User login
The Wells Rule and VTE Prophylaxis
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
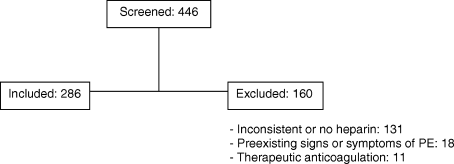
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).
| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).

| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
Symptoms, signs, chest radiograms, electrocardiograms and laboratory data have a low specificity for the diagnosis of pulmonary embolism (PE) when used in isolation, but when used in combination they can accurately identify patients with an increased likelihood of having a PE.17 The Wells score combines multiple variables into a prediction tool (Table 1). The original model identified three categories of patients with increasing likelihoods of having a PE,6 but a simpler, dichotomous version was subsequently proposed.7 A sequential diagnostic strategy combining the dichotomous Wells rule with a serum d‐dimer test has been validated against contrast‐enhanced spiral computed tomography (CTPE) on cohorts comprised largely of ambulatory outpatient and emergency room patients.815 This method, however, has never been tested in hospitalized patients who were receiving heparin in doses designed to prevent the development of venous thromboembolism (VTE). The purpose of this study was to evaluate the utility of the modified Wells score to predict the presence or absence of PE in hospitalized patients who were receiving prophylactic heparin.
| |
| Symptoms and signs of deep‐vein thrombosis | 3.0 |
| Heart rate >100 beats per minute | 1.5 |
| Recent immobilization or surgery (<4 weeks) | 1.5 |
| Previous VTE | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer | 1.0 |
| PE more likely than alternate diagnosis | 3.0 |
Methods
We screened consecutive patients who underwent CTPE studies from January 2006 through December 2007 at Denver Health, a university‐affiliated public hospital. Inclusion criteria were patients between 18 and 89 years of age who underwent CTPE imaging 2 or more days after being hospitalized, and had been receiving fractionated or unfractionated heparin in doses appropriate for preventing the development of deep venous thrombosis from the time of admission. Patients were excluded if they had signs or symptoms that were consistent with a diagnosis of PE at the time of admission, if they had a contraindication to prophylactic anticoagulation or if their prophylactic heparin therapy had been interrupted for any reason from the time prior to when the CTPE was ordered.
Patients were grouped depending on the service or location of their admission (ie, Medicine, Surgery, Orthopedics, Medical or Surgical Intensive Care Units). The objective elements of the Wells score were obtained by reviewing each patient's history and physical examination, progress notes and discharge summary. Patients were considered to have an alternate diagnosis of equal or greater likelihood than a PE if a d‐dimer was ordered, or if such a possibility was suggested by the treating clinician in the computerized order for the CTPE. The modified Wells score was used to classify patients into PE‐likely (total score 4) or PE‐unlikely (total score <4).7 Fisher's exact test was used to analyze the 2 2 table. P< 0.05 was taken to represent significance.
The Colorado Multiple Institutional Review Board approved this study with a waiver of informed consent.
Results
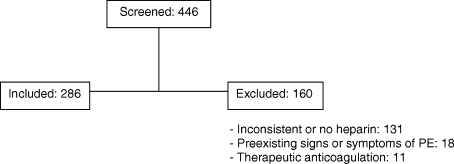
Of 446 patients who had CTPEs during the study period 286 (64%) met the inclusion criteria (Figure 1). Those who were excluded included 131 who did not receive continuous prophylactic anticoagulation from the time they were admitted to the time of the CT, 18 who had preexisting signs or symptoms and signs consistent with a diagnosis of PE at the time of admission, and 11 who were receiving therapeutic anticoagulation. The patients were hospitalized on different units and on a number of different services (Table 2).

| Total Patients | PE | PE Likely | |
|---|---|---|---|
| |||
| Medicine | 89 | 7 (8%) | 59 (66%) |
| Surgery | 55 | 0 (0%) | 43 (78%) |
| Orthopedics | 57 | 6 (11%) | 43 (75%) |
| MICU | 24 | 3 (13%) | 20 (83%) |
| SICU | 61 | 4 (7%) | 47 (77%) |
| Total | 286 | 20 (7%) | 212 (74%) |
Low molecular weight heparin was given to 165 patients (dalteparin, 5000 units, once daily), unfractionated heparin to 120 patients (104 receiving 5000 units twice daily and 16 receiving 5000 units 3 times a day) and 1 patient was given a Factor Xa inhibitor (fondaparinux 2.5 mg once daily) due to a history of heparin induced thrombocytopenia.
Hypoxia and tachycardia were the most common reasons for requesting a CTPE in instances in which an indication for CT imaging was documented. In almost 28% of patients, however, the reason for suspecting PE was not apparent on chart review (Table 3).
| Patients (%) | |
|---|---|
| |
| Hypoxia | 118 (41) |
| Hypoxia + tachycardia | 45 (16) |
| Tachycardia | 32 (11) |
| Chest pain | 10 (3) |
| Hemoptysis | 1 (0.3) |
| Not specified | 80 (28) |
| Total | 286 (100) |
The prevalence of PE was 20/286 (7.0%, 95% CI (confidence interval): 4.0‐10.0). On the basis of the Wells score 212 patients (74%) were classified as PE‐likely and 74 (26%) as PE‐unlikely. Immobility or recent surgery, tachycardia and the absence of a more plausible diagnosis were the most common contributors to the final score (Table 4).
| n (%) | |
|---|---|
| |
| Symptoms and signs of deep‐vein thrombosis | 12 (6) |
| Heart rate >100 beats per minute | 119 (60) |
| Recent immobilization or surgery (<4 weeks) | 179 (90) |
| Previous VTE | 10 (5) |
| Hemoptysis | 1 (<1) |
| Active cancer | 18 (9) |
| PE more likely than alternate diagnosis | 131 (66) |
Nineteen of the 20 patients (95%) who had PE diagnosed on the basis of a positive CTPE were risk‐stratified on the basis of the Wells score into the PE‐likely category, and 1 (5%) was classified as PE‐unlikely. Of the 266 patients whose CTPEs were negative 193 (73%) were classified as PE‐likely and 73 (27%) as PE‐unlikely (P < 0.03). Accordingly, the modified Wells score was 95% sensitive for having a diagnosis of PE confirmed on CTPE, the specificity was only 27%, the positive predictive value was only 9% and the negative predictive value was 99%(Table 5) with negative likelihood ratio of 0.19.
| Wells Rule | CTPE | Total | |
|---|---|---|---|
| Positive | Negative | ||
| |||
| PE likely | 19 | 193 | 272 |
| PE unlikely | 1 | 73 | 74 |
| Total | 20 | 266 | 286 |
| Sensitivity | 0.95 | ||
| Specificity | 0.27 | ||
| Positive predictive value | 0.09 | ||
| Negative predictive value | 0.99 | ||
| Positive likelihood ratio | 1.31 | ||
| Negative likelihood ratio | 0.18 | ||
| Two‐sided P value | 0.03 | ||
A d‐dimer was ordered for 70 of the 74 patients (95%) who were classified as PE‐unlikely. In 67 of these (96%) the test was positive, and in all but 1 the result was falsely positive. D‐dimer testing was also obtained in 8 of 212 (4%) of patients classified as PE‐likely and was positive in all 8.
Discussion
This retrospective cohort study demonstrated that in hospitalized patients who were receiving prophylactic doses of fractionated or unfractionated heparin and underwent CTPE studies for the clinical suspicion of PE, the prevalence of PE was very low, the modified Wells rule classified 26% of the patients as PE‐unlikely, and the PE‐unlikely category was associated with an extremely high negative predictive value and low negative likelihood ratio for PE. We also confirmed that the prevalence of a positive d‐dimer was so high in this population that the test did not add to the ability to risk‐stratify patients for the likelihood of having a PE. These findings lead to the conclusion that CTPE studies were performed excessively in this cohort of patients.
Previous studies validating the Wells score enrolled combinations of inpatients and outpatients813 or outpatients exclusively.14, 15 To our knowledge the present study is the first to validate the utility of the scoring system in inpatients receiving prophylactic anticoagulation. As would be expected, the prevalence of PE in our population was lower than the 9% to 30% that has previously been reported in patients not receiving prophylactic anticoagulation,815 consistent with the 68% to 76% reduction in the risk of deep venous thrombosis that occurs with use of low‐dose heparin or low molecular weight heparin.16
Similar to the findings of Arnason et al.17 a large proportion of this inpatient cohort was classified as PE‐likely on the basis of only 3 of the 7 variablestachycardia, immobility or previous surgery, and the absence of a more likely competing diagnosis.
The d‐dimer was elevated above the upper limit of normal in nearly all the cases in which it was tested (96%). Bounameaux et al.18 first suggested that conditions other than VTE could increase the plasma d‐dimer level. D‐dimer levels above the cutoff that excludes thrombosis have been documented in absence of thrombosis in the elderly and in patients with numerous other conditions including infections, cancer, coronary, cerebral and peripheral arterial vascular disease, heart failure, rheumatologic diseases, surgery, trauma burns, and pregnancy.1821 Van Beek et al.22 and Miron et al.23 demonstrated that d‐dimer testing was not useful in hospitalized patients. Kabrhel et al.24 reported similar results in an Emergency Department cohort and concluded that d‐dimer testing increased the percent of patients who were investigated for PE and the percent that were sent for pulmonary vascular imaging without increasing the percent of patients diagnosed as having a PE. In our cohort, 74 patients (26%) were classified as PE‐unlikely, and we theorize that 67 (90%) of these underwent CTPE studies solely on the basis of having a positive d‐dimer. All but one of the CTPEs in the patients with positive d‐dimers were negative for PE confirming the that the low specificity of d‐dimer testing in hospitalized patients also applies to those receiving prophylactic anticoagulation.
The Wells rule was associated with a high negative predictive value (99%) and a corresponding low negative likelihood ratio of 0.19, with both of these parameters likely being strongly influenced by the low prevalence of PE in this cohort.
In most longitudinal controlled studies of heparin‐based prophylaxis the incidence of VTE in all medical and most surgical patients approximates 5%.25,26 If this were taken to represent the pre‐test probability of VTE in patients on prophylaxis in whom the question of PE arises, then according to Bayesian theory, a PE‐unlikely classification with a negative likelihood ratio of 0.19 would result in a post‐test probability of less than 1%. This is well below the threshold at which diagnostic imaging delivers no benefit and in fact, may cause harm. Accordingly, PE can be safely excluded in those who are risk‐stratified to PE‐unlikely, with or without an accompanying negative d‐dimer. The average charge for a CTPE at our institution is $1800 and the 2009 cost/charge ratio was 54%. Accordingly, the cost savings to our hospital if CTPEs were not done on the 74 patients classified as PE‐unlikely would exceed $66,000/year.
Our study has a number of potential limitations. Because the data came from a single university‐affiliated public hospital the results might not generalize to other hospitals (teaching or nonteaching). Despite finding a very low prevalence of PE in patients receiving prophylactic heparin, the true prevalence of PE might have been overestimated since our sample size was small and Denver Health is a regional level I trauma center and has a busy joint arthroplasty service, i.e., services known to have an increased prevalence of venous thrombosis.16 If the prevalence of PE were indeed lower than what we observed, however, it would decrease the number of true positive and false negative CTPEs which would, in turn, further strengthen the conclusion that CTPEs are being overused in hospitalized patients receiving prophylactic heparin who are risk‐stratified to a PE‐unlikely category. Similarly, because our sample size was small we may have underestimated the prevalence of PE. Our narrow CIs, and the fact that the prevalence we observed is consistent with the effect of prophylaxic heparin on the incidence of VTE suggest that, if an error were made, it would not be large enough to alter our conclusions.
Our analysis did not include patients in whom PE was excluded without performing CTPE testing. If these patients had CTPEs the large majority would be negative because of a very low pretest probability and risk‐stratification would have placed them in a PE‐unlikely category (ie, true negatives), thereby also increasing the negative predictive value of the Wells score used in this setting.
We calculated the Wells score retrospectively as was previously done in studies by Chagnon et al.,11 Righini et al.,14 and Ranji et al.27 (although the methods used in these studies were not described in detail). We assumed that whenever a d‐dimer test was ordered the treating physician thought that PE was less likely than an alternate diagnosis reasoning that, if they thought PE were the most likely diagnosis, d‐dimers should not have been obtained as, in this circumstance, they are not recommended as part of the diagnostic algorithm.8 Conversely, we assumed that for patients who did not get d‐dimer testing, the treating physician thought that PE was the most likely diagnosis. Alternatively, the physicians might not have ordered a d‐dimer because they recognized that the test is of limited clinical utility in hospitalized patients. In this latter circumstance, the number of PE‐likely patients would be overestimated and the number of PE‐unlikely would be underestimated, reducing the strength of our conclusions or potentially invalidating them. Since the accuracy of prediction rules mirrors that of implicit clinical judgment, however, we suggest that, for most of the patients who had CTPEs performed without d‐dimers, the ordering physician had a high suspicion of PE28, 29 and that the large majority of PE‐likely patients were correctly classified.
In summary, we found that CTPE testing is frequently performed in hospitalized patients receiving prophylactic heparin despite there being a very low prevalence of PE in this cohort, and that risk‐stratifying patients into the PE‐unlikely category using the modified Wells score accurately excludes the diagnosis of PE. The problem of overuse of CTPEs is compounded by the well‐recognized misuse of d‐dimer testing in hospitalized patients. On the basis of our findings we recommend that, when hospitalized patients who are receiving heparin prophylaxis to prevent VTE develop signs or symptoms suggestive of PE they should be risk‐stratified using the modified Wells criteria. In those classified as PE‐unlikely PE can be safely excluded without further testing. Using this approach 26% of CTPEs done on the cohort of hospitalized patients we studied, and all d‐dimers could have been avoided. If the results of our study are duplicated in other centers these recommendations should be included in future guidelines summarizing the most cost‐effective ways to evaluate patients for possible PE.
Acknowledgements
Ms. Angela Keniston assisted in this study by identifying the initial population by using the hospital's computerized data warehouse.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
- ,Accuracy of the clinical diagnosis of pulmonary embolism.JAMA.1967;202(7):115–118.
- ,,, et al.Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease.Chest.1991;100(3):598–603.
- ,,,Chest Radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry.Chest.2000;118(1):33–38.
- ,,Alveolar‐arterial gradient in the assessment of acute pulmonary embolism.Chest.1995;107(1):139–143.
- ,,, et al.Diagnostic value of the electrocardiogram in suspected pulmonary embolism.Am J Cardiol.2000;86(7):807–809.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129(12):997–1005.
- ,,, et al.Derivation of a simple clinical model to categorize patient's probability of pulmonary embolism: increasing the models utility with the simpliRed D‐dimer.Thromb Haemost.2000;83(3):416–420.
- ,,, et al.Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d‐dimer testing and computer tomography.JAMA.2006;295(2):172–179.
- ,,,, et al.Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism.J Thromb Haemost.2008;6(1):40–44.
- ,,, et al.Further validation and simplification of the Wells clinical decision rule in pulmonary embolism.J Thromb Haemost.2008;99(1):229–234.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,,A prospective reassessment of the utility of the Wells score in identifying pulmonary embolism.Med J Aust.2007;187(6):333–336.
- ,,,et al.Performance of the Wells and Revised Geneva scores for predicting pulmonary embolism.Eur J Emerg Med.2009;16(1):49–52.
- ,,,Clinical probability assessment of pulmonary embolism by the Wells' score: is the easiest the best?J Thromb Haemost.2006;4(3):702–704
- ,,, et al.Simple and safe exclusion of pulmonary embolism in outpatients using quantitative D‐dimer and Wells' simplified decision rule.J Thromb Haemost.2007;97:146–150.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,Appropriateness of diagnostic strategies for evaluating suspected pulmonary embolism.J Thromb Haemost.2007;97(2):195–201.
- ,,, et al.Measurement of plasma D‐dimer for diagnosis of deep venous thrombosis.Am J Clin Path.1989;91(1):82–85.
- ,,,Plasma D‐dimer levels in elderly patients with suspected pulmonary embolism.Thromb Res.2000;98(6):577–579.
- ,,, et al.D‐dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism.Thromb Res.1993;69(1):125–130.
- ,,,D‐dimer for venous thromboembolism diagnosis: 20 years later.J Thromb Haemost.2008;6(7):1059–1071.
- ,,, et al.The role of plasma D‐dimer concentration in the exclusion of pulmonary embolism.Brit J Haematol.1996;92(3):725–732.
- ,,, et al.Contribution of non‐invasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients.Eur Respir J.1999;13(6):1365–1370.
- ,,A highly sensitive ELISA D‐dimer increases testing but not diagnosis of pulmonary embolism.Acad Emerg Med2006;13:519–524.
- ,,A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients.Canadian Collaborative Group.1998;114:115S–118S.
- ,,,,Impact of reliance on CT pulmonary angiography on diagnosis of pulmonary embolism: a Bayesian analysis.J Hosp Med.2006;1:81–87.
- The PIOPED investigators: Value of the ventilation‐perfusion scan in acute pulmonary embolism.JAMA.1990;263:2753–2759.
- ,,, et al.Non‐invasive diagnosis of venous thromboembolism in outpatients.Lancet.353:190–195.
Copyright © 2011 Society of Hospital Medicine