suspected, with 13 confirmed. None of the patients with the infection were immunocompromised, but two had diabetes. No additional cases were reported after June 17.
Mucormycosis can take many clinical forms, including rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated (most commonly in the brain). The fungi move aggressively into the bloodstream, causing everything from sinus pain and fever to black pus drainage from the eyes and cutaneous necrosis, she reported.
Coccidiomycosis
Coccidiomycosis, also known as valley fever, is a fungus found in the soil of arid areas. Inhalation of conidia from dust stirred up by human activity or a natural disaster can cause infection, said Dr. Bettencourt.
California, Arizona, Nevada, New Mexico, Texas, and Utah are U.S. endemic areas, where 10%-50% of the population will have evidence of exposure to Coccidioides species, according to the CDC.
An earthquake was the cause of the 1994 outbreak of coccidiomycosis in Northridge, Calif., where 203 cases of infection and 3 deaths were reported (JAMA 1997;277:904-8).
Symptoms of acute infection are flulike, occurring 1-3 weeks after exposure, with fever, cough, headache, rash, and muscle ache being the most common events. However, 60% of infected individuals will have no symptoms. Severe infection can cause lung problems, meningitis, skin ulcers, and bone and joint infection, but most patients make a full recovery, Dr. Bettencourt noted. Chronic pulmonary coccidioidomycosis may develop 20 years or more after infection.
Disseminated infection can spread to the skin, brain, bones, and heart. Some of the symptoms of disseminated skin infection are nodules, papules, plaques, furuncles, abscesses, and ulcers. Erythema nodosum is the most common skin presentation, she said.
Other natural disaster–related disease outbreaks include measles. Thirty-five cases were reported after the 2004 tsunami in Indonesia, and 400 cases were reported after the 2005 earthquake in Pakistan. "Malaria outbreaks are directly associated with flooding, while dengue transmission is influenced by rainfall and humidity, but is not directly associated with flooding," reported Dr. Bettencourt. Tetanus, hepatitis, and diarrhea from cholera and salmonella also can affect people after natural disasters.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway and conducting clinical trials for 3M Pharmaceuticals.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Most Derm Residents Believe Pharma Interaction Is Ethical
NAPA, CALIF. – More than 70% of dermatology residents and program directors say they believe that it is ethical for residents to interact with the pharmaceutical industry, according to a new study.
Dr. Erica Linnell, under the direction of Dr. Vincent DeLeo – both of the department of dermatology at St. Luke’s-Roosevelt Hospital Center in New York – assessed whether residents and program directions believe interactions with the pharmaceutical industry affect prescribing patterns among residents and whether they think such interactions are ethical.
They found that most residents have significant interactions with pharmaceutical representatives and most believe that such activities are acceptable in certain circumstances, which is similar to survey results of other specialties, said Dr. Linnell.
Of the 114 survey respondents from Accreditation Council for Graduate Medical Education (ACGME)-approved programs, 50% were chief residents, 37% were program directors, and 13% were first-, second-, or third-year dermatology residents or associate program directors.
The 17-question electronic survey found that 73% of residency programs accept pharmaceutical support, 68% accept educational materials, 55% accept drug samples, 55% accept funds for travel and registration at national meetings, 27% accept private lunches or dinners, 25% accept food for conferences, and 5% accept pens, notepads, and other office supplies.
A total of 87% of survey respondents reported that the residency program at their university had guidelines for interacting with the pharmaceutical industry. When asked about the guidelines, 60% of respondents reported that interacting with pharma was "acceptable in certain circumstances," 25% reported that it was "never acceptable," 8% reported that is was "not acceptable but not discouraged," and 3% reported that it was "acceptable and encouraged."
When it came to respondents’ personal beliefs, 71% reported that interacting with pharma is "acceptable in certain circumstances," 13% reported that it is "acceptable and encouraged," and 9% reported that it is "never acceptable."
Of the respondents, 55% reported having on-site meetings with pharmaceutical representatives, and 57% reported that they believe that such meetings are appropriate. Off-site meetings were reported by 62% of respondents, with 63% believing that such meetings are appropriate. Most off-site meetings (91%) were reported to be dinner with a lecture.
Survey participants were divided on whether they thought interactions with pharma affect prescribing patterns; 54% said yes, while 46% said no.
A total of 71% of residents reported that it is ethical to interact with pharma and explained that they believe it is important for residents to learn about new medications. The 29% who say they believe that it is not ethical to interact with pharma explained they believe it leads to biased prescribing, that residents are too easily influenced by pharma representatives, and that accepting gifts and food without educational value is wrong.
Residents may feel immune to the influence of pharma, said Dr. Linnell, but previous studies have found that prescribing patterns are affected. A curriculum for residents could help guide interactions with the pharmaceutical industry, as well as guidelines for ethical interactions.
She presented her findings during a resident forum during the meeting that was sponsored by Galderma. She did not report having other conflicts of interest.
Dr. Erica Linnell, Dr. Vincent DeLeo, interactions with the pharmaceutical industry, prescribing patterns, Accreditation Council for Graduate Medical Education, ACGME,
NAPA, CALIF. – More than 70% of dermatology residents and program directors say they believe that it is ethical for residents to interact with the pharmaceutical industry, according to a new study.
Dr. Erica Linnell, under the direction of Dr. Vincent DeLeo – both of the department of dermatology at St. Luke’s-Roosevelt Hospital Center in New York – assessed whether residents and program directions believe interactions with the pharmaceutical industry affect prescribing patterns among residents and whether they think such interactions are ethical.
They found that most residents have significant interactions with pharmaceutical representatives and most believe that such activities are acceptable in certain circumstances, which is similar to survey results of other specialties, said Dr. Linnell.
Of the 114 survey respondents from Accreditation Council for Graduate Medical Education (ACGME)-approved programs, 50% were chief residents, 37% were program directors, and 13% were first-, second-, or third-year dermatology residents or associate program directors.
The 17-question electronic survey found that 73% of residency programs accept pharmaceutical support, 68% accept educational materials, 55% accept drug samples, 55% accept funds for travel and registration at national meetings, 27% accept private lunches or dinners, 25% accept food for conferences, and 5% accept pens, notepads, and other office supplies.
A total of 87% of survey respondents reported that the residency program at their university had guidelines for interacting with the pharmaceutical industry. When asked about the guidelines, 60% of respondents reported that interacting with pharma was "acceptable in certain circumstances," 25% reported that it was "never acceptable," 8% reported that is was "not acceptable but not discouraged," and 3% reported that it was "acceptable and encouraged."
When it came to respondents’ personal beliefs, 71% reported that interacting with pharma is "acceptable in certain circumstances," 13% reported that it is "acceptable and encouraged," and 9% reported that it is "never acceptable."
Of the respondents, 55% reported having on-site meetings with pharmaceutical representatives, and 57% reported that they believe that such meetings are appropriate. Off-site meetings were reported by 62% of respondents, with 63% believing that such meetings are appropriate. Most off-site meetings (91%) were reported to be dinner with a lecture.
Survey participants were divided on whether they thought interactions with pharma affect prescribing patterns; 54% said yes, while 46% said no.
A total of 71% of residents reported that it is ethical to interact with pharma and explained that they believe it is important for residents to learn about new medications. The 29% who say they believe that it is not ethical to interact with pharma explained they believe it leads to biased prescribing, that residents are too easily influenced by pharma representatives, and that accepting gifts and food without educational value is wrong.
Residents may feel immune to the influence of pharma, said Dr. Linnell, but previous studies have found that prescribing patterns are affected. A curriculum for residents could help guide interactions with the pharmaceutical industry, as well as guidelines for ethical interactions.
She presented her findings during a resident forum during the meeting that was sponsored by Galderma. She did not report having other conflicts of interest.
NAPA, CALIF. – More than 70% of dermatology residents and program directors say they believe that it is ethical for residents to interact with the pharmaceutical industry, according to a new study.
Dr. Erica Linnell, under the direction of Dr. Vincent DeLeo – both of the department of dermatology at St. Luke’s-Roosevelt Hospital Center in New York – assessed whether residents and program directions believe interactions with the pharmaceutical industry affect prescribing patterns among residents and whether they think such interactions are ethical.
They found that most residents have significant interactions with pharmaceutical representatives and most believe that such activities are acceptable in certain circumstances, which is similar to survey results of other specialties, said Dr. Linnell.
Of the 114 survey respondents from Accreditation Council for Graduate Medical Education (ACGME)-approved programs, 50% were chief residents, 37% were program directors, and 13% were first-, second-, or third-year dermatology residents or associate program directors.
The 17-question electronic survey found that 73% of residency programs accept pharmaceutical support, 68% accept educational materials, 55% accept drug samples, 55% accept funds for travel and registration at national meetings, 27% accept private lunches or dinners, 25% accept food for conferences, and 5% accept pens, notepads, and other office supplies.
A total of 87% of survey respondents reported that the residency program at their university had guidelines for interacting with the pharmaceutical industry. When asked about the guidelines, 60% of respondents reported that interacting with pharma was "acceptable in certain circumstances," 25% reported that it was "never acceptable," 8% reported that is was "not acceptable but not discouraged," and 3% reported that it was "acceptable and encouraged."
When it came to respondents’ personal beliefs, 71% reported that interacting with pharma is "acceptable in certain circumstances," 13% reported that it is "acceptable and encouraged," and 9% reported that it is "never acceptable."
Of the respondents, 55% reported having on-site meetings with pharmaceutical representatives, and 57% reported that they believe that such meetings are appropriate. Off-site meetings were reported by 62% of respondents, with 63% believing that such meetings are appropriate. Most off-site meetings (91%) were reported to be dinner with a lecture.
Survey participants were divided on whether they thought interactions with pharma affect prescribing patterns; 54% said yes, while 46% said no.
A total of 71% of residents reported that it is ethical to interact with pharma and explained that they believe it is important for residents to learn about new medications. The 29% who say they believe that it is not ethical to interact with pharma explained they believe it leads to biased prescribing, that residents are too easily influenced by pharma representatives, and that accepting gifts and food without educational value is wrong.
Residents may feel immune to the influence of pharma, said Dr. Linnell, but previous studies have found that prescribing patterns are affected. A curriculum for residents could help guide interactions with the pharmaceutical industry, as well as guidelines for ethical interactions.
She presented her findings during a resident forum during the meeting that was sponsored by Galderma. She did not report having other conflicts of interest.
Dr. Erica Linnell, Dr. Vincent DeLeo, interactions with the pharmaceutical industry, prescribing patterns, Accreditation Council for Graduate Medical Education, ACGME,
Dr. Erica Linnell, Dr. Vincent DeLeo, interactions with the pharmaceutical industry, prescribing patterns, Accreditation Council for Graduate Medical Education, ACGME,
FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Major Finding: When it came to respondents’ personal beliefs, 71% felt that interacting with pharma was "acceptable in certain circumstances," 13% felt that it is "acceptable and encouraged," and 9% reported that it is "never acceptable."
Data Source: An electronic survey of 114 residents and program directors from ACGME-approved programs.
Disclosures: She presented her findings during a resident forum during a meeting that was sponsored by Galderma. She did not report having other conflicts of interest.
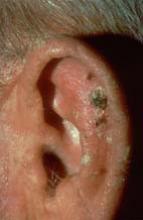
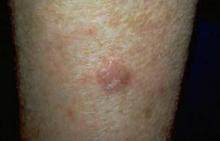
Expert: Risk of AK Progression to SCC Hard to Predict
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – Because actinic keratoses are the most common lesions with premalignant potential in the skin, knowing how to predict their malignancy can be life saving, according to Dr. Miriam S. Bettencourt.
Most AKs do not progress to squamous cell carcinoma (SCC), but most invasive SCCs have evidence of preexisting AK, she said. Of the AKs that progress, 10% will metastasize, giving patients a 5-year survival rate, Dr. Bettencourt of the University of Nevada, Las Vegas.
"Major risk factors for AK malignancy include induration and inflammation, a diameter of over 1 cm, rapid enlargement, bleeding, erythema, and ulceration. Minor risk factors include pigmentation changes, palpability, pain, pruritus, and hyperkeratosis.
Hyperkeratotic AKs greater than 1 cm on the hands, wrists, or forearms have a 50% or greater risk for malignancy, she noted.
Other risk factors include age at diagnosis, human papillomavirus status, skin type, sunburns during childhood, proximity to the Equator, amount of outdoor activity, and a history of skin cancer.
Organ-transplant recipients have a 250-fold greater risk for developing AKs, and a 100-fold risk for SCC; 40% of organ-transplant patients with an AK will show progression to SCC, compared with 10% of the average population, she said.
However, the wide range of rates for the risk of AK progression to SCC found in the literature highlights the difficulty of putting risk into perspective, she noted.
In one study, of 169 patients with AKs, 65% developed an SCC from an AK; 97% of the patients had a contiguous AK near the site of the SCC (Dermatol. Surg. 1995;21:184). But a review of 875 studies of AKs found a much lower rate; 11 studies were identified that predicted the risk of malignancy. The review found the rate to be 0.025% to 10% per lesion, per year (Eur. J. Dermatol. 2006;16:335-9).
In another study, 10% of AKs were found to transform into SCC in 2 years (Dermatol. Surg. 2007;33:1099-101).
A more recent report suggests that AKs also may progress to basal cell carcinomas (BCCs), she reported. In a study of 7,784 lesions on the face and ears of 169 patients, 65% of SCCs and 36% of BCCs arose in lesions that were previously diagnosed as AKs (Cancer 2009;115:2523-30). The study also found the risk of progression of AK to SCC to be 0.6% at year 1 and 2.6% at year 4.
It is important to remember that risk applies to each individual AK and that the fate of any one AK is impossible to predict, said Dr. Bettencourt. "Although the risk [of progression] is small, since most [patients] have multiple AKs, the risk of transformation is much greater than the risk of any individual lesion."
She recommended treating all AKs because some may be difficult to distinguish from SCC and lentigo maligna.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSISUM
Treat All AKs to Prevent Skin Cancer
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
NAPA, CALIF. – A preventive approach is needed in the treatment of actinic keratoses to curtail the potential emergence of nonmelanoma skin cancer, according to Dr. Miriam S. Bettencourt.
"In addition to the visible AKs that we see, there’s a lot more in the field area of the AK below the skin surface," she said. "AKs are just the tip of the iceberg."
Because nonmelanoma skin cancer can be easily missed in AK patients, Dr. Bettencourt, of the University of Nevada in Las Vegas, recommended treating all AK lesions.
Many factors should be taken into consideration when determining the best AK treatment for patients, she said, including the number of lesions and location, patient compliance, cosmetic implications, age of patient, history of skin cancer, and insurance coverage.
The treatment of AK involves lesion-directed destructive procedures, topical field-directed therapies, or combination treatment.
Lesion Destruction Therapies
For treating nonspecific lesions, Dr. Bettencourt recommended considering cryosurgery, noting an efficacy rate of 67%-88% and a 1-year recurrence rate of 1.2%-12%. Side effects are mild, the most common being pain, redness, edema, and hypo- or hyperpigmentation.
Chemical peels are an option for extensive AKs, she said, noting an efficacy rate of 75% and a recurrence rate of 25%-35% at 1 year, depending on the agent. Side effects with peels are mild, the most common being pain, inflammation, and pigment alterations.
For single lesions on the face, scalp, or neck, or for full-face resurfacing, CO2 and YAG lasers can be used. While device dependent, 90% of patients achieve lesion remission; however, the recurrence rate is 10%-15% at 3-6 months.
Photodynamic therapy (PDT) offers selective destruction of atypical cells through light activation of a photosensitizer. She noted an efficacy rate of 70%-78% after one treatment session, and a rate of 90% after two sessions. However, there is a risk of photosensitivity and pain from treatment, and it tends to cost more than other therapies, she noted.
Topical Treatment
Topical 5-fluorouracil "inhibits thymidylate synthetase and causes cell death in actively proliferating cells," reported Dr. Bettencourt. A 5% cream or solution is the most popular form of 5-FU, but it is also available in 2%, 1%, and 0.5% formulas.
The 5% cream should be applied twice a day for a month. Common side effects include temporary ulcers, erythema, crusting, and scarring. She highly recommended reviewing the adverse effects with patients before treatment. She noted that she has patients sign the package insert before she begins treatment with 5-FU.
Imiquimod is another topical treatment available for AKs that upregulates a variety of cytokines, she said. "Experimental evidence suggests that patients may develop T-cell memory after treatment, and thus develop less AKs in the future." She recommended Aldara 5%, which is applied 2-3 times per week for up to 4 months, or Zyclara 3.75%, which is applied once a day for two cycles of 2 weeks (with a 2-week free interval in the middle).
The last topical agent she recommended was diclofenac sodium 3% gel (Solaraze), which is a nonsteroidal anti-inflammatory drug that is well tolerated. "The treatment inhibits cyclooxygenase, which is essential for biosynthesis of prostaglandins," she said. Hyaluronic acid delays uptake of the drug, which leads to higher concentrations in the skin. Patients should be treated with the gel twice a day for 3 months. Little to no inflammation may occur during treatment; however, the drug has been known to cause hepatotoxicity.
She noted that diclofenac is her treatment of choice for AKs on the lips.
Combination Therapy
Consider combination, sequential therapy with 0.5% micronized 5-FU before cryotherapy for better results than cryotherapy alone, said Dr. Bettencourt. In a study of 144 patients, 5-FU plus cryotherapy resulted in partial remission in 62% of patients, and complete remission in 17% of patients after 4 weeks of treatment (Arch. Dermatol. 2004;140:813-6).
Low-dose 5-FU plus salicylic acid has also been found to be an effective treatment for AKs, she said. A 2009 study found that 5-FU combined with Jessner’s solution or a 70% glycolic acid peel every other week had an 80% clearance rate and offered photodamage improvement (Int. J. Dermatol. 2009;48:902-7).
PDT followed by treatment with imiquimod 5% was found to reduce lesions by 89.9%, compared with 74.5% with placebo at 12 months (J. Drugs Dermatol. 2009;8:35-9). The randomized vehicle-controlled study enrolled 25 patients (24 completed the study), with a at least 10 AKs. The entire face of each patient was treated with PDT and aminolevulinic acid 20% at baseline and at 1 month; then, at month 2, imiquimod 5% was applied to one-half of each patient’s face and a vehicle to the other half two times a week for 16 weeks. Erythema and flaking were the most serious skin reactions reported during the study.
Positive results have also been seen when imiquimod is combined with cryosurgery, noted Dr. Bettencourt. She also recommended considering diclofenac gel after cryosurgery.
Whatever treatment is deemed best for the AK patient, he or she should be strongly encouraged to avoid the sun whenever possible, to wear protective clothing, to use sunscreen, and to limit outdoor activity between 10 a.m. and 3 p.m., she concluded. A low-fat diet may also lead to a greater resolution of existent AKs and may limit the development of future lesions.
Dr. Bettencourt reported being on the speakers bureaus of PharmaDerm and Graceway, and conducting clinical trials for 3M Pharmaceuticals.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Shot of Sunscreen a Day Helps Keep Skin Cancer Away
NAPA, CALIF. – When counseling patients to use sunscreens, instructions should also be given on how much to use, recommended Dr. Vincent DeLeo.
Sunscreens should prevent sunburn, photoimmune suppression, photoaging, photocarcinogenicity, and photosensitive disorders – but considering studies have found that people only apply 25%-75% of the testing quantity, sunscreens can’t effectively do their job, noted Dr. DeLeo, chairman of the department of dermatology at St. Luke’s-Roosevelt Hospital Center and Beth Israel Medical Center in New York.
Two tablespoons of sunscreen should cover an adult’s skin surface, he said. And an 8-oz bottle of sunscreen contains 16 tablespoons. Therefore, a family of four at the beach should use one bottle of sunscreen every 2 days, assuming they spend 4 hours a day in the sun (based on American Academy of Dermatology recommendations that sunscreen be applied at least every 2 hours).
Dr. DeLeo noted that a shot glass is 2.5 tablespoons, so this is a good guide for patients. Spray-on sunscreens are a good choice, because they allow patients to self apply to their backs and are a quicker way to coat impatient children. However, 2 tablespoons still need to be used with the sprays.
A recent sunscreen cost analysis found that during a 1-week beach vacation, a family of four should be spending $178-$238 a week on sunscreen, he said. This cost can be brought down by 33% if sun protective clothing is worn, and by 44% if large volumes of sunscreen are purchased. The same cost analysis also found that a transplant patient should be spending $249-$292 per year on sunscreen (J. Am. Acad. Dermatol. 2011;65:e73-9).
It is also important to remind patients that mixing an SPF 10 sunscreen with an SPF 20 will only give them an SPF of 15, he said. And while an SPF 30 may allow patients to stay in the sun twice as long as an SPF 15 with the same amount of protection, it does not offer 100% protection from burning rays.
Sunscreens with an SPF 10 block 90% of damaging rays, SPF 15 blocks 92.5%, SPF 20 blocks 95%, and SPF 40 blocks 97.5%, he noted. Always recommend a high SPF sunscreen to patients, because chances are they are not using enough of whatever they are putting on, he said.
Dr. DeLeo reported relationships with La Roche-Posay, Estée Lauder, Mary Kay, Galderma, Johnson & Johnson, and Pfizer.
NAPA, CALIF. – When counseling patients to use sunscreens, instructions should also be given on how much to use, recommended Dr. Vincent DeLeo.
Sunscreens should prevent sunburn, photoimmune suppression, photoaging, photocarcinogenicity, and photosensitive disorders – but considering studies have found that people only apply 25%-75% of the testing quantity, sunscreens can’t effectively do their job, noted Dr. DeLeo, chairman of the department of dermatology at St. Luke’s-Roosevelt Hospital Center and Beth Israel Medical Center in New York.
Two tablespoons of sunscreen should cover an adult’s skin surface, he said. And an 8-oz bottle of sunscreen contains 16 tablespoons. Therefore, a family of four at the beach should use one bottle of sunscreen every 2 days, assuming they spend 4 hours a day in the sun (based on American Academy of Dermatology recommendations that sunscreen be applied at least every 2 hours).
Dr. DeLeo noted that a shot glass is 2.5 tablespoons, so this is a good guide for patients. Spray-on sunscreens are a good choice, because they allow patients to self apply to their backs and are a quicker way to coat impatient children. However, 2 tablespoons still need to be used with the sprays.
A recent sunscreen cost analysis found that during a 1-week beach vacation, a family of four should be spending $178-$238 a week on sunscreen, he said. This cost can be brought down by 33% if sun protective clothing is worn, and by 44% if large volumes of sunscreen are purchased. The same cost analysis also found that a transplant patient should be spending $249-$292 per year on sunscreen (J. Am. Acad. Dermatol. 2011;65:e73-9).
It is also important to remind patients that mixing an SPF 10 sunscreen with an SPF 20 will only give them an SPF of 15, he said. And while an SPF 30 may allow patients to stay in the sun twice as long as an SPF 15 with the same amount of protection, it does not offer 100% protection from burning rays.
Sunscreens with an SPF 10 block 90% of damaging rays, SPF 15 blocks 92.5%, SPF 20 blocks 95%, and SPF 40 blocks 97.5%, he noted. Always recommend a high SPF sunscreen to patients, because chances are they are not using enough of whatever they are putting on, he said.
Dr. DeLeo reported relationships with La Roche-Posay, Estée Lauder, Mary Kay, Galderma, Johnson & Johnson, and Pfizer.
NAPA, CALIF. – When counseling patients to use sunscreens, instructions should also be given on how much to use, recommended Dr. Vincent DeLeo.
Sunscreens should prevent sunburn, photoimmune suppression, photoaging, photocarcinogenicity, and photosensitive disorders – but considering studies have found that people only apply 25%-75% of the testing quantity, sunscreens can’t effectively do their job, noted Dr. DeLeo, chairman of the department of dermatology at St. Luke’s-Roosevelt Hospital Center and Beth Israel Medical Center in New York.
Two tablespoons of sunscreen should cover an adult’s skin surface, he said. And an 8-oz bottle of sunscreen contains 16 tablespoons. Therefore, a family of four at the beach should use one bottle of sunscreen every 2 days, assuming they spend 4 hours a day in the sun (based on American Academy of Dermatology recommendations that sunscreen be applied at least every 2 hours).
Dr. DeLeo noted that a shot glass is 2.5 tablespoons, so this is a good guide for patients. Spray-on sunscreens are a good choice, because they allow patients to self apply to their backs and are a quicker way to coat impatient children. However, 2 tablespoons still need to be used with the sprays.
A recent sunscreen cost analysis found that during a 1-week beach vacation, a family of four should be spending $178-$238 a week on sunscreen, he said. This cost can be brought down by 33% if sun protective clothing is worn, and by 44% if large volumes of sunscreen are purchased. The same cost analysis also found that a transplant patient should be spending $249-$292 per year on sunscreen (J. Am. Acad. Dermatol. 2011;65:e73-9).
It is also important to remind patients that mixing an SPF 10 sunscreen with an SPF 20 will only give them an SPF of 15, he said. And while an SPF 30 may allow patients to stay in the sun twice as long as an SPF 15 with the same amount of protection, it does not offer 100% protection from burning rays.
Sunscreens with an SPF 10 block 90% of damaging rays, SPF 15 blocks 92.5%, SPF 20 blocks 95%, and SPF 40 blocks 97.5%, he noted. Always recommend a high SPF sunscreen to patients, because chances are they are not using enough of whatever they are putting on, he said.
Dr. DeLeo reported relationships with La Roche-Posay, Estée Lauder, Mary Kay, Galderma, Johnson & Johnson, and Pfizer.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
FDA: TNF Blockers Up Risk of Legionella, Listeria Infection
The Food and Drug Administration has updated the boxed warning label of all tumor necrosis factor–alpha blockers to include information on the risk of infection from Legionella and Listeria.
The warnings and precautions sections of the drug labels also have been revised to include information on the risk for serious infections that could lead to death from multiple disease-causing pathogens.
The TNF blockers that will receive the warnings include Remicade (infliximab), Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab pegol), and Simponi (golimumab), according to the FDA announcement.
The FDA advised that risks and benefits should be weighed before initiating TNF blocker therapy in patients with chronic or reoccurring infections, or in patients with conditions that may put them at higher risk for infection.
Reports of serious adverse events associated with these and other drugs should be reported to MedWatch at 800-332-1088.
The Food and Drug Administration has updated the boxed warning label of all tumor necrosis factor–alpha blockers to include information on the risk of infection from Legionella and Listeria.
The warnings and precautions sections of the drug labels also have been revised to include information on the risk for serious infections that could lead to death from multiple disease-causing pathogens.
The TNF blockers that will receive the warnings include Remicade (infliximab), Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab pegol), and Simponi (golimumab), according to the FDA announcement.
The FDA advised that risks and benefits should be weighed before initiating TNF blocker therapy in patients with chronic or reoccurring infections, or in patients with conditions that may put them at higher risk for infection.
Reports of serious adverse events associated with these and other drugs should be reported to MedWatch at 800-332-1088.
The Food and Drug Administration has updated the boxed warning label of all tumor necrosis factor–alpha blockers to include information on the risk of infection from Legionella and Listeria.
The warnings and precautions sections of the drug labels also have been revised to include information on the risk for serious infections that could lead to death from multiple disease-causing pathogens.
The TNF blockers that will receive the warnings include Remicade (infliximab), Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab pegol), and Simponi (golimumab), according to the FDA announcement.
The FDA advised that risks and benefits should be weighed before initiating TNF blocker therapy in patients with chronic or reoccurring infections, or in patients with conditions that may put them at higher risk for infection.
Reports of serious adverse events associated with these and other drugs should be reported to MedWatch at 800-332-1088.
FDA: Nature Relief's Instant Wart and Mole Remover Recalled
The Food and Drug Administration and Nature Relief have issued a joint recall notice for Instant Wart and Mole Remover.
The product's active ingredient, calcium oxide, may cause severe burns of the skin, particularly to areas where the skin is thin.
The FDA received a report of an injury requiring medical attention after use of the product, which is sold as a kit.
Consumers using the product should immediately discontinue use and dispose of the product. The FDA also advised consumers to have their moles checked by a clinician to rule our cancer.
At press time, the company's website was still live and promoting the product.
Adverse events should be reported to MedWatch.
The Food and Drug Administration and Nature Relief have issued a joint recall notice for Instant Wart and Mole Remover.
The product's active ingredient, calcium oxide, may cause severe burns of the skin, particularly to areas where the skin is thin.
The FDA received a report of an injury requiring medical attention after use of the product, which is sold as a kit.
Consumers using the product should immediately discontinue use and dispose of the product. The FDA also advised consumers to have their moles checked by a clinician to rule our cancer.
At press time, the company's website was still live and promoting the product.
Adverse events should be reported to MedWatch.
The Food and Drug Administration and Nature Relief have issued a joint recall notice for Instant Wart and Mole Remover.
The product's active ingredient, calcium oxide, may cause severe burns of the skin, particularly to areas where the skin is thin.
The FDA received a report of an injury requiring medical attention after use of the product, which is sold as a kit.
Consumers using the product should immediately discontinue use and dispose of the product. The FDA also advised consumers to have their moles checked by a clinician to rule our cancer.
At press time, the company's website was still live and promoting the product.
Adverse events should be reported to MedWatch.
FDA Sends Warning Letters to Four Hand Sanitizer Manufacturers
The Food and Drug Administration has sent warning letters to four companies that manufacture or distribute hand sanitizers carrying an unproven message that they can prevent MRSA.
The four companies are: Tec Laboratories (Staphaseptic First Aid Antiseptic/Pain Relieving Gel); JD Nelson and Associates (Safe4Hours Hand Sanitizing Lotion and Safe4Hours First Aid Antiseptic Skin Protectant); Dr. G.H. Tichenor Antiseptic Co. (Dr. Tichenor’s Antiseptic Gel); and Oh So Clean Inc., also known as CleanWell Company (Clean Well All-Natural Hand Sanitizer, Clean Well All-Natural Hand Sanitizing Wipes, and Clean Well All-Natural Antibacterial Foaming Hand Soap).
The companies have 15 days from the date of the FDA's Warning Letter (April 20) to correct product labeling, or they risk other legal action and/or product seizure.
The FDA also issued advice to consumers that they should not buy over-the-counter products that claim to prevent infection. "Consumers are being misled if they think these products you can buy in a drugstore or from other places will protect them from a potentially deadly infection," Deborah Autor, compliance director at the FDA’s Center for Drug Evaluation and Research, said in a press release.
Examples of claims found on the labels include: "Kills over 99.9% of MRSA," "helps prevent skin infections caused by MRSA and other germs," and "is effective against a broad spectrum of pathogens, including MRSA."
Any side effects believed to be caused by the use of these hand sanitizing products should be reported to MedWatch.
The Food and Drug Administration has sent warning letters to four companies that manufacture or distribute hand sanitizers carrying an unproven message that they can prevent MRSA.
The four companies are: Tec Laboratories (Staphaseptic First Aid Antiseptic/Pain Relieving Gel); JD Nelson and Associates (Safe4Hours Hand Sanitizing Lotion and Safe4Hours First Aid Antiseptic Skin Protectant); Dr. G.H. Tichenor Antiseptic Co. (Dr. Tichenor’s Antiseptic Gel); and Oh So Clean Inc., also known as CleanWell Company (Clean Well All-Natural Hand Sanitizer, Clean Well All-Natural Hand Sanitizing Wipes, and Clean Well All-Natural Antibacterial Foaming Hand Soap).
The companies have 15 days from the date of the FDA's Warning Letter (April 20) to correct product labeling, or they risk other legal action and/or product seizure.
The FDA also issued advice to consumers that they should not buy over-the-counter products that claim to prevent infection. "Consumers are being misled if they think these products you can buy in a drugstore or from other places will protect them from a potentially deadly infection," Deborah Autor, compliance director at the FDA’s Center for Drug Evaluation and Research, said in a press release.
Examples of claims found on the labels include: "Kills over 99.9% of MRSA," "helps prevent skin infections caused by MRSA and other germs," and "is effective against a broad spectrum of pathogens, including MRSA."
Any side effects believed to be caused by the use of these hand sanitizing products should be reported to MedWatch.
The Food and Drug Administration has sent warning letters to four companies that manufacture or distribute hand sanitizers carrying an unproven message that they can prevent MRSA.
The four companies are: Tec Laboratories (Staphaseptic First Aid Antiseptic/Pain Relieving Gel); JD Nelson and Associates (Safe4Hours Hand Sanitizing Lotion and Safe4Hours First Aid Antiseptic Skin Protectant); Dr. G.H. Tichenor Antiseptic Co. (Dr. Tichenor’s Antiseptic Gel); and Oh So Clean Inc., also known as CleanWell Company (Clean Well All-Natural Hand Sanitizer, Clean Well All-Natural Hand Sanitizing Wipes, and Clean Well All-Natural Antibacterial Foaming Hand Soap).
The companies have 15 days from the date of the FDA's Warning Letter (April 20) to correct product labeling, or they risk other legal action and/or product seizure.
The FDA also issued advice to consumers that they should not buy over-the-counter products that claim to prevent infection. "Consumers are being misled if they think these products you can buy in a drugstore or from other places will protect them from a potentially deadly infection," Deborah Autor, compliance director at the FDA’s Center for Drug Evaluation and Research, said in a press release.
Examples of claims found on the labels include: "Kills over 99.9% of MRSA," "helps prevent skin infections caused by MRSA and other germs," and "is effective against a broad spectrum of pathogens, including MRSA."
Any side effects believed to be caused by the use of these hand sanitizing products should be reported to MedWatch.