User login
Defending access to reproductive health care
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
Office hysteroscopic evaluation of postmenopausal bleeding
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
Postmenopausal bleeding: Its risk for cancer
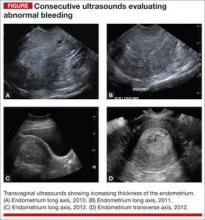
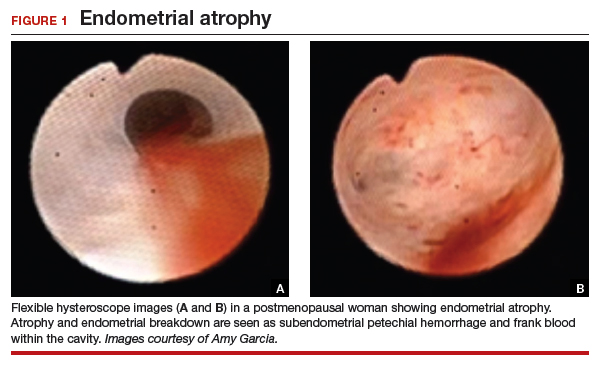
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
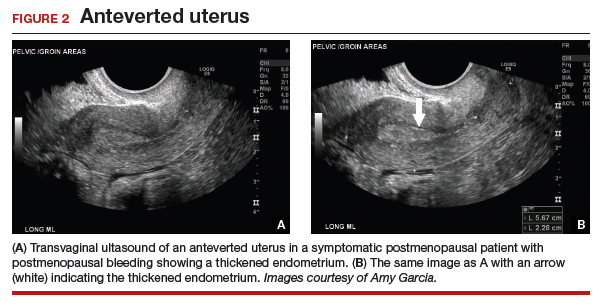
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
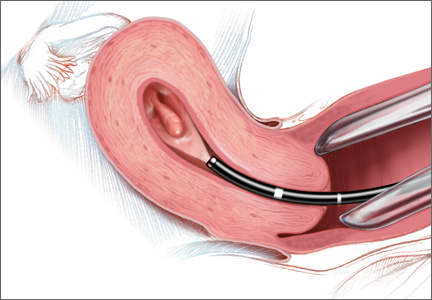
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
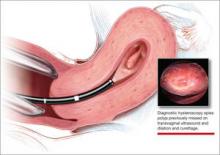
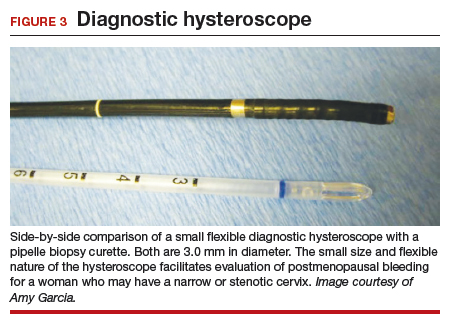
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
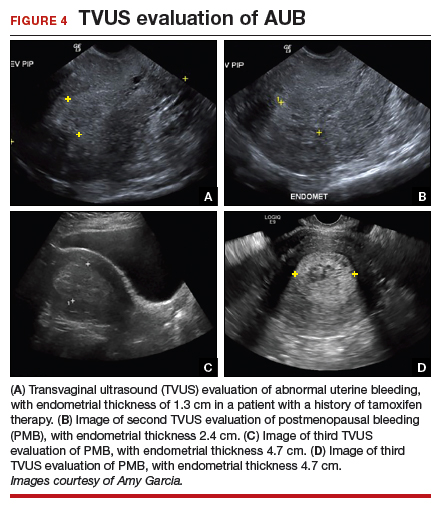
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

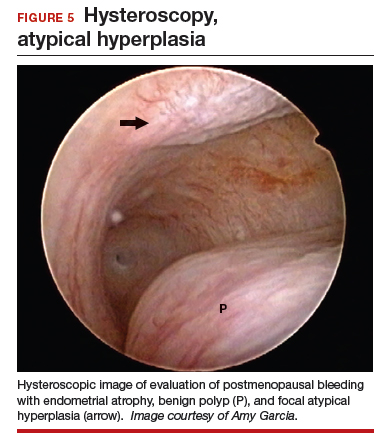
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

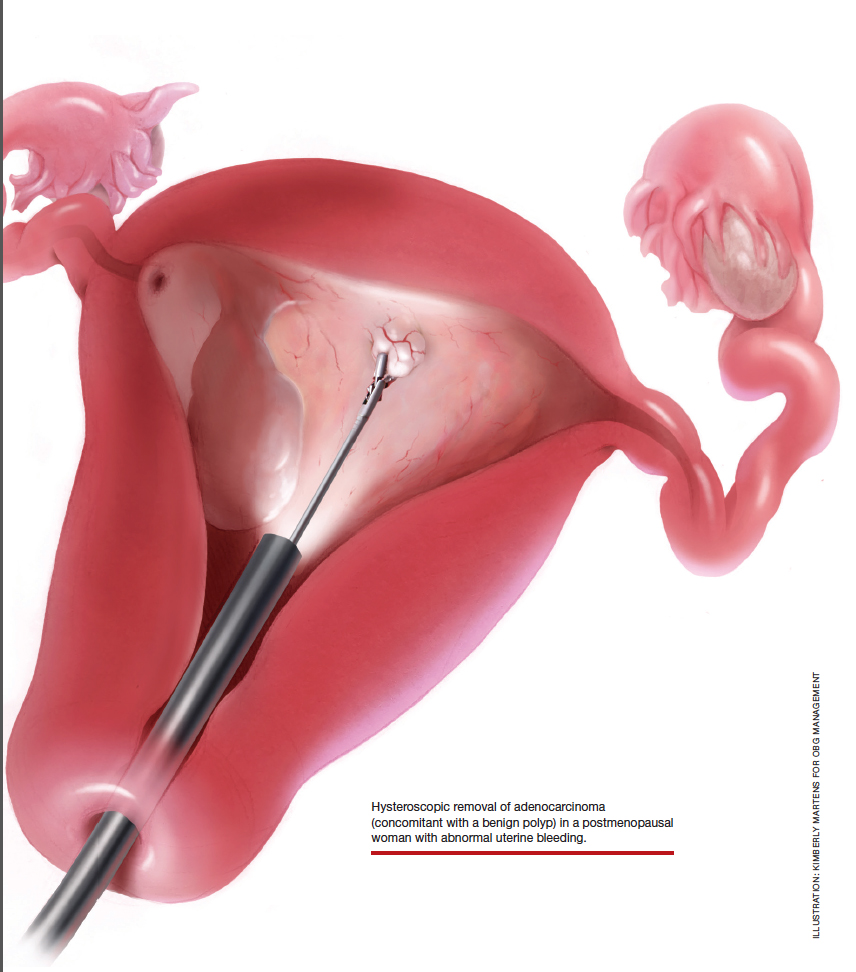
At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

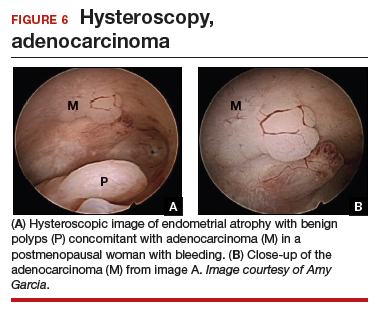
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
2014 Update on minimally invasive gynecology
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
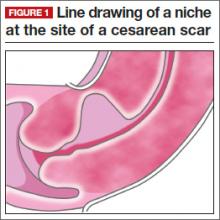
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
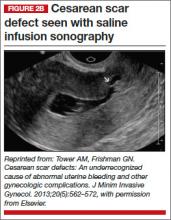
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
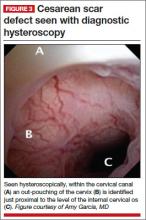
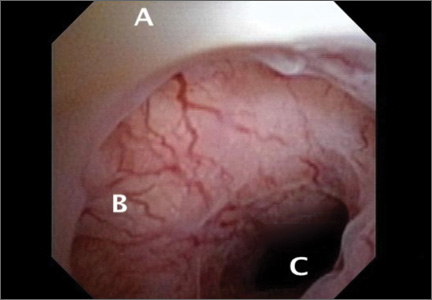
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
CASE: POSTMENSTRUAL BLEEDING, HISTORY OF CESAREAN DELIVERIES
A 36-year-old woman (G3P3) reports prolonged and postmenstrual bleeding. Her cycles are regular, every 28 to 30 days, and are associated with ovulatory symptoms. She bleeds for 8 to 10 days with each cycle, having heavy bleeding on cycle day 2 requiring use of super tampons every 3 hours. Beginning on day 5 of the cycle, the blood becomes much darker and scant requiring a small pad, which she changes twice daily. Often, she experiences dark bleeding with physical activity—specifically, running—usually several days after her cycle has ended. She is otherwise healthy and uses no medications. She uses condoms for contraception. She has had a prior vaginal delivery followed by two cesarean sections. Physical examination is normal.
What is causing this patient’s abnormal bleeding pattern?
From 1996 to 2009, the total US cesarean delivery rate increased steadily from 20.7% to 32.9% and has remained stable at 32.8% through 2012.1 With 3,952,841 registered births in 2012, the number of operative procedures performed annually approximates 1.3 million.2 This means, potentially, that one-third of pregnant American women will undergo cesarean delivery annually, translating into an increasing prevalence of long-term sequelae of this surgery.
An increasingly recognized etiology of AUB
One long-term complication of cesarean delivery, not often discussed, is the presence of a defect within the uterine scar that is directly associated with a type of abnormal uterine bleeding (AUB) referred to as postmenstrual bleeding. Stewart first reported this post–cesarean delivery phenomenon in 1975.3 It is postulated that the cesarean scar defect (CSD)4 forms a pocket, which holds the menstrual effluent, allowing bleeding to occur after regular menstrual cycle bleeding has concluded. Often, remnant menstrual blood is extruded slowly over several days, and is generally dark brown, indicating old blood. Physical activity sometimes can initiate expulsion of the old blood even after the regular cycle has ceased (FIGURE 1).
As early as 1995, Morris reported the histopathologic changes within the cesarean scar in a series of 51 hysterectomy specimens with scar present for 2 to 15 years. His findings included distortion and widening of the lower uterine segment (75%), congested endometrium above the scar recess (61%), marked lymphocytic infiltration (65%), capillary dilation (65%), residual suture material with foreign body giant cell reaction (92%), fragmentation and breakdown of the endometrium of the scar (37%), and iatrogenic adenomyosis confined to the scar (28%). Morris concluded that in addition to AUB, these scar abnormalities could give rise to clinical symptoms such as pelvic pain, dyspareunia, and dysmenorrhea.5 It also has been suggested that otherwise unexplained infertility is associated with anatomic and physiologic changes seen with CSD.6 A recent review article published by Tower summarized additional clinical outcomes of CSD, such as ectopic pregnancy and increased surgical risks for such gynecologic procedures as uterine evacuation in the nonpregnant or postpartum state, hysterectomy, endometrial ablation, and intrauterine device placement.4
The CSD generally is described as a triangular or circular sonographically anechoic area in the myometrium of the anterior lower uterine segment or cervix at the site of a previous cesarean section. In nonpregnant patients, the defect is best evaluated with contrast infusion sonography (CIS), such as saline infusion or gel infusion, versus transvaginal ultrasound (TVUS) alone (FIGURE 2).4,7,8 However, the precise dimensions and definition of the scar defect vary among investigators.4,6,7,8,10
The reported prevalence of CSD has varied in the literature and appears to depend on the modality of diagnosis and the population studied. For instance, van der Voet and colleagues reported that in random populations of women who had undergone cesarean delivery, the defect was evident in 24% to 69% of women evaluated with transvaginal noncontrast ultrasound; the defect was evident in 56% to 78% of women evaluated with transvaginal contrast sonography.8
The scar defect also has been identified with magnetic resonance imaging (MRI) and found to be equal in sensitivity to TVUS.9,10 When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”6 Hysteroscopically, the defect also is visualized commonly within the cervical canal, indicating that cesarean incisions often are made through cervical tissue at the time of delivery (FIGURE 3, VIDEO 1, VIDEO 2 [see below]). Not all women with CSD report bleeding abnormalities, but it appears that the deeper and wider the defect, the more likely a woman is to present with postmenstrual AUB.7 According to the International Federation of Gynecology and Obstetrics (FIGO) Classification of AUB, CSD-associated postmenstrual bleeding falls into the “iatrogenic” category in the PALM-COIEN pneumonic.11
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.
MINIMALLY INVASIVE THERAPY FOR GYNECOLOGIC SYMPTOMS
van der Voet LF, Vervoort AJ, Veersema S, Bij de Vatte AJ, Brolmann HAM, Huirne JAF. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145-156.
CSD-related bleeding issues may not respond to hormonal management and are frequently underdiagnosed. This scenario often leads to hysterectomy. Because there are women who desire uterine preservation, van der Voet and colleagues sought to evaluate the results of nonhysterectomy treatments of CSD-related AUB. They limited this systematic review to include only published studies that were randomized controlled trials, cohort studies, case-control studies, and case series of at least five patients.
Additionally, they included only studies that reported on conservative therapies (hysteroscopic resection, laparoscopic repair, abdominal repair, vaginal repair, endometrial ablation, LNG-IUS, or medical management) as well as at least one of the following outcomes: AUB, pain relief, sexual function, quality of life, surgical outcome, anatomic reconstruction, fertility or pregnancy outcome. Of 1,629 publications that were screened, 12 ultimately met inclusion criteria for the review. The studies, 11 of which were peer reviewed and 1 abstract, were published between 1996 and 2013 and reported on a total of 455 women with postcesarean AUB.
Weaknesses of the study
The most poignant statements made by the investigators pertain to the methodologic quality of the included articles. No study met requisite quality criteria. A clear definition of outcomes, including standardized measurements, was lacking in most studies. Most of the studies reviewed did not report CSD measurements, and only one study provided an objective reproducible method of CSD measurement. Few studies reported AUB symptom evaluation methodology, and no study used validated questionnaires. In the majority of studies, methods of posttreatment outcome measurements either were not reported or differed from pretreatment evaluation methods, potentiating verification bias. Because their literature review yielded primarily small case series publications that reported positive effects of interventions, and because of a lack of large RCT and prospective cohort trials, little could be gleaned regarding the viability of treatment interventions for CSD-related AUB.
Only three studies provided sufficient data to be included in a meta-analysis. The number of days of bleeding was reduced with hysteroscopic defect resection by 2 to 4 days in two studies, and in one study, vaginal repair decreased days of bleeding by 4 to 7 days. Only one study with laparoscopic repair compared CSD characteristics before and after surgery. Residual myometrial thickness increased for laparoscopic repair to greater than 8.3 mm; however, it is not known if this will make a clinical difference in the risk of scar dehiscence or improved functionality of the lower uterine segment.
Two studies reported on the laparoscopic repair of scar defects in asymptomatic patients, which is not recommended by these investigators. It is not known what ramifications hysteroscopic resection of the scar will have for the risk of uterine rupture, malplacentation or cervical incompetence for women who conceive after hysteroscopic repair.
Meaningful conclusions are lacking
Despite the high success rates reported by investigators of various surgical intervention case series involving hysteroscopic resection, vaginal repair, or laparoscopic repair, van der Voet and colleagues ultimately state that the methodologies of these studies do not allow meaningful conclusions to be drawn regarding the effectiveness of any of these interventions. Consequently, the authors recommend that the outcomes of their meta-analysis be scrutinized. They also point out that the LNG-IUS has proven benefit for AUB and yet has not been studied in the treatment of AUB associated with a CSD.
They finally propose that women who are symptomatic be treated with oral contraceptives unless immediate fertility is desired, or by expectant management without intervention. While their primary focus was to assess AUB, given the stated shortcomings of the included studies and lack of long-term follow-up, the authors also warn against hysteroscopic, laparoscopic, or vaginal repair for fertility, as the risk to pregnancy or delivery after these therapies is unknown.
CASE RESOLVED
Suspecting a cesarean scar defect, you perform a saline infusion sonography and diagnose a 14 mm x 19 mm anechoic region within the scar, with no other intracavitary abnormalities found. You first reassure the patient that this is a benign finding and inform her why she likely is experiencing this type of bleeding pattern. After an informed discussion with you regarding the risks and benefits of possible surgical or nonsurgical options for management, she chooses to use oral contraceptive pills in a continuous fashion.
CONCLUSION
Consider a history of cesarean section in the evaluation of AUB, and be cognizant of the prevalence of CSD with cesarean delivery and the association of postmenstrual bleeding with CSD.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• A critical systematic review of available data suggests that there is not enough clinical evidence to support surgical intervention for the treatment of CSD for women symptomatic with AUB.
• Recommended nonhysterectomy treatments for AUB associated with CSD include oral contraceptives or expectant management.
• Surgical treatment should be limited to the research environment in the form of RCT to assess the long-term outcomes of intervention.
• An RCT of the LNG-IUS for the treatment of AUB associated with CSD is needed.
Acknowledgments
The author would like to thank Andrew Brill, MD, Lee Sloan-Garcia, MD, and William Parker, MD, for their thoughtful review of this manuscript.
We want to hear from you!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: obg@frontlinemedcom.com Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
- Osterman MJK, Martin JA. Primary cesarean delivery rates, by state: Results from the revised birth certificate, 2006-2012. Natl Vital Stat Rep. 2014;63(1):1–11.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013;62(9). Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. Accessed March 19, 2014.
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975;82(8):682–686.
- Tower AM, Frishman GN. Cesarean scar defects: An underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
- Morris H. Surgical pathology of the lower uterine segment cesarean section scar: Is the scar a source of clinical symptoms? Intl J Gynecol Pathol. 1995;14(1):16–20.
- Gubbini G, Centini G, Nascetti D, et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: Prospective study. J Minim Invasive Gynecol. 2011;18(2):234–237.
- van der Voet LF, Bijde Vaate AM, Veersema S, Brolmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
- van der Voet LF, Vervoort AJ, Veersema S, Bijde Vatte AJ, Brolmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: A systematic review. BJOG. 2014;121(2):145–156.
- Maldjian C, Adam R, Maldjian J, Smith R. MRI appearance of the pelvis in the post cesarean-section patient. Magn Reson Imaging. 1999;17(2):223–227.
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-Cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013;20(3):386–391.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COIEN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13.
CESAREAN SCAR DEFECT DIAGNOSED WITH HYSTEROSCOPY
![]()
![]()
Videos courtesy of Amy Garcia, MD
Fiberoptic flexible hysteroscopy diagnoses cesarean scar defect
Fiberoptic flexible hysteroscopy showing cesarean scar defect in the anterior lower uterine segment of a patient evaluated for abnormal uterine bleeding. When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”
Related video: Digital flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Fiberoptic flexible hysteroscopy showing cesarean scar defect in the anterior lower uterine segment of a patient evaluated for abnormal uterine bleeding. When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”
Related video: Digital flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Fiberoptic flexible hysteroscopy showing cesarean scar defect in the anterior lower uterine segment of a patient evaluated for abnormal uterine bleeding. When identified hysteroscopically, a definitive out-pouching is visualized in the lower uterine segment, where the defect has been termed an “isthmocele.”
Related video: Digital flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Digital flexible hysteroscopy diagnoses cesarean scar defect
Seen at office diagnostic hysteroscopy during an evaluation of abnormal uterine bleeding, an out-pouching of the cervix is identified within the cervical canal just proximal to the level of the internal cervical os. A small amount of dark blood is seen emanating from the defect within the cervix.
Related video: Fiberoptic flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Seen at office diagnostic hysteroscopy during an evaluation of abnormal uterine bleeding, an out-pouching of the cervix is identified within the cervical canal just proximal to the level of the internal cervical os. A small amount of dark blood is seen emanating from the defect within the cervix.
Related video: Fiberoptic flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Seen at office diagnostic hysteroscopy during an evaluation of abnormal uterine bleeding, an out-pouching of the cervix is identified within the cervical canal just proximal to the level of the internal cervical os. A small amount of dark blood is seen emanating from the defect within the cervix.
Related video: Fiberoptic flexible hysteroscopy for diagnosing cesarean scar defect Amy Garcia, MD (April 2014)
Related article: Update on minimally invasive gynecology Amy Garcia, MD (April 2014)
Supracervical hysterectomy: Indications and why women prefer it
At the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium held December 13–15, 2012, in Las Vegas, Amy Garcia, MD, presented a session on laparoscopic supracervical hysterectomy.
In her presentation, Dr. Garcia described the indications, technique, benefits, and risks associated with this procedure. "As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common," noted Dr. Garcia.
In this audiocast, Dr. Garcia discusses the indications for laparoscopic supracervical hysterectomy, and why women prefer it.
At the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium held December 13–15, 2012, in Las Vegas, Amy Garcia, MD, presented a session on laparoscopic supracervical hysterectomy.
In her presentation, Dr. Garcia described the indications, technique, benefits, and risks associated with this procedure. "As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common," noted Dr. Garcia.
In this audiocast, Dr. Garcia discusses the indications for laparoscopic supracervical hysterectomy, and why women prefer it.
At the Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium held December 13–15, 2012, in Las Vegas, Amy Garcia, MD, presented a session on laparoscopic supracervical hysterectomy.
In her presentation, Dr. Garcia described the indications, technique, benefits, and risks associated with this procedure. "As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common," noted Dr. Garcia.
In this audiocast, Dr. Garcia discusses the indications for laparoscopic supracervical hysterectomy, and why women prefer it.
STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding
CASE: In-office hysteroscopy spies previously missed polyp
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
CASE: In-office hysteroscopy spies previously missed polyp
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
CASE: In-office hysteroscopy spies previously missed polyp
A 51-year-old woman with a history of breast cancer completed 5 years of tamoxifen. During her treatment she had a 3-year history of abnormal vaginal bleeding. Results from consecutive pelvic ultrasounds indicated that the patient had progressively thickening endometrium (from 1.4 cm to 2.5 cm to 4.7 cm). In-office biopsy was negative for endometrial pathology. An ultimate dilation and curettage (D&C) was performed with negative histologic diagnosis. The patient is seen in consultation, and the ultrasound images are reviewed (FIGURE).
These images show an increasing thickness of the ednometrium with definitive intracavitary pathology that was missed with the prevous clinical evaluation with enodmetrial biopsy and D&C. An in-office hysteroscopy is performed, and a large 5 x 4 x 7 cm cystic and fibrous polyp is identified with normal endometrium (VIDEO 1).
Hysteroscopy reveals massive polyp extending into a dilated lower uterine segment
Abnormal uterine bleeding
The evaluation of abnormal uterine bleeding. (AUB), as described in this clinical scenario, is quite common. As a consequence, many patients have a missed or inaccurate diagnosis and undergo unnecessary invasive procedures under general anesthesia.
AUB is one of the primary indications for a gynecologic consultation, accounting for approximately 33% of all gynecology visits, and for 69% of visits among postmenopausal women.1 Confirming the etiology and planning appropriate intervention is important in the clinical management of AUB because accurate diagnosis may result in avoiding major gynecologic surgery in favor of minimally invasive hysteroscopic management.
Diagnostic hysteroscopy is proven in AUB evaluation
Drawbacks of other diagnostic tools. It is generally accepted that the initial evaluation of women with AUB is performed with noninvasive transvaginal ultrasound (TVUS).1-3 As illustrated by the opening case, however, the accuracy of TVUS is limited in the diagnosis of focal endometrial lesions, and further investigation of the uterine cavity is warranted.
Evaluation of the uterine cavity with sonohysterography (SH)—vaginal ultrasound with the instillation of saline into the uterine cavity—is more accurate than TVUS alone. Yet, diagnostic hysteroscopy (DH) has proven to be superior to either modality for the accurate evaluation of intracavitary pathology.1-3
Evidence of DH superiority. Farquhar and colleagues reported results of a systematic review of studies published from 1980 to July 2001 that examined TVUS versus SH and DH for the investigation of AUB in premenopausal women. The researchers found that TVUS had a higher rate of false negatives for detecting intrauterine pathology, compared with SH and DH. They also found that DH was superior to SH in diagnosing submucous myomas.2
In 2010, results of a prospective comparison of TVUS, SH, and DH for detecting endometrial pathology, showed that DH had a significantly better diagnostic performance than SH and TVUS and that hysteroscopy was significantly more precise in the diagnosis of intracavitary masses.3
Again, in 2012, a prospective comparison of TVUS, SH, and DH in the diagnosis of AUB revealed that hysteroscopy provided the most accurate diagnosis.1
In a systematic review and meta-analysis, van Dongen found the accuracy of DH to be estimated at 96.9%.4
Hysteroscopy is also considered to be more comfortable for patients than SH.2
blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
A procedure performed “blind” limits its usefulness in AUB evaluation
This statement is not a new realization. As far back as 1989, Loffer showed that blind D&C was less accurate for diagnosing AUB than was hysteroscopy with visually directed biopsy. The sensitivity of diagnosing the etiology of AUB with D&C was 65%, compared to a sensitivity of 98% with hysteroscopy with directed biopsy. Hysteroscopy was shown to be better because blinded sampling missed intracavitary lesions such as polyps and myomas that accounted for bleeding abnormalities.5
The limitations of D&C are evident in the opening case, as the D&C performed in the operating room under general anesthesia, without visualization of the uterine cavity, failed to identify the patient’s intrauterine pathology. D&C is seldom necessary to evaluate AUB and has significant surgical risks beyond general anesthesia, including cervical or uterine trauma that can occur with cervical dilation and instrumentation of the uterus.
As with D&C, endometrial biopsy has limitations in diagnosing abnormalities within the uterine cavity. In a 2008 prospective comparative study of hysteroscopy versus blind biopsy with a suction biopsy curette, Angioni and colleagues showed a significant difference in the capability of these two procedures to accurately diagnose the etiology of bleeding for menopausal women. Blinded biopsy had a sensitivity for diagnosing polyps, myomas, and hyperplasia of 11%, 13%, and 25%, respectively. The sensitivity of hysteroscopy to diagnose the same intracavitary pathology was 89%, 100%, and 74%, respectively.6
This does not mean, however, that the endometrial biopsy is not beneficial in the evaluation of AUB. Several authors recommend that in a clinically relevant situation, endometrial biopsy with a small suction curette should be performed concomitantly with hysteroscopy to improve the sensitivity of the overall evaluation with histology.7,8 And, as Loffer showed, the hysteroscopic evaluation with a visually directed biopsy is extremely accurate (VIDEO 2, VIDEO 3).5
The bottom line for D&C use in AUB evaluation
Sometimes a D&C is needed. For instance, when more tissue is needed for histologic evaluation than can be obtained with small suction curette at endometrial biopsy. However, there are several shortcomings of D&C for the evaluation of AUB:
- Most clinicians perform D&C in the operating room under general anesthesia.
- It is often done without concomitant hysteroscopy.
- There is significant potential to miss pathology, such as polyps or myomas.
- There is risk of uterine perforation with cervical dilation and uterine instrumentation.
- Hysteroscopy with visually directed biopsy provides a method that offers a more accurate diagnosis, and the procedure can be performed in the office.
In-office AUB evaluation using hysteroscopy is possible and advantageous
Hysteroscopy not only has increased accuracy for identifying the etiology of AUB, compared with D&C, but also offers the possibility of in-office use. Newer hysteroscopes with small diameters and decreasing costs of hysteroscopic equipment allow gynecologists to perform hysteroscopy economically and safely in the office.
Office evaluation of the uterine cavity and preoperative decision-making before a patient is taken to the operating room (OR) improve the likelihood that the appropriate procedure will be performed. They also provide an opportunity for the patient to see inside her own uterine cavity and for the surgeon to discuss management options with her (VIDEO 4: Diagnostic hysteroscopy with fundal myoma, VIDEO 5: Diagnostic hysteroscopy in a menopausal patient with atypical hyperplasia, VIDEO 6: Diagnostic hysteroscopy in a menopausal patient with polyps). If pathology is noted, there is a potential to treat abnormalities such as endometrial polyps at the same time, thus avoiding the OR altogether (VIDEO 7).
The small diameter of the hysteroscope allows evaluation in most menopausal and nulliparous patients comfortably without first having to dilate or soften the cervix. Paracervical placement of local anesthetic can be used as needed for patient comfort (VIDEO 8).9 A vaginoscopic approach will eliminate the discomfort of having to place a speculum (VIDEO 9).
It offers:
- a familiar and comfortable environment for the procedure
- saved time for patient and physician
- saved money for the patient with a large deductible or coinsurance
- no requirement for general anesthesia
- local anesthesia can be used but is not necessary
- immediate visual affirmation for the patient and physician
- a see and treat possibility
- possibility of preoperative decision-making
- saved trip to the OR if significant precancer or cancer is identified
- use in menopausal and nulliparous patients with no cervical preparation necessary when a small-diameter hysteroscope or flexible hysteroscope is used
- minimized discomfort from a speculum with the vaginoscopic approach for awake patients
- possibility of cervical access when needed (VIDEO 10).
My clinical recommendation
Use office hysteroscopy with endometrial biopsy as needed with the opportunity to perform a directed biopsy.
Coding for in-office hysteroscopy
Some procedures now can be performed in the office setting. Among these is operative hysteroscopy, for things such as abnormal uterine bleeding (AUB), foreign body removal, and tubal occlusion. When performing hysteroscopic evaluation of AUB, the Current Procedural Terminology (CPT) code 58558 (Hysteroscopy, surgical; with sampling (biopsy) of endometrium and/or polypectomy, with or without D&C) should be reported. This code is reported whether polyp(s) are removed or a sampling of the uterine lining or a full D&C is performed.
Under the Resource-Based Relative Value System (RBRVS), used by the majority of payers for reimbursement, there is a payment differential for site of service. In other words, when performed in the office setting, reimbursement will be higher than in the hospital setting to offset the increased practice expenses incurred. In the office setting, 58558 has 11.93 relative value units. In comparison, a D&C performed without hysteroscopy has 7.75 relative value units in the office setting. Keep in mind, however, that all supplies used in performing this procedure are included in the reimbursement amount.
Some payers will reimburse separately for administering a regional anesthetic, but a local anesthetic is considered integral to the procedure. Under CPT rules, you may bill separately for regional anesthesia. When performing office hysteroscopy, the most common regional anesthesia would be a paracervical nerve block (CPT code 66435—Injection, anesthetic agent; paracervical [uterine] nerve). Under CPT rules, coding should go in as 58558-47, 64435-51. The modifier -47 lets the payer know that the physician performed the regional block, and the modifier -51 identifies the regional block as a multiple procedure.
Medicare, however, will never reimburse separately for regional anesthesia performed by the operating physician, and because of this, Medicare’s Correct Coding Initiative (CCI) permanently bundles 64435 when billed with 58558. Medicare will not permit a modifier to be used to bypass this bundling edit, and separate payment is never allowed. If your payer has adopted this Medicare policy, separate payment will also not be made.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
- Soguktas S, Cogendez E, Kayatas SE, Asoglu MR, Selcuk S, Ertekin A. Comparison of saline infusion sonohysterography and hysteroscopy in diagnosis of premenopausal women with abnormal uterine bleeding. Eur J Obstet Gynecol Repro Biol. 2012;161(1):66−70.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal bleeding. Acta Obstet Gynecol Scand. 2003;82(6):493–504.
- Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril. 2010;94(7):2721−2725.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114(6):664–75.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D & C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73(1):16–20.
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol. 2008;15(1):87–91.
- Lo KY, Yuen PM. The role of outpatient diagnostic hysteroscopy in identifying anatomic pathology and histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc. 2000;7(3):381–385.
- Garuti G, Sambruni I, Colonneli M, Luerti M. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc. 2001;8(2):207–213.
- Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
Vaginoscopic hysteroscopy
UPDATE: MINIMALLY INVASIVE GYNECOLOGY
LARCs: Why they should be first-line contraceptive options
for your patients
Elizabeth O. Schmidt, MD; Tessa Madden, MD, MPH; Jeffrey F. Piepert, MD, PhD
(November 2012)
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2012)
Your surgical toolbox should include topical hemostatic
agents—here is why
Lisa A. dos Santos, MD; Andrew W. Menzin, MD (Surgical Techniques, April 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2011)
The proliferation of terms to describe heavy menstrual bleeding sometimes seems never-ending. From “menometrorrhagia” to “uterine hemorrhage,” these terms pop up quickly and confuse discussion of one of the most widespread problems in gynecology.
Enter the International Federation of Gynecology and Obstetrics (FIGO), which decided to tackle the inconsistent terminology and lack of classification of causes of abnormal uterine bleeding (AUB) with an eye toward standardizing research, facilitating discussion, and informing management decisions.
In this article, I focus on three aspects of this effort:
- FIGO’s revamping of terminology and classification
- comparisons of outcomes of hysterectomy versus endometrial ablation and the levonorgestrel-releasing intrauterine system
- guidelines on management of AUB related to ovulatory disorders and endometrial hemostatic dysfunction.
FIGO revamps nomenclature for abnormal uterine bleeding
Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? Am J Obstet Gynecol. 2012;207(4):259–265.
As early as 2004, FIGO began a process to standardize the nomenclature for defining both normal and abnormal uterine bleeding in reproductive-aged women who are not pregnant.1 This process was a response to a lack of consistency and continuity in the design and interpretation of basic science and clinical investigation related to the problem of AUB. Inconsistent definitions of AUB, such as “menorrhagia,” “metrorrhagia,” and “dysfunctional uterine bleeding,” along with the absence of standard categorization of the causes of AUB, have led to confusion and difficulties in comparing clinical trials and in finding significant, relevant, and even meaningful correlations among investigations of AUB. Applying information from asynchronous and often incomplete investigations to evidence-based clinical practice then becomes a challenge for the gynecologist.
Munro and colleagues summarize the process by which FIGO developed both a nomenclature system and a classification system of the causes of AUB, which were formally adopted by FIGO in 2010 and endorsed in 2012 by the American College of Obstetricians and Gynecologists (ACOG).1-6 The arduous process led to:
- a refined definition of chronic AUB
- a new category called acute AUB
- a method for describing the clinical dimensions of menstruation and the menstrual cycle according to the following parameters:
- regularity of onset
- frequency of onset
- duration of menstrual flow
- heaviness, or volume, of menstrual flow.
Wherever appropriate, the definitions of normal for these parameters were based on statistics from large population studies that used medians and 5th and 95th percentiles.
The term “heavy menstrual bleeding” (HMB) is used to describe a woman’s perception of increased menstrual volume, regardless of regularity, frequency, or duration. AUB is the overarching term to describe any departure from normal menstruation, as defined by the parameters listed above. A group of misleading terms commonly used to describe AUB were eliminated from the FIGO nomenclature system, including “dysfunctional uterine bleeding,” “menorrhagia,” “hypermenorrhea,” “menometrorrhagia,” “polymenorrhagia,” and “metrorrhagia.”
The causes of AUB are classified in nine categories that are arranged according to the acronym PALM-COEIN:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
Leiomyoma are subclassified as submucous or other, with tertiary subcategorization for intramural, subserosal, and transmural lesions
In general, the components of the PALM group are discrete (structural) entities that are measurable visually via imaging or histopathology, or both, while the COEI (of the COEIN group) includes women for whom the AUB is unrelated to structural abnormalities.
The classification system provides the infrastructure for a thorough investigative process and a means to characterize AUB for an individual who may have one or more potential causes or contributors. Such a comprehensive assessment allows the basic scientist to identify pure populations for tissue and molecular studies, the clinical scientist to identify potential confounders when defining populations for clinical investigation, and the clinician, educator, and trainee to consider the multidimensional nature of AUB where asymptomatic “red herrings” may coexist with otherwise invisible disorders of menstrual function.
The FIGO Menstrual Disorders Working Group anticipates that widespread, international acceptance of the recommended terms, definitions, and classification for AUB will lead to improved and more meaningful communication in clinical trials and published research and will enhance communication between health-care providers and patients, leading to better management of AUB.
Use of the FIGO-recommended terms, definitions, and classification of AUB will lead to higher-quality clinical research and thorough clinical investigation into the causes of AUB, with improved management of patients.
How hysterectomy for AUB compares with less invasive treatment options
Matteson KA, Abed H, Wheeler TL II, et al; Society of Gynecologic Surgeons Systematic Review Group. A systematic review comparing hysterectomy with less invasive treatments for abnormal uterine bleeding. J Minim Invasive Gynecol. 2012;19(1):13–28.
To create reliable treatment recommendations for AUB, as defined by the FIGO classification system just described, in women with ovulatory disorders, endometrial hemostatic dysfunction, and concomitant leiomyoma, the Systematic Review Group (SRG) of the Society of Gynecologic Surgeons performed a systematic review of treatments. The analysis was intended to compare hysterectomy with less invasive treatment modalities. The SRG reviewed randomized, controlled trials of AUB treatment that compared hysterectomy with:
- endometrial ablation by resectoscopic loop, rollerball, or thermal balloon
- the LNG-IUS
- medical therapy.
This comprehensive review of literature published between 1950 and January 14, 2011 led the SRG to create seven categories of clinical outcomes:
- bleeding control
- quality of life
- pain
- sexual health
- patient satisfaction
- need for additional treatment
- adverse events.
Of the initial 5,503 titles identified, only 18 articles, representing nine clinical trials, contained data of adequate quality to meet criteria for review. Seven of the trials compared hysterectomy with ablation, one compared hysterectomy with the LNG-IUS, and one compared hysterectomy with medical therapy. As FIGO has pointed out, the lack of homogeneity of terminology used to describe AUB and classification of its causes prevented clinically applicable comparative analyses of treatment outcomes.
Here are some of the SRG’s findings:
- Control of bleeding. Only data regarding amenorrhea were sufficient for comparative analysis. The SRG was able to conclude only that there was moderate strength of evidence supporting the statement that bleeding is better controlled following hysterectomy than following ablation.
- Quality of life. Overall, studies that evaluated quality of life showed improvement after ablation and hysterectomy. The strength of evidence demonstrating no difference between hysterectomy and ablation in postoperative quality of life was moderate.
- Pain, general health, vitality, and social function. Three studies found statistically significant differences in validated dimensions of the SF-36 questionnaire favoring hysterectomy for pain, general health, vitality, and social function. Two of these three studies evaluated minimally invasive hysterectomy by the laparoscopic supracervical or vaginal approach. The strength of evidence on pain beyond the postoperative time period was low and favored hysterectomy over ablation.
- Sexual health. The strength of evidence related to sexual health was low and revealed no differences between hysterectomy and ablation.
- Patient satisfaction. Overall, the quality of evidence was very low, showing no difference between hysterectomy and ablation.
- Need for additional treatment. The quality of evidence was moderate and favored hysterectomy over ablation.
- Adverse events. Evidence of moderate quality favored ablation and the LNG-IUS over hysterectomy, and low-quality evidence favored medical therapy over hysterectomy (TABLES 1, 2).
TABLE 1
What the data reveal about hysterectomy versus ablation
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | Ablation | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Low (F) | — |
| Sexual health | Low (S) | Low (S) |
| Patient satisfaction | Very low (S) | Very low (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
TABLE 2
What the data reveal about hysterectomy versus the levonorgestrel-releasing intrauterine system (LNG-IUS)
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | LNG-IUS | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Moderate (S) | Moderate (S) |
| Sexual health | Moderate (S) | Moderate (S) |
| Patient satisfaction | Moderate (S) | Moderate (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
The SRG concluded that there are tradeoffs between treatment effectiveness and the risk of serious adverse events between hysterectomy, ablation, and the LNG-IUS. It recommended that clinicians be educated about the relative advantages and disadvantages of each option so that they can discuss them with patients.
The SRG developed clinical practice guidelines for the treatment of ovulatory disorders and endometrial hemostatic dysfunction associated with AUB (see below).
Gynecologists should educate each patient about the efficacy and risks of options available for the management of AUB in the context of specific symptoms to facilitate an informed choice.
Wheeler TL II, Murphy M, Rogers RG, et al; Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines for abnormal uterine bleeding: hysterectomy versus alternative therapy. J Minim Invasive Gynecol. 2012;19(1):81–87.
The SRG used the results of the systematic review just summarized to formulate clinical guidelines for the treatment of AUB related to ovulatory disorders and endometrial hemostatic dysfunction. Recommendations were assigned a grade for their strength on the basis of the quality of supporting evidence, the size of the net medical benefit, and other considerations, including values and preferences applied in judgments. The strength of the clinical recommendation is either “strong” or “weak” and indicates the degree to which one can be confident that adherence to the recommendation will do more good than harm. All of the clinical recommendations described below received a grade of “weak.”
One primary suggestion from the study group is patient counseling that must first determine the type of AUB and the degree of burden or distress for the patient, as well as the presence of any additional cycle-related symptoms. Consideration should be given to variables that may modify the inherent risks or benefits of each intervention for the particular patient, as well as her values and preferences regarding treatment harms, benefits, and potential outcomes. Counseling should assess the patient’s need for contraception, desire for future childbearing, and proximity to menopause, as well as any cultural preferences for management.
Based on the clinical evidence related to hysterectomy versus endometrial ablation, the SRG made the following recommendations:
- If the patient desires amenorrhea and less pain and wants to avoid additional therapy, hysterectomy is preferred
- If the patient wants to avoid adverse events and seeks a shorter hospital stay, endometrial ablation is preferred
- If the patient’s main desire is for improvement in overall quality of life or sexual health, either intervention is appropriate, depending on patient preferences.
There were no data available in the systematic review concerning newer technologies for nonhysteroscopic endometrial ablation versus hysterectomy.
Based on the clinical evidence related to hysterectomy versus the LNG-IUS, the SRG made the following recommendations:
- If the patient desires amenorrhea or seeks to avoid additional therapy, hysterectomy is preferred
- If the patient’s main preference is to avoid adverse events, the LNG-IUS is preferred
- If her preference is for improved quality of life or sexual health, either treatment can be offered.
Based on the clinical evidence related to hysterectomy versus systemic medication, the SRG made the following recommendations:
- If the patient wants to become amenorrheic or hopes to avoid further intervention, hysterectomy is recommended
- If she wants to avoid adverse events, medications are recommended
- If her main preference is overall improvement in quality of life, less pain, or improvement in sexual health, either hysterectomy or medication is appropriate.
Note that no standard therapy was given; medical agents included combined oral contraceptive pills, cyclic or continuous progestin, conjugated estrogen with or without progestin, and prostaglandin synthetase inhibitors, usually with hormonal therapy. There are no randomized, controlled trials of other medications such as nonsteroidal anti-inflammatory drugs or tranexamic acid versus hysterectomy.
The SRG cited three main difficulties in the development of clinical guidelines:
- a lack of well-developed randomized, controlled trials of alternative management versus hysterectomy, as well as inconsistent measurement and reporting among the few trials that exist
- a lack of uniformity in AUB diagnoses among the randomized, controlled trials evaluated
- inconsistent use of terminology related to AUB within the trials.
All of these challenges were addressed by the FIGO nomenclature and AUB classification recommendations. Adherence to the FIGO guidelines for future clinical research would eliminate the difficulties faced by this study group and lead to higher-quality clinical evidence that could form the basis of solid clinical recommendations for the treatment of AUB related to ovulatory disorders or endometrial hemostatic dysfunction.
“Decision-making about treatments of AUB requires discussion so a patient can choose a therapy that best fits her disease, her values, and her preferences and optimizes her chance for treatment success while minimizing risks,” the SRG concluded.
ACKNOWLEDGMENT. Thank you to Dr. Malcolm Munro and Dr. Anita Lee Sloan for their thoughtful reviews of this manuscript.
We want to hear from you! Tell us what you think.
TECHNIQUE ARTICLES?
CLICK HERE to access Surgical Technique articles published recently in OBG Management.
1. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3-13.
2. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. The FIGO classification of causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204-2208.
3. Critchley HO, Munro MG, Broder M, Fraser IS. A five-year international review process concerning terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):377-382.
4. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-390.
5. Munro MG, Critchley HO, Fraser IS. The flexible FIGO classification concept for underlying causes of abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):391-399.
6. American College of Obstetricians and Gynecologists. Practice bulletin #128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):207-211.
LARCs: Why they should be first-line contraceptive options
for your patients
Elizabeth O. Schmidt, MD; Tessa Madden, MD, MPH; Jeffrey F. Piepert, MD, PhD
(November 2012)
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2012)
Your surgical toolbox should include topical hemostatic
agents—here is why
Lisa A. dos Santos, MD; Andrew W. Menzin, MD (Surgical Techniques, April 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2011)
The proliferation of terms to describe heavy menstrual bleeding sometimes seems never-ending. From “menometrorrhagia” to “uterine hemorrhage,” these terms pop up quickly and confuse discussion of one of the most widespread problems in gynecology.
Enter the International Federation of Gynecology and Obstetrics (FIGO), which decided to tackle the inconsistent terminology and lack of classification of causes of abnormal uterine bleeding (AUB) with an eye toward standardizing research, facilitating discussion, and informing management decisions.
In this article, I focus on three aspects of this effort:
- FIGO’s revamping of terminology and classification
- comparisons of outcomes of hysterectomy versus endometrial ablation and the levonorgestrel-releasing intrauterine system
- guidelines on management of AUB related to ovulatory disorders and endometrial hemostatic dysfunction.
FIGO revamps nomenclature for abnormal uterine bleeding
Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? Am J Obstet Gynecol. 2012;207(4):259–265.
As early as 2004, FIGO began a process to standardize the nomenclature for defining both normal and abnormal uterine bleeding in reproductive-aged women who are not pregnant.1 This process was a response to a lack of consistency and continuity in the design and interpretation of basic science and clinical investigation related to the problem of AUB. Inconsistent definitions of AUB, such as “menorrhagia,” “metrorrhagia,” and “dysfunctional uterine bleeding,” along with the absence of standard categorization of the causes of AUB, have led to confusion and difficulties in comparing clinical trials and in finding significant, relevant, and even meaningful correlations among investigations of AUB. Applying information from asynchronous and often incomplete investigations to evidence-based clinical practice then becomes a challenge for the gynecologist.
Munro and colleagues summarize the process by which FIGO developed both a nomenclature system and a classification system of the causes of AUB, which were formally adopted by FIGO in 2010 and endorsed in 2012 by the American College of Obstetricians and Gynecologists (ACOG).1-6 The arduous process led to:
- a refined definition of chronic AUB
- a new category called acute AUB
- a method for describing the clinical dimensions of menstruation and the menstrual cycle according to the following parameters:
- regularity of onset
- frequency of onset
- duration of menstrual flow
- heaviness, or volume, of menstrual flow.
Wherever appropriate, the definitions of normal for these parameters were based on statistics from large population studies that used medians and 5th and 95th percentiles.
The term “heavy menstrual bleeding” (HMB) is used to describe a woman’s perception of increased menstrual volume, regardless of regularity, frequency, or duration. AUB is the overarching term to describe any departure from normal menstruation, as defined by the parameters listed above. A group of misleading terms commonly used to describe AUB were eliminated from the FIGO nomenclature system, including “dysfunctional uterine bleeding,” “menorrhagia,” “hypermenorrhea,” “menometrorrhagia,” “polymenorrhagia,” and “metrorrhagia.”
The causes of AUB are classified in nine categories that are arranged according to the acronym PALM-COEIN:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
Leiomyoma are subclassified as submucous or other, with tertiary subcategorization for intramural, subserosal, and transmural lesions
In general, the components of the PALM group are discrete (structural) entities that are measurable visually via imaging or histopathology, or both, while the COEI (of the COEIN group) includes women for whom the AUB is unrelated to structural abnormalities.
The classification system provides the infrastructure for a thorough investigative process and a means to characterize AUB for an individual who may have one or more potential causes or contributors. Such a comprehensive assessment allows the basic scientist to identify pure populations for tissue and molecular studies, the clinical scientist to identify potential confounders when defining populations for clinical investigation, and the clinician, educator, and trainee to consider the multidimensional nature of AUB where asymptomatic “red herrings” may coexist with otherwise invisible disorders of menstrual function.
The FIGO Menstrual Disorders Working Group anticipates that widespread, international acceptance of the recommended terms, definitions, and classification for AUB will lead to improved and more meaningful communication in clinical trials and published research and will enhance communication between health-care providers and patients, leading to better management of AUB.
Use of the FIGO-recommended terms, definitions, and classification of AUB will lead to higher-quality clinical research and thorough clinical investigation into the causes of AUB, with improved management of patients.

How hysterectomy for AUB compares with less invasive treatment options
Matteson KA, Abed H, Wheeler TL II, et al; Society of Gynecologic Surgeons Systematic Review Group. A systematic review comparing hysterectomy with less invasive treatments for abnormal uterine bleeding. J Minim Invasive Gynecol. 2012;19(1):13–28.
To create reliable treatment recommendations for AUB, as defined by the FIGO classification system just described, in women with ovulatory disorders, endometrial hemostatic dysfunction, and concomitant leiomyoma, the Systematic Review Group (SRG) of the Society of Gynecologic Surgeons performed a systematic review of treatments. The analysis was intended to compare hysterectomy with less invasive treatment modalities. The SRG reviewed randomized, controlled trials of AUB treatment that compared hysterectomy with:
- endometrial ablation by resectoscopic loop, rollerball, or thermal balloon
- the LNG-IUS
- medical therapy.
This comprehensive review of literature published between 1950 and January 14, 2011 led the SRG to create seven categories of clinical outcomes:
- bleeding control
- quality of life
- pain
- sexual health
- patient satisfaction
- need for additional treatment
- adverse events.
Of the initial 5,503 titles identified, only 18 articles, representing nine clinical trials, contained data of adequate quality to meet criteria for review. Seven of the trials compared hysterectomy with ablation, one compared hysterectomy with the LNG-IUS, and one compared hysterectomy with medical therapy. As FIGO has pointed out, the lack of homogeneity of terminology used to describe AUB and classification of its causes prevented clinically applicable comparative analyses of treatment outcomes.
Here are some of the SRG’s findings:
- Control of bleeding. Only data regarding amenorrhea were sufficient for comparative analysis. The SRG was able to conclude only that there was moderate strength of evidence supporting the statement that bleeding is better controlled following hysterectomy than following ablation.
- Quality of life. Overall, studies that evaluated quality of life showed improvement after ablation and hysterectomy. The strength of evidence demonstrating no difference between hysterectomy and ablation in postoperative quality of life was moderate.
- Pain, general health, vitality, and social function. Three studies found statistically significant differences in validated dimensions of the SF-36 questionnaire favoring hysterectomy for pain, general health, vitality, and social function. Two of these three studies evaluated minimally invasive hysterectomy by the laparoscopic supracervical or vaginal approach. The strength of evidence on pain beyond the postoperative time period was low and favored hysterectomy over ablation.
- Sexual health. The strength of evidence related to sexual health was low and revealed no differences between hysterectomy and ablation.
- Patient satisfaction. Overall, the quality of evidence was very low, showing no difference between hysterectomy and ablation.
- Need for additional treatment. The quality of evidence was moderate and favored hysterectomy over ablation.
- Adverse events. Evidence of moderate quality favored ablation and the LNG-IUS over hysterectomy, and low-quality evidence favored medical therapy over hysterectomy (TABLES 1, 2).
TABLE 1
What the data reveal about hysterectomy versus ablation
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | Ablation | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Low (F) | — |
| Sexual health | Low (S) | Low (S) |
| Patient satisfaction | Very low (S) | Very low (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
TABLE 2
What the data reveal about hysterectomy versus the levonorgestrel-releasing intrauterine system (LNG-IUS)
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | LNG-IUS | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Moderate (S) | Moderate (S) |
| Sexual health | Moderate (S) | Moderate (S) |
| Patient satisfaction | Moderate (S) | Moderate (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
The SRG concluded that there are tradeoffs between treatment effectiveness and the risk of serious adverse events between hysterectomy, ablation, and the LNG-IUS. It recommended that clinicians be educated about the relative advantages and disadvantages of each option so that they can discuss them with patients.
The SRG developed clinical practice guidelines for the treatment of ovulatory disorders and endometrial hemostatic dysfunction associated with AUB (see below).
Gynecologists should educate each patient about the efficacy and risks of options available for the management of AUB in the context of specific symptoms to facilitate an informed choice.
Wheeler TL II, Murphy M, Rogers RG, et al; Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines for abnormal uterine bleeding: hysterectomy versus alternative therapy. J Minim Invasive Gynecol. 2012;19(1):81–87.
The SRG used the results of the systematic review just summarized to formulate clinical guidelines for the treatment of AUB related to ovulatory disorders and endometrial hemostatic dysfunction. Recommendations were assigned a grade for their strength on the basis of the quality of supporting evidence, the size of the net medical benefit, and other considerations, including values and preferences applied in judgments. The strength of the clinical recommendation is either “strong” or “weak” and indicates the degree to which one can be confident that adherence to the recommendation will do more good than harm. All of the clinical recommendations described below received a grade of “weak.”
One primary suggestion from the study group is patient counseling that must first determine the type of AUB and the degree of burden or distress for the patient, as well as the presence of any additional cycle-related symptoms. Consideration should be given to variables that may modify the inherent risks or benefits of each intervention for the particular patient, as well as her values and preferences regarding treatment harms, benefits, and potential outcomes. Counseling should assess the patient’s need for contraception, desire for future childbearing, and proximity to menopause, as well as any cultural preferences for management.
Based on the clinical evidence related to hysterectomy versus endometrial ablation, the SRG made the following recommendations:
- If the patient desires amenorrhea and less pain and wants to avoid additional therapy, hysterectomy is preferred
- If the patient wants to avoid adverse events and seeks a shorter hospital stay, endometrial ablation is preferred
- If the patient’s main desire is for improvement in overall quality of life or sexual health, either intervention is appropriate, depending on patient preferences.
There were no data available in the systematic review concerning newer technologies for nonhysteroscopic endometrial ablation versus hysterectomy.
Based on the clinical evidence related to hysterectomy versus the LNG-IUS, the SRG made the following recommendations:
- If the patient desires amenorrhea or seeks to avoid additional therapy, hysterectomy is preferred
- If the patient’s main preference is to avoid adverse events, the LNG-IUS is preferred
- If her preference is for improved quality of life or sexual health, either treatment can be offered.
Based on the clinical evidence related to hysterectomy versus systemic medication, the SRG made the following recommendations:
- If the patient wants to become amenorrheic or hopes to avoid further intervention, hysterectomy is recommended
- If she wants to avoid adverse events, medications are recommended
- If her main preference is overall improvement in quality of life, less pain, or improvement in sexual health, either hysterectomy or medication is appropriate.
Note that no standard therapy was given; medical agents included combined oral contraceptive pills, cyclic or continuous progestin, conjugated estrogen with or without progestin, and prostaglandin synthetase inhibitors, usually with hormonal therapy. There are no randomized, controlled trials of other medications such as nonsteroidal anti-inflammatory drugs or tranexamic acid versus hysterectomy.
The SRG cited three main difficulties in the development of clinical guidelines:
- a lack of well-developed randomized, controlled trials of alternative management versus hysterectomy, as well as inconsistent measurement and reporting among the few trials that exist
- a lack of uniformity in AUB diagnoses among the randomized, controlled trials evaluated
- inconsistent use of terminology related to AUB within the trials.
All of these challenges were addressed by the FIGO nomenclature and AUB classification recommendations. Adherence to the FIGO guidelines for future clinical research would eliminate the difficulties faced by this study group and lead to higher-quality clinical evidence that could form the basis of solid clinical recommendations for the treatment of AUB related to ovulatory disorders or endometrial hemostatic dysfunction.
“Decision-making about treatments of AUB requires discussion so a patient can choose a therapy that best fits her disease, her values, and her preferences and optimizes her chance for treatment success while minimizing risks,” the SRG concluded.
ACKNOWLEDGMENT. Thank you to Dr. Malcolm Munro and Dr. Anita Lee Sloan for their thoughtful reviews of this manuscript.
We want to hear from you! Tell us what you think.
TECHNIQUE ARTICLES?
CLICK HERE to access Surgical Technique articles published recently in OBG Management.
LARCs: Why they should be first-line contraceptive options
for your patients
Elizabeth O. Schmidt, MD; Tessa Madden, MD, MPH; Jeffrey F. Piepert, MD, PhD
(November 2012)
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2012)
Your surgical toolbox should include topical hemostatic
agents—here is why
Lisa A. dos Santos, MD; Andrew W. Menzin, MD (Surgical Techniques, April 2012)
Minimally invasive surgery
Amy Garcia, MD (Update, April 2011)
The proliferation of terms to describe heavy menstrual bleeding sometimes seems never-ending. From “menometrorrhagia” to “uterine hemorrhage,” these terms pop up quickly and confuse discussion of one of the most widespread problems in gynecology.
Enter the International Federation of Gynecology and Obstetrics (FIGO), which decided to tackle the inconsistent terminology and lack of classification of causes of abnormal uterine bleeding (AUB) with an eye toward standardizing research, facilitating discussion, and informing management decisions.
In this article, I focus on three aspects of this effort:
- FIGO’s revamping of terminology and classification
- comparisons of outcomes of hysterectomy versus endometrial ablation and the levonorgestrel-releasing intrauterine system
- guidelines on management of AUB related to ovulatory disorders and endometrial hemostatic dysfunction.
FIGO revamps nomenclature for abnormal uterine bleeding
Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? Am J Obstet Gynecol. 2012;207(4):259–265.
As early as 2004, FIGO began a process to standardize the nomenclature for defining both normal and abnormal uterine bleeding in reproductive-aged women who are not pregnant.1 This process was a response to a lack of consistency and continuity in the design and interpretation of basic science and clinical investigation related to the problem of AUB. Inconsistent definitions of AUB, such as “menorrhagia,” “metrorrhagia,” and “dysfunctional uterine bleeding,” along with the absence of standard categorization of the causes of AUB, have led to confusion and difficulties in comparing clinical trials and in finding significant, relevant, and even meaningful correlations among investigations of AUB. Applying information from asynchronous and often incomplete investigations to evidence-based clinical practice then becomes a challenge for the gynecologist.
Munro and colleagues summarize the process by which FIGO developed both a nomenclature system and a classification system of the causes of AUB, which were formally adopted by FIGO in 2010 and endorsed in 2012 by the American College of Obstetricians and Gynecologists (ACOG).1-6 The arduous process led to:
- a refined definition of chronic AUB
- a new category called acute AUB
- a method for describing the clinical dimensions of menstruation and the menstrual cycle according to the following parameters:
- regularity of onset
- frequency of onset
- duration of menstrual flow
- heaviness, or volume, of menstrual flow.
Wherever appropriate, the definitions of normal for these parameters were based on statistics from large population studies that used medians and 5th and 95th percentiles.
The term “heavy menstrual bleeding” (HMB) is used to describe a woman’s perception of increased menstrual volume, regardless of regularity, frequency, or duration. AUB is the overarching term to describe any departure from normal menstruation, as defined by the parameters listed above. A group of misleading terms commonly used to describe AUB were eliminated from the FIGO nomenclature system, including “dysfunctional uterine bleeding,” “menorrhagia,” “hypermenorrhea,” “menometrorrhagia,” “polymenorrhagia,” and “metrorrhagia.”
The causes of AUB are classified in nine categories that are arranged according to the acronym PALM-COEIN:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
Leiomyoma are subclassified as submucous or other, with tertiary subcategorization for intramural, subserosal, and transmural lesions
In general, the components of the PALM group are discrete (structural) entities that are measurable visually via imaging or histopathology, or both, while the COEI (of the COEIN group) includes women for whom the AUB is unrelated to structural abnormalities.
The classification system provides the infrastructure for a thorough investigative process and a means to characterize AUB for an individual who may have one or more potential causes or contributors. Such a comprehensive assessment allows the basic scientist to identify pure populations for tissue and molecular studies, the clinical scientist to identify potential confounders when defining populations for clinical investigation, and the clinician, educator, and trainee to consider the multidimensional nature of AUB where asymptomatic “red herrings” may coexist with otherwise invisible disorders of menstrual function.
The FIGO Menstrual Disorders Working Group anticipates that widespread, international acceptance of the recommended terms, definitions, and classification for AUB will lead to improved and more meaningful communication in clinical trials and published research and will enhance communication between health-care providers and patients, leading to better management of AUB.
Use of the FIGO-recommended terms, definitions, and classification of AUB will lead to higher-quality clinical research and thorough clinical investigation into the causes of AUB, with improved management of patients.

How hysterectomy for AUB compares with less invasive treatment options
Matteson KA, Abed H, Wheeler TL II, et al; Society of Gynecologic Surgeons Systematic Review Group. A systematic review comparing hysterectomy with less invasive treatments for abnormal uterine bleeding. J Minim Invasive Gynecol. 2012;19(1):13–28.
To create reliable treatment recommendations for AUB, as defined by the FIGO classification system just described, in women with ovulatory disorders, endometrial hemostatic dysfunction, and concomitant leiomyoma, the Systematic Review Group (SRG) of the Society of Gynecologic Surgeons performed a systematic review of treatments. The analysis was intended to compare hysterectomy with less invasive treatment modalities. The SRG reviewed randomized, controlled trials of AUB treatment that compared hysterectomy with:
- endometrial ablation by resectoscopic loop, rollerball, or thermal balloon
- the LNG-IUS
- medical therapy.
This comprehensive review of literature published between 1950 and January 14, 2011 led the SRG to create seven categories of clinical outcomes:
- bleeding control
- quality of life
- pain
- sexual health
- patient satisfaction
- need for additional treatment
- adverse events.
Of the initial 5,503 titles identified, only 18 articles, representing nine clinical trials, contained data of adequate quality to meet criteria for review. Seven of the trials compared hysterectomy with ablation, one compared hysterectomy with the LNG-IUS, and one compared hysterectomy with medical therapy. As FIGO has pointed out, the lack of homogeneity of terminology used to describe AUB and classification of its causes prevented clinically applicable comparative analyses of treatment outcomes.
Here are some of the SRG’s findings:
- Control of bleeding. Only data regarding amenorrhea were sufficient for comparative analysis. The SRG was able to conclude only that there was moderate strength of evidence supporting the statement that bleeding is better controlled following hysterectomy than following ablation.
- Quality of life. Overall, studies that evaluated quality of life showed improvement after ablation and hysterectomy. The strength of evidence demonstrating no difference between hysterectomy and ablation in postoperative quality of life was moderate.
- Pain, general health, vitality, and social function. Three studies found statistically significant differences in validated dimensions of the SF-36 questionnaire favoring hysterectomy for pain, general health, vitality, and social function. Two of these three studies evaluated minimally invasive hysterectomy by the laparoscopic supracervical or vaginal approach. The strength of evidence on pain beyond the postoperative time period was low and favored hysterectomy over ablation.
- Sexual health. The strength of evidence related to sexual health was low and revealed no differences between hysterectomy and ablation.
- Patient satisfaction. Overall, the quality of evidence was very low, showing no difference between hysterectomy and ablation.
- Need for additional treatment. The quality of evidence was moderate and favored hysterectomy over ablation.
- Adverse events. Evidence of moderate quality favored ablation and the LNG-IUS over hysterectomy, and low-quality evidence favored medical therapy over hysterectomy (TABLES 1, 2).
TABLE 1
What the data reveal about hysterectomy versus ablation
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | Ablation | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Low (F) | — |
| Sexual health | Low (S) | Low (S) |
| Patient satisfaction | Very low (S) | Very low (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
TABLE 2
What the data reveal about hysterectomy versus the levonorgestrel-releasing intrauterine system (LNG-IUS)
| Parameter | Strength of evidence (comparison) | |
|---|---|---|
| Hysterectomy | LNG-IUS | |
| Bleeding control | Moderate (F) | — |
| Quality of life | Moderate (S) | Moderate (S) |
| Lower pain | Moderate (S) | Moderate (S) |
| Sexual health | Moderate (S) | Moderate (S) |
| Patient satisfaction | Moderate (S) | Moderate (S) |
| Need for additional treatment | Moderate (F) | — |
| Adverse events | — | Moderate (F) |
| F=Evidence favors comparator; S=no difference between comparators | ||
The SRG concluded that there are tradeoffs between treatment effectiveness and the risk of serious adverse events between hysterectomy, ablation, and the LNG-IUS. It recommended that clinicians be educated about the relative advantages and disadvantages of each option so that they can discuss them with patients.
The SRG developed clinical practice guidelines for the treatment of ovulatory disorders and endometrial hemostatic dysfunction associated with AUB (see below).
Gynecologists should educate each patient about the efficacy and risks of options available for the management of AUB in the context of specific symptoms to facilitate an informed choice.
Wheeler TL II, Murphy M, Rogers RG, et al; Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines for abnormal uterine bleeding: hysterectomy versus alternative therapy. J Minim Invasive Gynecol. 2012;19(1):81–87.
The SRG used the results of the systematic review just summarized to formulate clinical guidelines for the treatment of AUB related to ovulatory disorders and endometrial hemostatic dysfunction. Recommendations were assigned a grade for their strength on the basis of the quality of supporting evidence, the size of the net medical benefit, and other considerations, including values and preferences applied in judgments. The strength of the clinical recommendation is either “strong” or “weak” and indicates the degree to which one can be confident that adherence to the recommendation will do more good than harm. All of the clinical recommendations described below received a grade of “weak.”
One primary suggestion from the study group is patient counseling that must first determine the type of AUB and the degree of burden or distress for the patient, as well as the presence of any additional cycle-related symptoms. Consideration should be given to variables that may modify the inherent risks or benefits of each intervention for the particular patient, as well as her values and preferences regarding treatment harms, benefits, and potential outcomes. Counseling should assess the patient’s need for contraception, desire for future childbearing, and proximity to menopause, as well as any cultural preferences for management.
Based on the clinical evidence related to hysterectomy versus endometrial ablation, the SRG made the following recommendations:
- If the patient desires amenorrhea and less pain and wants to avoid additional therapy, hysterectomy is preferred
- If the patient wants to avoid adverse events and seeks a shorter hospital stay, endometrial ablation is preferred
- If the patient’s main desire is for improvement in overall quality of life or sexual health, either intervention is appropriate, depending on patient preferences.
There were no data available in the systematic review concerning newer technologies for nonhysteroscopic endometrial ablation versus hysterectomy.
Based on the clinical evidence related to hysterectomy versus the LNG-IUS, the SRG made the following recommendations:
- If the patient desires amenorrhea or seeks to avoid additional therapy, hysterectomy is preferred
- If the patient’s main preference is to avoid adverse events, the LNG-IUS is preferred
- If her preference is for improved quality of life or sexual health, either treatment can be offered.
Based on the clinical evidence related to hysterectomy versus systemic medication, the SRG made the following recommendations:
- If the patient wants to become amenorrheic or hopes to avoid further intervention, hysterectomy is recommended
- If she wants to avoid adverse events, medications are recommended
- If her main preference is overall improvement in quality of life, less pain, or improvement in sexual health, either hysterectomy or medication is appropriate.
Note that no standard therapy was given; medical agents included combined oral contraceptive pills, cyclic or continuous progestin, conjugated estrogen with or without progestin, and prostaglandin synthetase inhibitors, usually with hormonal therapy. There are no randomized, controlled trials of other medications such as nonsteroidal anti-inflammatory drugs or tranexamic acid versus hysterectomy.
The SRG cited three main difficulties in the development of clinical guidelines:
- a lack of well-developed randomized, controlled trials of alternative management versus hysterectomy, as well as inconsistent measurement and reporting among the few trials that exist
- a lack of uniformity in AUB diagnoses among the randomized, controlled trials evaluated
- inconsistent use of terminology related to AUB within the trials.
All of these challenges were addressed by the FIGO nomenclature and AUB classification recommendations. Adherence to the FIGO guidelines for future clinical research would eliminate the difficulties faced by this study group and lead to higher-quality clinical evidence that could form the basis of solid clinical recommendations for the treatment of AUB related to ovulatory disorders or endometrial hemostatic dysfunction.
“Decision-making about treatments of AUB requires discussion so a patient can choose a therapy that best fits her disease, her values, and her preferences and optimizes her chance for treatment success while minimizing risks,” the SRG concluded.
ACKNOWLEDGMENT. Thank you to Dr. Malcolm Munro and Dr. Anita Lee Sloan for their thoughtful reviews of this manuscript.
We want to hear from you! Tell us what you think.
TECHNIQUE ARTICLES?
CLICK HERE to access Surgical Technique articles published recently in OBG Management.
1. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3-13.
2. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. The FIGO classification of causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204-2208.
3. Critchley HO, Munro MG, Broder M, Fraser IS. A five-year international review process concerning terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):377-382.
4. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-390.
5. Munro MG, Critchley HO, Fraser IS. The flexible FIGO classification concept for underlying causes of abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):391-399.
6. American College of Obstetricians and Gynecologists. Practice bulletin #128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):207-211.
1. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3-13.
2. Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. The FIGO classification of causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204-2208.
3. Critchley HO, Munro MG, Broder M, Fraser IS. A five-year international review process concerning terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):377-382.
4. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383-390.
5. Munro MG, Critchley HO, Fraser IS. The flexible FIGO classification concept for underlying causes of abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):391-399.
6. American College of Obstetricians and Gynecologists. Practice bulletin #128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):207-211.
UPDATE: MINIMALLY INVASIVE SURGERY
Dr. Garcia serves as a consultant to IOGYN, Minerva Surgical, Conceptus, Ethicon EndoSurgery, and Ethicon Women’s Health & Urology. She is a speaker for Conceptus.
Two-thirds of the almost one-half million hysterectomies performed annually in the United States for benign conditions take the abdominal route—even though less invasive transvaginal and laparoscopic approaches are available. Compared with abdominal hysterectomy, vaginal and laparoscopic hysterectomies are, on the whole, associated with less morbidity, a shorter hospital stay, and more rapid return to physical activity.
Over the past year, our understanding of the comparative advantages and risks of the various approaches to hysterectomy has been deepened by new research and by guidance from AAGL. Here is what we’ve learned, and here is how our surgical practices ought to be evolving for the long-term good of our patients.
Hysterectomy should be performed only rarely abdominally
AAGL Position Statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3. [To link to the AAGL Position Statement, click here.]
In 2011, AAGL, which has been an international leader in promoting minimally invasive gynecologic surgery for longer than 40 years, issued a position statement on hysterectomy for the treatment of benign disease. AAGL’s position is a clear assertion that, when vaginal hysterectomy is not possible, laparoscopic hysterectomy should be performed—thus leaving few clinical indications for an abdominal hysterectomy.
Historically established contraindications to vaginal or laparoscopic hysterectomy—prior cesarean delivery, need for oophorectomy, an enlarged uterus—have been invalidated by recent studies. In competent hands, ovarian removal can be accomplished in 65% to 98.5% of vaginal hysterectomies.1 Vaginal morcellation techniques can facilitate removal of a large uterus vaginally and mechanical tissue morcellators enable laparoscopic removal.
In 2011, ACOG reaffirmed its 1999 Committee Opinion on Gynecologic Practice,1 which recommends that the vaginal approach for hysterectomy be the preferred route. ACOG asserts that, when vaginal hysterectomy is impossible, the laparoscopic and abdominal routes are alternatives.
How do these positions differ?
The difference in the AAGL Position Statement and the ACOG Committee Opinion lies in the surgeon’s ability to perform laparoscopic or vaginal hysterectomy. Although it might seem admirable for a surgeon to choose abdominal hysterectomy because he, or she, lacks the training and skills to perform the procedure laparoscopically or vaginally, AAGL does not hold this position. AAGL has established the expectation that, if a surgeon is unable to perform a hysterectomy safely vaginally or laparoscopically, he should refer the patient to a gynecologic surgeon who can.
Furthermore, AAGL recommends that abdominal hysterectomy be reserved for four broad situations, when:
- a patient has a medical condition, such as cardiopulmonary disease, in which the risk of general anesthesia or increased intraperitoneal pressure that is associated with laparoscopy is deemed unacceptable
- morcellation is known, or likely, to be required for vaginal or laparoscopic hysterectomy and uterine malignancy is either known or suspected
- hysterectomy is indicated but there is no access to surgeons or facilities required for vaginal or laparoscopic hysterectomy and referral is not feasible
- anatomy is so distorted by uterine disease or adhesions that the vaginal and laparoscopic approaches are deemed unsafe or unreasonable by a recognized expert in vaginal or laparoscopic hysterectomy techniques.
When hysterectomy is necessary, therefore, the demonstrated safety, efficacy, and cost-effectiveness of vaginal and laparoscopic approaches to surgical removal of the uterus mandate that these procedures be 1) the ones of choice and 2) presented as options to all appropriate candidates.
Whenever feasible for benign disease, perform hysterectomy vaginally or laparoscopically. Make the effort to facilitate these approaches based on the underlying principles of 1) informed patient choice and 2) preferential provision of minimally invasive options.
If you have not had the requisite training or learned the skills required to perform vaginal or laparoscopic hysterectomy, you should enlist the assistance of colleagues who do or refer your patients to those colleagues for surgical care. You should also, for the long term, seek to acquire those skills through formal training.
Nieboer T, Hendriks J, Bongers MY, Vierhout ME, Kluivers KB. Quality of life after laparoscopic and abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;119(1):85–91.
Nieboer and colleagues have presented their long-term data from a prospective, randomized evaluation of quality of life (QOL) after abdominal hysterectomy compared with QOL after laparoscopic hysterectomy. Other researchers have compared hysterectomy approaches, but most of those studies focused on such outcome measures as operation time, surgical intraoperative and postoperative complications, hospital stay, and rate of recurrence of the condition that prompted the surgery. This is the first study to address QOL parameters that are more patient-centered —using a validated questionnaire and having a median follow-up of 4.7 years (range, 188 to 303 weeks).
In 2007, investigators published the findings of a randomized comparison of QOL measures after total laparoscopic hysterectomy and total abdominal hysterectomy. Their assessment in that study utilized eight QOL measures from the RAND-36 Measure of Health-Related Quality of Life (the Dutch version of the validated QOL questionnaire, the SF-36 Health Survey)2 (TABLE).
8 key RAND-36 measures of quality of life in women who have had a hysterectomy
|
The current (Nieboer and co-workers) study evaluated 59 randomized patients from the 2007 work: 27 to laparoscopic hysterectomy and 32 to abdominal hysterectomy. The overall response rate after 4 years was 83% (N=49).
The QOL questionnaire addressed eight RAND-36 (SF-36) measures, with each measure having a possible score of 0 to 100 (maximum possible total score, 800); the higher the number, the better the QOL. The researchers considered a difference of 15 points between the two surgical approaches on any single parameter to be statistically significant.
Findings. The mean total RAND-36 (SF-36) score was 50.4 points (95% confidence interval, 1.0–99.7) higher in the laparoscopic hysterectomy group at each point of measurement in the weeks postoperatively, up to 4 years of follow-up. Higher scores at 4 years were also seen in the laparoscopy group for vitality, physical functioning, and social functioning.
From these findings, the authors surmise that QOL remains better 4 years after laparoscopic hysterectomy than it does after abdominal hysterectomy.
Why these findings? The Nieboer team offers several explanations for ongoing improvement in QOL scores among laparoscopic hysterectomy patients.
First, it is conceivable that laparoscopic patients scored higher on the Body Image Scale, benefiting from the knowledge that they underwent what, even in layman’s terms, would be called the “minimally invasive approach.”
Second, chronic abdominal or pelvic pain could affect QOL scores. It has been shown that, for other laparotomy procedures, the incidence of postop chronic pain ranges from 3% to 56%. Risk factors for postop chronic pain are female gender, younger age, and surgery for benign disease—similar to those that characterized the patient population in this study.
Some weaknesses. The authors acknowledge that the study has shortcomings, including 1) a small sample and 2) their inability to discriminate QOL that reflects subjects’ surgical outcome from QOL related to typical life events—the death of a spouse, for example.
Nieboer and colleagues conclude by saying that, given the apparent improved QOL after laparoscopic hysterectomy compared with abdominal hysterectomy, all patients in whom vaginal hysterectomy is not feasible should be able to opt for laparoscopic hysterectomy.
Vaginal and laparoscopic approaches to hysterectomy have significant short-term advantages over abdominal hysterectomy by traditionally compared measures of surgical outcome. Taking the less-invasive approach allows you to offer greater long-lasting improvement in your surgical patients’ quality of life.
Einarsson J, Suzuki Y, Vellinga T, et al. Prospective evaluation of quality of life in total versus supracervical laparoscopic hysterectomy. J Minim Invasive Gynecol. 2011;18(5):617–621.
Einarsson and colleagues sought to prospectively evaluate a cohort of patients undergoing total laparoscopic hysterectomy (TLH) or laparoscopic supracervical hysterectomy (LSH) for 1) time to recovery and 2) short-term QOL after surgery. In all, 122 women underwent surgery (TLH: N=71; LSH: N=51) for benign indications. A QOL questionnaire (again, the SF-36) was administered immediately preoperatively, as a baseline, and at 3 to 4 weeks postoperatively.
Preoperatively, patients were presented with the two surgical options, without being influenced with information about any benefit to removing or retaining the cervix at laparoscopic hysterectomy. Patients then chose which surgery they wanted, and were neither randomized nor blinded to the procedure that was performed.
Findings. The data show greater patient self-selection and more patients with endometriosis in the TLH group; other preoperative baseline characteristics were similar across groups. More operative and postoperative complications were seen in the TLH group (vaginal cuff bleeding requiring return to the operating room, 2 patients; cuff cellulitis, 1; intraoperative vaginal laceration, 1; urinary tract infection, 1), although the difference did not reach statistical significance. There were no significant differences group to group in postop nausea, pain, narcotic use, or return to daily activities.
Regarding the eight QOL parameters, however, a statistically significant difference was observed in six of them to favor laparoscopic supracervical hysterectomy: physical functioning, physical role, bodily pain, vitality, social functioning, and physical component summary.
Study has shortcomings. The authors address two limitations of their study: namely, that the participants were neither blinded nor randomized. They acknowledge that these limitations might have biased QOL measurements in a way that showed improved QOL among the supracervical hysterectomy group. They raise the possibility that not being blinded to whether the cervix was removed may have affected subjects’ bodily perception. (Patients also returned to their daily activities 5 days earlier in the supracervical group, but this finding was found to be statistically insignificant.)
It is possible, however, to look at these limitations not as shortcomings of the study but as an important insight into the validity of patient choice and the benefits of patient education and autonomy in decision-making. Perhaps patients who have chosen to keep their cervix have a discernable advantage in regard to their perception of a higher QOL after hysterectomy.
An additional critique. Although the authors addressed a return to several daily activities that are outside the SF-36 questionnaire (e.g., a return to household chores, driving, work, exercise, and normal activities) they did not address sexual activity.
It has been the generally accepted practice to instruct patients not to place anything in the vagina, and to avoid vaginal intercourse, for at least 6 weeks after the cervix has been removed—regardless of the route of removal. After supracervical hysterectomy, however, patients can resume intercourse as early as 2 weeks. I think that it would be realistic for the authors to have stated that a quicker return to sexual activity after surgery might improve QOL scores for women, but they did not specifically address this domain.
When you’ve determined that hysterectomy is indicated for treatment of a patient’s benign disease and plan a laparoscopic approach, consider that education and autonomy of choice about whether to keep the cervix might improve quality of life postoperatively.
Acknowledgment
Andrew I. Brill, MD, and William H. Parker, MD, reviewed the manuscript of this article before it was submitted for publication.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease (reaffirmed 2011). Obstet Gynecol. 2009;114(5):1156-1158.
2. Kluivers K, Hendriks J, Mol BW, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: A randomized, controlled trial. J Minim Invasive Gynecol. 2007;14(2):145-152.
Dr. Garcia serves as a consultant to IOGYN, Minerva Surgical, Conceptus, Ethicon EndoSurgery, and Ethicon Women’s Health & Urology. She is a speaker for Conceptus.
Two-thirds of the almost one-half million hysterectomies performed annually in the United States for benign conditions take the abdominal route—even though less invasive transvaginal and laparoscopic approaches are available. Compared with abdominal hysterectomy, vaginal and laparoscopic hysterectomies are, on the whole, associated with less morbidity, a shorter hospital stay, and more rapid return to physical activity.
Over the past year, our understanding of the comparative advantages and risks of the various approaches to hysterectomy has been deepened by new research and by guidance from AAGL. Here is what we’ve learned, and here is how our surgical practices ought to be evolving for the long-term good of our patients.
Hysterectomy should be performed only rarely abdominally
AAGL Position Statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3. [To link to the AAGL Position Statement, click here.]
In 2011, AAGL, which has been an international leader in promoting minimally invasive gynecologic surgery for longer than 40 years, issued a position statement on hysterectomy for the treatment of benign disease. AAGL’s position is a clear assertion that, when vaginal hysterectomy is not possible, laparoscopic hysterectomy should be performed—thus leaving few clinical indications for an abdominal hysterectomy.
Historically established contraindications to vaginal or laparoscopic hysterectomy—prior cesarean delivery, need for oophorectomy, an enlarged uterus—have been invalidated by recent studies. In competent hands, ovarian removal can be accomplished in 65% to 98.5% of vaginal hysterectomies.1 Vaginal morcellation techniques can facilitate removal of a large uterus vaginally and mechanical tissue morcellators enable laparoscopic removal.
In 2011, ACOG reaffirmed its 1999 Committee Opinion on Gynecologic Practice,1 which recommends that the vaginal approach for hysterectomy be the preferred route. ACOG asserts that, when vaginal hysterectomy is impossible, the laparoscopic and abdominal routes are alternatives.
How do these positions differ?
The difference in the AAGL Position Statement and the ACOG Committee Opinion lies in the surgeon’s ability to perform laparoscopic or vaginal hysterectomy. Although it might seem admirable for a surgeon to choose abdominal hysterectomy because he, or she, lacks the training and skills to perform the procedure laparoscopically or vaginally, AAGL does not hold this position. AAGL has established the expectation that, if a surgeon is unable to perform a hysterectomy safely vaginally or laparoscopically, he should refer the patient to a gynecologic surgeon who can.
Furthermore, AAGL recommends that abdominal hysterectomy be reserved for four broad situations, when:
- a patient has a medical condition, such as cardiopulmonary disease, in which the risk of general anesthesia or increased intraperitoneal pressure that is associated with laparoscopy is deemed unacceptable
- morcellation is known, or likely, to be required for vaginal or laparoscopic hysterectomy and uterine malignancy is either known or suspected
- hysterectomy is indicated but there is no access to surgeons or facilities required for vaginal or laparoscopic hysterectomy and referral is not feasible
- anatomy is so distorted by uterine disease or adhesions that the vaginal and laparoscopic approaches are deemed unsafe or unreasonable by a recognized expert in vaginal or laparoscopic hysterectomy techniques.
When hysterectomy is necessary, therefore, the demonstrated safety, efficacy, and cost-effectiveness of vaginal and laparoscopic approaches to surgical removal of the uterus mandate that these procedures be 1) the ones of choice and 2) presented as options to all appropriate candidates.
Whenever feasible for benign disease, perform hysterectomy vaginally or laparoscopically. Make the effort to facilitate these approaches based on the underlying principles of 1) informed patient choice and 2) preferential provision of minimally invasive options.
If you have not had the requisite training or learned the skills required to perform vaginal or laparoscopic hysterectomy, you should enlist the assistance of colleagues who do or refer your patients to those colleagues for surgical care. You should also, for the long term, seek to acquire those skills through formal training.
Nieboer T, Hendriks J, Bongers MY, Vierhout ME, Kluivers KB. Quality of life after laparoscopic and abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;119(1):85–91.
Nieboer and colleagues have presented their long-term data from a prospective, randomized evaluation of quality of life (QOL) after abdominal hysterectomy compared with QOL after laparoscopic hysterectomy. Other researchers have compared hysterectomy approaches, but most of those studies focused on such outcome measures as operation time, surgical intraoperative and postoperative complications, hospital stay, and rate of recurrence of the condition that prompted the surgery. This is the first study to address QOL parameters that are more patient-centered —using a validated questionnaire and having a median follow-up of 4.7 years (range, 188 to 303 weeks).
In 2007, investigators published the findings of a randomized comparison of QOL measures after total laparoscopic hysterectomy and total abdominal hysterectomy. Their assessment in that study utilized eight QOL measures from the RAND-36 Measure of Health-Related Quality of Life (the Dutch version of the validated QOL questionnaire, the SF-36 Health Survey)2 (TABLE).
8 key RAND-36 measures of quality of life in women who have had a hysterectomy
|
The current (Nieboer and co-workers) study evaluated 59 randomized patients from the 2007 work: 27 to laparoscopic hysterectomy and 32 to abdominal hysterectomy. The overall response rate after 4 years was 83% (N=49).
The QOL questionnaire addressed eight RAND-36 (SF-36) measures, with each measure having a possible score of 0 to 100 (maximum possible total score, 800); the higher the number, the better the QOL. The researchers considered a difference of 15 points between the two surgical approaches on any single parameter to be statistically significant.
Findings. The mean total RAND-36 (SF-36) score was 50.4 points (95% confidence interval, 1.0–99.7) higher in the laparoscopic hysterectomy group at each point of measurement in the weeks postoperatively, up to 4 years of follow-up. Higher scores at 4 years were also seen in the laparoscopy group for vitality, physical functioning, and social functioning.
From these findings, the authors surmise that QOL remains better 4 years after laparoscopic hysterectomy than it does after abdominal hysterectomy.
Why these findings? The Nieboer team offers several explanations for ongoing improvement in QOL scores among laparoscopic hysterectomy patients.
First, it is conceivable that laparoscopic patients scored higher on the Body Image Scale, benefiting from the knowledge that they underwent what, even in layman’s terms, would be called the “minimally invasive approach.”
Second, chronic abdominal or pelvic pain could affect QOL scores. It has been shown that, for other laparotomy procedures, the incidence of postop chronic pain ranges from 3% to 56%. Risk factors for postop chronic pain are female gender, younger age, and surgery for benign disease—similar to those that characterized the patient population in this study.
Some weaknesses. The authors acknowledge that the study has shortcomings, including 1) a small sample and 2) their inability to discriminate QOL that reflects subjects’ surgical outcome from QOL related to typical life events—the death of a spouse, for example.
Nieboer and colleagues conclude by saying that, given the apparent improved QOL after laparoscopic hysterectomy compared with abdominal hysterectomy, all patients in whom vaginal hysterectomy is not feasible should be able to opt for laparoscopic hysterectomy.
Vaginal and laparoscopic approaches to hysterectomy have significant short-term advantages over abdominal hysterectomy by traditionally compared measures of surgical outcome. Taking the less-invasive approach allows you to offer greater long-lasting improvement in your surgical patients’ quality of life.
Einarsson J, Suzuki Y, Vellinga T, et al. Prospective evaluation of quality of life in total versus supracervical laparoscopic hysterectomy. J Minim Invasive Gynecol. 2011;18(5):617–621.
Einarsson and colleagues sought to prospectively evaluate a cohort of patients undergoing total laparoscopic hysterectomy (TLH) or laparoscopic supracervical hysterectomy (LSH) for 1) time to recovery and 2) short-term QOL after surgery. In all, 122 women underwent surgery (TLH: N=71; LSH: N=51) for benign indications. A QOL questionnaire (again, the SF-36) was administered immediately preoperatively, as a baseline, and at 3 to 4 weeks postoperatively.
Preoperatively, patients were presented with the two surgical options, without being influenced with information about any benefit to removing or retaining the cervix at laparoscopic hysterectomy. Patients then chose which surgery they wanted, and were neither randomized nor blinded to the procedure that was performed.
Findings. The data show greater patient self-selection and more patients with endometriosis in the TLH group; other preoperative baseline characteristics were similar across groups. More operative and postoperative complications were seen in the TLH group (vaginal cuff bleeding requiring return to the operating room, 2 patients; cuff cellulitis, 1; intraoperative vaginal laceration, 1; urinary tract infection, 1), although the difference did not reach statistical significance. There were no significant differences group to group in postop nausea, pain, narcotic use, or return to daily activities.
Regarding the eight QOL parameters, however, a statistically significant difference was observed in six of them to favor laparoscopic supracervical hysterectomy: physical functioning, physical role, bodily pain, vitality, social functioning, and physical component summary.
Study has shortcomings. The authors address two limitations of their study: namely, that the participants were neither blinded nor randomized. They acknowledge that these limitations might have biased QOL measurements in a way that showed improved QOL among the supracervical hysterectomy group. They raise the possibility that not being blinded to whether the cervix was removed may have affected subjects’ bodily perception. (Patients also returned to their daily activities 5 days earlier in the supracervical group, but this finding was found to be statistically insignificant.)
It is possible, however, to look at these limitations not as shortcomings of the study but as an important insight into the validity of patient choice and the benefits of patient education and autonomy in decision-making. Perhaps patients who have chosen to keep their cervix have a discernable advantage in regard to their perception of a higher QOL after hysterectomy.
An additional critique. Although the authors addressed a return to several daily activities that are outside the SF-36 questionnaire (e.g., a return to household chores, driving, work, exercise, and normal activities) they did not address sexual activity.
It has been the generally accepted practice to instruct patients not to place anything in the vagina, and to avoid vaginal intercourse, for at least 6 weeks after the cervix has been removed—regardless of the route of removal. After supracervical hysterectomy, however, patients can resume intercourse as early as 2 weeks. I think that it would be realistic for the authors to have stated that a quicker return to sexual activity after surgery might improve QOL scores for women, but they did not specifically address this domain.
When you’ve determined that hysterectomy is indicated for treatment of a patient’s benign disease and plan a laparoscopic approach, consider that education and autonomy of choice about whether to keep the cervix might improve quality of life postoperatively.
Acknowledgment
Andrew I. Brill, MD, and William H. Parker, MD, reviewed the manuscript of this article before it was submitted for publication.
We want to hear from you! Tell us what you think.
Dr. Garcia serves as a consultant to IOGYN, Minerva Surgical, Conceptus, Ethicon EndoSurgery, and Ethicon Women’s Health & Urology. She is a speaker for Conceptus.
Two-thirds of the almost one-half million hysterectomies performed annually in the United States for benign conditions take the abdominal route—even though less invasive transvaginal and laparoscopic approaches are available. Compared with abdominal hysterectomy, vaginal and laparoscopic hysterectomies are, on the whole, associated with less morbidity, a shorter hospital stay, and more rapid return to physical activity.
Over the past year, our understanding of the comparative advantages and risks of the various approaches to hysterectomy has been deepened by new research and by guidance from AAGL. Here is what we’ve learned, and here is how our surgical practices ought to be evolving for the long-term good of our patients.
Hysterectomy should be performed only rarely abdominally
AAGL Position Statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3. [To link to the AAGL Position Statement, click here.]
In 2011, AAGL, which has been an international leader in promoting minimally invasive gynecologic surgery for longer than 40 years, issued a position statement on hysterectomy for the treatment of benign disease. AAGL’s position is a clear assertion that, when vaginal hysterectomy is not possible, laparoscopic hysterectomy should be performed—thus leaving few clinical indications for an abdominal hysterectomy.
Historically established contraindications to vaginal or laparoscopic hysterectomy—prior cesarean delivery, need for oophorectomy, an enlarged uterus—have been invalidated by recent studies. In competent hands, ovarian removal can be accomplished in 65% to 98.5% of vaginal hysterectomies.1 Vaginal morcellation techniques can facilitate removal of a large uterus vaginally and mechanical tissue morcellators enable laparoscopic removal.
In 2011, ACOG reaffirmed its 1999 Committee Opinion on Gynecologic Practice,1 which recommends that the vaginal approach for hysterectomy be the preferred route. ACOG asserts that, when vaginal hysterectomy is impossible, the laparoscopic and abdominal routes are alternatives.
How do these positions differ?
The difference in the AAGL Position Statement and the ACOG Committee Opinion lies in the surgeon’s ability to perform laparoscopic or vaginal hysterectomy. Although it might seem admirable for a surgeon to choose abdominal hysterectomy because he, or she, lacks the training and skills to perform the procedure laparoscopically or vaginally, AAGL does not hold this position. AAGL has established the expectation that, if a surgeon is unable to perform a hysterectomy safely vaginally or laparoscopically, he should refer the patient to a gynecologic surgeon who can.
Furthermore, AAGL recommends that abdominal hysterectomy be reserved for four broad situations, when:
- a patient has a medical condition, such as cardiopulmonary disease, in which the risk of general anesthesia or increased intraperitoneal pressure that is associated with laparoscopy is deemed unacceptable
- morcellation is known, or likely, to be required for vaginal or laparoscopic hysterectomy and uterine malignancy is either known or suspected
- hysterectomy is indicated but there is no access to surgeons or facilities required for vaginal or laparoscopic hysterectomy and referral is not feasible
- anatomy is so distorted by uterine disease or adhesions that the vaginal and laparoscopic approaches are deemed unsafe or unreasonable by a recognized expert in vaginal or laparoscopic hysterectomy techniques.
When hysterectomy is necessary, therefore, the demonstrated safety, efficacy, and cost-effectiveness of vaginal and laparoscopic approaches to surgical removal of the uterus mandate that these procedures be 1) the ones of choice and 2) presented as options to all appropriate candidates.
Whenever feasible for benign disease, perform hysterectomy vaginally or laparoscopically. Make the effort to facilitate these approaches based on the underlying principles of 1) informed patient choice and 2) preferential provision of minimally invasive options.
If you have not had the requisite training or learned the skills required to perform vaginal or laparoscopic hysterectomy, you should enlist the assistance of colleagues who do or refer your patients to those colleagues for surgical care. You should also, for the long term, seek to acquire those skills through formal training.
Nieboer T, Hendriks J, Bongers MY, Vierhout ME, Kluivers KB. Quality of life after laparoscopic and abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;119(1):85–91.
Nieboer and colleagues have presented their long-term data from a prospective, randomized evaluation of quality of life (QOL) after abdominal hysterectomy compared with QOL after laparoscopic hysterectomy. Other researchers have compared hysterectomy approaches, but most of those studies focused on such outcome measures as operation time, surgical intraoperative and postoperative complications, hospital stay, and rate of recurrence of the condition that prompted the surgery. This is the first study to address QOL parameters that are more patient-centered —using a validated questionnaire and having a median follow-up of 4.7 years (range, 188 to 303 weeks).
In 2007, investigators published the findings of a randomized comparison of QOL measures after total laparoscopic hysterectomy and total abdominal hysterectomy. Their assessment in that study utilized eight QOL measures from the RAND-36 Measure of Health-Related Quality of Life (the Dutch version of the validated QOL questionnaire, the SF-36 Health Survey)2 (TABLE).
8 key RAND-36 measures of quality of life in women who have had a hysterectomy
|
The current (Nieboer and co-workers) study evaluated 59 randomized patients from the 2007 work: 27 to laparoscopic hysterectomy and 32 to abdominal hysterectomy. The overall response rate after 4 years was 83% (N=49).
The QOL questionnaire addressed eight RAND-36 (SF-36) measures, with each measure having a possible score of 0 to 100 (maximum possible total score, 800); the higher the number, the better the QOL. The researchers considered a difference of 15 points between the two surgical approaches on any single parameter to be statistically significant.
Findings. The mean total RAND-36 (SF-36) score was 50.4 points (95% confidence interval, 1.0–99.7) higher in the laparoscopic hysterectomy group at each point of measurement in the weeks postoperatively, up to 4 years of follow-up. Higher scores at 4 years were also seen in the laparoscopy group for vitality, physical functioning, and social functioning.
From these findings, the authors surmise that QOL remains better 4 years after laparoscopic hysterectomy than it does after abdominal hysterectomy.
Why these findings? The Nieboer team offers several explanations for ongoing improvement in QOL scores among laparoscopic hysterectomy patients.
First, it is conceivable that laparoscopic patients scored higher on the Body Image Scale, benefiting from the knowledge that they underwent what, even in layman’s terms, would be called the “minimally invasive approach.”
Second, chronic abdominal or pelvic pain could affect QOL scores. It has been shown that, for other laparotomy procedures, the incidence of postop chronic pain ranges from 3% to 56%. Risk factors for postop chronic pain are female gender, younger age, and surgery for benign disease—similar to those that characterized the patient population in this study.
Some weaknesses. The authors acknowledge that the study has shortcomings, including 1) a small sample and 2) their inability to discriminate QOL that reflects subjects’ surgical outcome from QOL related to typical life events—the death of a spouse, for example.
Nieboer and colleagues conclude by saying that, given the apparent improved QOL after laparoscopic hysterectomy compared with abdominal hysterectomy, all patients in whom vaginal hysterectomy is not feasible should be able to opt for laparoscopic hysterectomy.
Vaginal and laparoscopic approaches to hysterectomy have significant short-term advantages over abdominal hysterectomy by traditionally compared measures of surgical outcome. Taking the less-invasive approach allows you to offer greater long-lasting improvement in your surgical patients’ quality of life.
Einarsson J, Suzuki Y, Vellinga T, et al. Prospective evaluation of quality of life in total versus supracervical laparoscopic hysterectomy. J Minim Invasive Gynecol. 2011;18(5):617–621.
Einarsson and colleagues sought to prospectively evaluate a cohort of patients undergoing total laparoscopic hysterectomy (TLH) or laparoscopic supracervical hysterectomy (LSH) for 1) time to recovery and 2) short-term QOL after surgery. In all, 122 women underwent surgery (TLH: N=71; LSH: N=51) for benign indications. A QOL questionnaire (again, the SF-36) was administered immediately preoperatively, as a baseline, and at 3 to 4 weeks postoperatively.
Preoperatively, patients were presented with the two surgical options, without being influenced with information about any benefit to removing or retaining the cervix at laparoscopic hysterectomy. Patients then chose which surgery they wanted, and were neither randomized nor blinded to the procedure that was performed.
Findings. The data show greater patient self-selection and more patients with endometriosis in the TLH group; other preoperative baseline characteristics were similar across groups. More operative and postoperative complications were seen in the TLH group (vaginal cuff bleeding requiring return to the operating room, 2 patients; cuff cellulitis, 1; intraoperative vaginal laceration, 1; urinary tract infection, 1), although the difference did not reach statistical significance. There were no significant differences group to group in postop nausea, pain, narcotic use, or return to daily activities.
Regarding the eight QOL parameters, however, a statistically significant difference was observed in six of them to favor laparoscopic supracervical hysterectomy: physical functioning, physical role, bodily pain, vitality, social functioning, and physical component summary.
Study has shortcomings. The authors address two limitations of their study: namely, that the participants were neither blinded nor randomized. They acknowledge that these limitations might have biased QOL measurements in a way that showed improved QOL among the supracervical hysterectomy group. They raise the possibility that not being blinded to whether the cervix was removed may have affected subjects’ bodily perception. (Patients also returned to their daily activities 5 days earlier in the supracervical group, but this finding was found to be statistically insignificant.)
It is possible, however, to look at these limitations not as shortcomings of the study but as an important insight into the validity of patient choice and the benefits of patient education and autonomy in decision-making. Perhaps patients who have chosen to keep their cervix have a discernable advantage in regard to their perception of a higher QOL after hysterectomy.
An additional critique. Although the authors addressed a return to several daily activities that are outside the SF-36 questionnaire (e.g., a return to household chores, driving, work, exercise, and normal activities) they did not address sexual activity.
It has been the generally accepted practice to instruct patients not to place anything in the vagina, and to avoid vaginal intercourse, for at least 6 weeks after the cervix has been removed—regardless of the route of removal. After supracervical hysterectomy, however, patients can resume intercourse as early as 2 weeks. I think that it would be realistic for the authors to have stated that a quicker return to sexual activity after surgery might improve QOL scores for women, but they did not specifically address this domain.
When you’ve determined that hysterectomy is indicated for treatment of a patient’s benign disease and plan a laparoscopic approach, consider that education and autonomy of choice about whether to keep the cervix might improve quality of life postoperatively.
Acknowledgment
Andrew I. Brill, MD, and William H. Parker, MD, reviewed the manuscript of this article before it was submitted for publication.
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease (reaffirmed 2011). Obstet Gynecol. 2009;114(5):1156-1158.
2. Kluivers K, Hendriks J, Mol BW, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: A randomized, controlled trial. J Minim Invasive Gynecol. 2007;14(2):145-152.
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease (reaffirmed 2011). Obstet Gynecol. 2009;114(5):1156-1158.
2. Kluivers K, Hendriks J, Mol BW, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: A randomized, controlled trial. J Minim Invasive Gynecol. 2007;14(2):145-152.
IN THIS ARTICLE