User login
Hospitalist‐Run Postdischarge Clinic
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
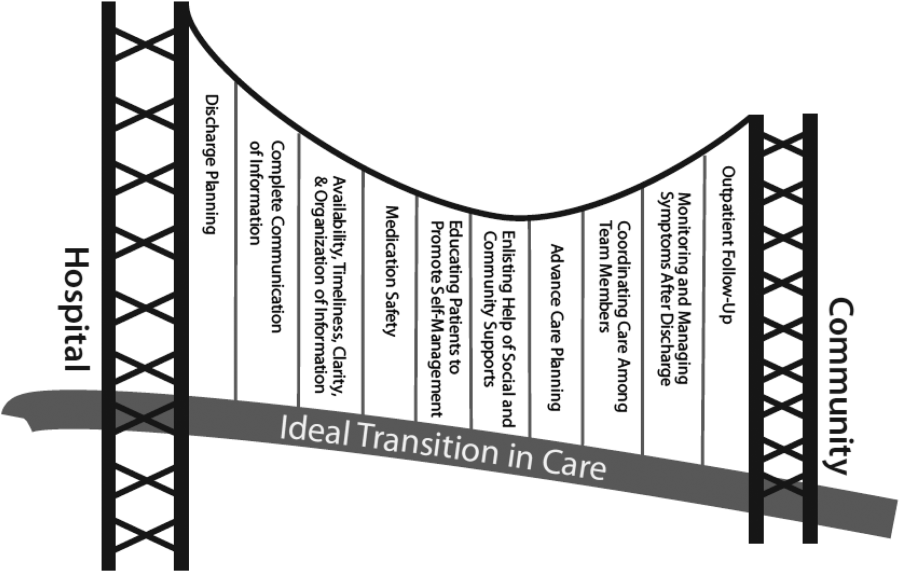
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.
The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
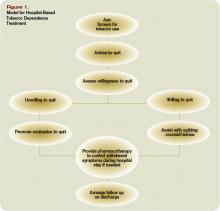
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.

The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.

The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
© 2013 Society of Hospital Medicine
How Should Hospitalized Patients with Long QT Syndrome Be Managed?
Case
You are asked to admit a 63-year-old male with a history of hypertension and osteoarthritis. The patient, who fell at home, is scheduled for open repair of his femoral neck fracture the following day. The patient reports tripping over his granddaughter’s toys and denies any associated symptoms around the time of his fall. An electrocardiogram (ECG) reveals a QTc (QT) interval of 480 ms. How should this hospitalized patient’s prolonged QT interval be managed?
Overview
Patients with a prolonged QT interval on routine ECG present an interesting dilemma for clinicians. Although QT prolongation—either congenital or acquired—has been associated with dysrhythmias, the risk of torsades de pointes and sudden cardiac death varies considerably based on myriad underlying factors.1 Therefore, the principle job of the clinician who has recognized QT prolongation is to assess and minimize the risk of the development of clinically significant dysrhythmias, and to be prepared to manage them should they arise.
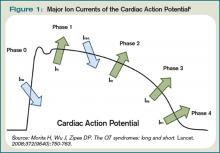
The QT interval encompasses ventricular depolarization and repolarization. This ventricular action potential proceeds through five phases. The initial upstroke (phase 0) of depolarization occurs with the opening of Na+ channels, triggering the inward Na+ current (INa), and causes the interior of the myocytes to become positively charged. This is followed by initial repolarization (phase 1) when the opening of K+ channels causes an outward K+ current (Ito). Next, the plateau phase (phase 2) of the action potential follows with a balance of inward current through Ca2+channels (Ica-L) and outward current through slow rectifier K+ channels (IKs), and then later through delayed, rapid K+ rectifier channels (IKr). Then, the inward current is deactivated, while the outward current increases through the rapid delayed rectifier (IKr) and opening of inward rectifier channels (IK1) to complete repolarization (phase 3). Finally, the action potential returns to baseline (phase 4) and Na+ begins to enter the cell again (see Figure 1, above).
The long QT syndrome (LQTS) is defined by a defect in these cardiac ion channels, which leads to abnormal repolarization, usually lengthening the QT interval and thus predisposing to ventricular dysrhythmias.2 It is estimated that as many as 85% of these syndromes are inherited, and up to 15% are acquired or sporadic.3 Depending on the underlying etiology of the LQTS, manifestations might first be appreciated at any time from in utero through adulthood.4 Symptoms including palpitations, syncope, seizures, or cardiac arrest bring these patients to medical attention.3 These symptoms frequently elicit physical or emotional stress, but they can occur without obvious inciting triggers.5 A 20% mortality risk exists in patients who are symptomatic and untreated in the first year following diagnosis, and up to 50% within 10 years following diagnosis.4
How is Long QT Syndrome Diagnosed?
The LQTS diagnosis is based on clinical history in combination with ECG abnormalities.6 Important historical elements include symptoms of palpitations, syncope, seizures, or cardiac arrest.3 In addition, a family history of unexplained syncope or sudden death, especially at a young age, should raise LQTS suspicion.5
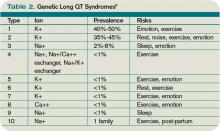
A variety of ECG findings can be witnessed in LQTS patients.4,5 Although the majority of patients have a QTc >440 ms, approximately one-third have a QTc ≤460 ms, and about 10% have normal QTc intervals.5 Other ECG abnormalities include notched, biphasic, or prolonged T-waves, and the presence of U-waves.4,5 Schwartz et al used these elements to publish criteria (see Table 1, right) that physicians can use to assess the probability that a patient has LQTS.7
Types of Long QT Syndromes
Because the risk of developing significant dysrhythmias with LQTS is dependent on both the actual QT interval, with risk for sudden cardiac death increased two to three times with QT >440 ms compared with QT <440 ms and the specific underlying genotype, it is important to have an understanding of congenital and acquired LQTS and the associated triggers for torsades de pointes.
Congenital LQTS
Congenital LQTS is caused by mutations in cardiac ion channel proteins, primarily sodium, and potassium channels.5,6 These defects either slow depolarization or lengthen repolarization, leading to heterogeneity of repolarization of the membrane.5 This, in turn, predisposes to ventricular dysrhythmias, including torsades de pointes and subsequent ventricular fibrillation and death.2 Currently, 12 genetic defects have been identified in LQTS. Hundreds of mutations have been described to cause these defects (see Table 2, right).8 Approximately 70% of congenital LQTS are caused by mutations in three genes and are classified as LQTS 1, LQTS 2, and LQTS 3.8 The other seven mutation types account for about 5% of cases; a quarter of LQTS cases have no identified genetic mutations.8
LQTS usually can be distinguished by clinical features and some ECG characteristics, but diagnosis of the specific type requires genetic testing.8,9 The most common types of LQTS are discussed below.
- Long QT1 is the most common type, occurring in approximately 40% to 50% of patients diagnosed with LQTS. It is characterized by a defect in the potassium channel alpha subunit leading to IKs reduction.9 These patients typically present with syncope or adrenergic-induced torsades, might have wide, broad-based T-waves on ECG, and respond well to beta-blocker therapy.6 Triggers for these patients include physical exertion or emotional stressors, particularly exercise and swimming. These patients typically present in early childhood.1
- Long QT2 occurs in 35% to 40% of patients and is characterized by a different defect in the alpha subunit of the potassium channel, which leads to reduced IKr.9 ECGs in LQTS2 can demonstrate low-amplitude and notched T-waves. Sudden catecholamine surges related to emotional stress or loud noises and bradycardia can trigger dysrhythmias in Long QT2.6 Thus, beta blockers reduce overall cardiac events in LQTS2 but less effectively than in LQTS1.6 These patients also present in childhood but typically are older than patients with LQTS1.6
- Long QT3 is less common than LQTS1 or LQTS2, at 2% to 8% of LQTS patients, but carries a higher mortality and is not treated effectively with beta blockers. LQTS3 is characterized by a defect in a sodium channel, causing a gain-of-function in the INa.4,9 These patients are more likely to have a fatal dysrhythmia while sleeping, are less susceptible to exercise-induced events, and have increased morbidity and mortality associated with bradycardia.4,9 ECG frequently reveals a relatively long ST segment, followed by a peaked and tall T-wave. Beta-blocker therapy can predispose to dysrhythmias in these patients; therefore, many of these patients will have pacemakers implanted as first-line therapy.6
While less common, Jervell and Lange Nielson syndrome is an autosomal recessive form of LQTS in which affected patients have homozygous mutations in the KCNQ1 or KCNE1 genes. This syndrome occurs in approximately 1% to 7% of LQTS patients, displays a typical QTc >550 ms, can be triggered by exercise and emotional stress, is associated with deafness, and carries a high risk of cardiac events at a young age.6
Acquired Syndromes
In addition to congenital LQTS, certain patients can acquire LQTS after being treated with particular drugs or having metabolic abnormalities, namely hypomagnesemia, hypocalcemia, and hypokalemia. Most experts think patients who “acquire” LQTS that predisposes to torsades de pointes have underlying structural heart disease or LQTS genetic carrier mutations that combine with transient initiating events (e.g., drugs or metabolic abnormalities) to induce dysrhythmias.1 In addition to certain drugs, cardiac ischemia, and electrolyte abnormalities, cocaine abuse, HIV, and subarachnoid hemorrhage can induce dysrhythmias in susceptible patients.5
Many types of drugs can cause a prolonged QT interval, and others should be avoided in patients with pre-existing prolonged QT (see Table 3, p. 17). Potentially offending drugs that are frequently encountered by inpatient physicians include amiodarone, diltiazem, erythromycin, clarithromycin, ciprofloxacin, fluoxetine, paroxetine, sertraline, haloperidol, ritonavir, and methadone.1 Additionally, drugs that cause electrolyte abnormalities (e.g., diuretics and lithium) should be monitored closely.
Overall, the goals of therapy in LQTS are:
- Decrease the risk of dysrhythmic events;
- Minimize adrenergic response;
- Shorten the QTc;
- Decrease the dispersion of refractoriness; and
- Improve the function of the ion channels.3
Supportive measures should be taken for patients who are acutely symptomatic from LQTS and associated torsades de pointes. In addition to immediate cardioversion for ongoing and hemodynamically significant torsades, intravenous magnesium should be administered, electrolytes corrected, and offending drugs discontinued.5 Temporary transvenous pacing at rates of approximately 100 beats per minute is highly effective in preventing short-term recurrence of torsades in congenital and acquired LQTS, especially in bradycardic patients.5 Isoproterenol infusion increases the heart rate and effectively prevents acute recurrence of torsades in patients with acquired LQTS, but it should be used with caution in patients with structural heart disease.5
Long-term strategies to manage LQTS include:
- Minimizing the risk of triggering cardiac events via adrenergic stimulation;
- Preventing ongoing dysrhythmias;
- Avoiding medications known to prolong the QT interval; and
- Maintaining normal electrolytes and minerals.5
Most patients with congenital long QT should be discouraged from participating in competitive sports, and patients should attempt to eliminate exposures to stress or sudden awakening, though this is not practical in all cases.5 Beta blockers generally are the first-line therapy and are more effective for LQT1 than LQT2 or LQT3.4,5 If patients are still symptomatic despite adequate medical therapy, or have survived cardiac arrest, they should be considered for ICD therapy.4,5 In addition, patients with profound bradycardia benefit from pacemaker implantation.5 Patients who remain symptomatic despite both beta blockade and ICD placement might find cervicothoracic sympathectomy curative.4,5
Perioperative Considerations
Although little data is available to guide physicians in the prevention of torsades de pointes during the course of anesthesia, there are a number of considerations that may reduce the chances of symptomatic dysrhythmias.
First, care should be taken to avoid dysrhythmia triggers in LQTS by providing a calm, quiet environment during induction, monitoring, and treating metabolic abnormalities, and providing an appropriate level of anesthesia.10 Beta-blocker therapy should be continued and potentially measured preoperatively by assessing heart rate response during stress testing.5 An implantable cardioverter-defibrillator (AICD) should be interrogated prior to surgery and inactivated during the operation.5
Finally, Kies et al have recommended general anesthesia with propofol for induction (or throughout), isoflurane as the volatile agent, vecuronium for muscle relaxation, and intravenous fentanyl for analgesia when possible.10
Back to the Case
While the patient had no genetic testing for LQTS, evaluation of previous ECGs demonstrated a prolonged QT interval. The hip fracture repair was considered an urgent procedure, which precluded the ability to undertake genetic testing and consideration for device implantation. The only medication that was known to increase the risk for dysrhythmias in this patient was his diuretic, with the attendant risk of electrolyte abnormalities.
Thus, the patient’s hydrochlorothiazide was discontinued and his pre-existing atenolol continued. The patient’s electrolytes and minerals were monitored closely, and magnesium was administered on the day of surgery. Anesthesia was made aware of the prolonged QT interval, such that they were able to minimize the risk for and anticipate the treatment of dysrhythmias. The patient tolerated the surgery and post-operative period without complication and was scheduled for an outpatient workup and management for his prolonged QT interval.
Bottom Line
Long QT syndrome is frequently genetic in origin, but it can be caused by certain medications and perturbations of electrolytes. Beta blockers are the first-line therapy for the majority of LQTS cases, along with discontinuation of drugs that might induce or aggravate the QT prolongation.
Patients who have had cardiac arrest or who remain symptomatic despite beta-blocker therapy should have an ICD implanted.
In the perioperative period, patients’ electrolytes should be monitored and kept within normal limits. If the patient is on a beta blocker, it should be continued, and the anesthesiologist should be made aware of the diagnosis so that the anesthethic plan can be optimized to prevent arrhythmic complications. TH
Dr. Kamali is a medical resident at the University of Colorado Denver. Dr. Stickrath is a hospitalist at the Veterans Affairs Medical Center in Denver and an instructor of medicine at UC Denver. Dr. Prochazka is director of ambulatory care at the Denver VA and professor of medicine at UC Denver. Dr. Varosy is director of cardiac electrophysiology at the Denver VA and assistant professor of medicine at UC Denver.
References
- Kao LW, Furbee BR. Drug-induced q-T prolongation. Med Clin North Am. 2005;89(6):1125-1144.
- Marchlinski F. Chapter 226, The Tachyarrhythmias; Harrison's Principles of Internal Medicine, 17e. Available at: www.accessmedicine.com/resourceTOC .aspx?resourceID=4. Accessed Nov. 21, 2009.
- Zareba W, Cygankiewicz I. Long QT syndrome and short QT syndrome. Prog Cardiovasc Dis. 2008; 51(3):264-278.
- Booker PD, Whyte SD, Ladusans EJ. Long QT syndrome and anaesthesia. Br J Anaesth. 2003;90(3):349-366.
- Khan IA. Long QT syndrome: diagnosis and management. Am Heart J. 2002;143(1):7-14.
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372(9640):750-763.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88(2):782-784.
- Kapa S, Tester DJ, Salisbury BA, et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752-1760.
- Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: a HuGE review. Genet Med. 2006;8(3):143-155.
- Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005;102(1):204-210.
- Wisely NA, Shipton EA. Long QT syndrome and anaesthesia. Eur J Anaesthesiol. 2002;19(12):853-859.
Case
You are asked to admit a 63-year-old male with a history of hypertension and osteoarthritis. The patient, who fell at home, is scheduled for open repair of his femoral neck fracture the following day. The patient reports tripping over his granddaughter’s toys and denies any associated symptoms around the time of his fall. An electrocardiogram (ECG) reveals a QTc (QT) interval of 480 ms. How should this hospitalized patient’s prolonged QT interval be managed?
Overview
Patients with a prolonged QT interval on routine ECG present an interesting dilemma for clinicians. Although QT prolongation—either congenital or acquired—has been associated with dysrhythmias, the risk of torsades de pointes and sudden cardiac death varies considerably based on myriad underlying factors.1 Therefore, the principle job of the clinician who has recognized QT prolongation is to assess and minimize the risk of the development of clinically significant dysrhythmias, and to be prepared to manage them should they arise.
The QT interval encompasses ventricular depolarization and repolarization. This ventricular action potential proceeds through five phases. The initial upstroke (phase 0) of depolarization occurs with the opening of Na+ channels, triggering the inward Na+ current (INa), and causes the interior of the myocytes to become positively charged. This is followed by initial repolarization (phase 1) when the opening of K+ channels causes an outward K+ current (Ito). Next, the plateau phase (phase 2) of the action potential follows with a balance of inward current through Ca2+channels (Ica-L) and outward current through slow rectifier K+ channels (IKs), and then later through delayed, rapid K+ rectifier channels (IKr). Then, the inward current is deactivated, while the outward current increases through the rapid delayed rectifier (IKr) and opening of inward rectifier channels (IK1) to complete repolarization (phase 3). Finally, the action potential returns to baseline (phase 4) and Na+ begins to enter the cell again (see Figure 1, above).
The long QT syndrome (LQTS) is defined by a defect in these cardiac ion channels, which leads to abnormal repolarization, usually lengthening the QT interval and thus predisposing to ventricular dysrhythmias.2 It is estimated that as many as 85% of these syndromes are inherited, and up to 15% are acquired or sporadic.3 Depending on the underlying etiology of the LQTS, manifestations might first be appreciated at any time from in utero through adulthood.4 Symptoms including palpitations, syncope, seizures, or cardiac arrest bring these patients to medical attention.3 These symptoms frequently elicit physical or emotional stress, but they can occur without obvious inciting triggers.5 A 20% mortality risk exists in patients who are symptomatic and untreated in the first year following diagnosis, and up to 50% within 10 years following diagnosis.4
How is Long QT Syndrome Diagnosed?
The LQTS diagnosis is based on clinical history in combination with ECG abnormalities.6 Important historical elements include symptoms of palpitations, syncope, seizures, or cardiac arrest.3 In addition, a family history of unexplained syncope or sudden death, especially at a young age, should raise LQTS suspicion.5
A variety of ECG findings can be witnessed in LQTS patients.4,5 Although the majority of patients have a QTc >440 ms, approximately one-third have a QTc ≤460 ms, and about 10% have normal QTc intervals.5 Other ECG abnormalities include notched, biphasic, or prolonged T-waves, and the presence of U-waves.4,5 Schwartz et al used these elements to publish criteria (see Table 1, right) that physicians can use to assess the probability that a patient has LQTS.7
Types of Long QT Syndromes
Because the risk of developing significant dysrhythmias with LQTS is dependent on both the actual QT interval, with risk for sudden cardiac death increased two to three times with QT >440 ms compared with QT <440 ms and the specific underlying genotype, it is important to have an understanding of congenital and acquired LQTS and the associated triggers for torsades de pointes.
Congenital LQTS
Congenital LQTS is caused by mutations in cardiac ion channel proteins, primarily sodium, and potassium channels.5,6 These defects either slow depolarization or lengthen repolarization, leading to heterogeneity of repolarization of the membrane.5 This, in turn, predisposes to ventricular dysrhythmias, including torsades de pointes and subsequent ventricular fibrillation and death.2 Currently, 12 genetic defects have been identified in LQTS. Hundreds of mutations have been described to cause these defects (see Table 2, right).8 Approximately 70% of congenital LQTS are caused by mutations in three genes and are classified as LQTS 1, LQTS 2, and LQTS 3.8 The other seven mutation types account for about 5% of cases; a quarter of LQTS cases have no identified genetic mutations.8
LQTS usually can be distinguished by clinical features and some ECG characteristics, but diagnosis of the specific type requires genetic testing.8,9 The most common types of LQTS are discussed below.
- Long QT1 is the most common type, occurring in approximately 40% to 50% of patients diagnosed with LQTS. It is characterized by a defect in the potassium channel alpha subunit leading to IKs reduction.9 These patients typically present with syncope or adrenergic-induced torsades, might have wide, broad-based T-waves on ECG, and respond well to beta-blocker therapy.6 Triggers for these patients include physical exertion or emotional stressors, particularly exercise and swimming. These patients typically present in early childhood.1
- Long QT2 occurs in 35% to 40% of patients and is characterized by a different defect in the alpha subunit of the potassium channel, which leads to reduced IKr.9 ECGs in LQTS2 can demonstrate low-amplitude and notched T-waves. Sudden catecholamine surges related to emotional stress or loud noises and bradycardia can trigger dysrhythmias in Long QT2.6 Thus, beta blockers reduce overall cardiac events in LQTS2 but less effectively than in LQTS1.6 These patients also present in childhood but typically are older than patients with LQTS1.6
- Long QT3 is less common than LQTS1 or LQTS2, at 2% to 8% of LQTS patients, but carries a higher mortality and is not treated effectively with beta blockers. LQTS3 is characterized by a defect in a sodium channel, causing a gain-of-function in the INa.4,9 These patients are more likely to have a fatal dysrhythmia while sleeping, are less susceptible to exercise-induced events, and have increased morbidity and mortality associated with bradycardia.4,9 ECG frequently reveals a relatively long ST segment, followed by a peaked and tall T-wave. Beta-blocker therapy can predispose to dysrhythmias in these patients; therefore, many of these patients will have pacemakers implanted as first-line therapy.6
While less common, Jervell and Lange Nielson syndrome is an autosomal recessive form of LQTS in which affected patients have homozygous mutations in the KCNQ1 or KCNE1 genes. This syndrome occurs in approximately 1% to 7% of LQTS patients, displays a typical QTc >550 ms, can be triggered by exercise and emotional stress, is associated with deafness, and carries a high risk of cardiac events at a young age.6
Acquired Syndromes
In addition to congenital LQTS, certain patients can acquire LQTS after being treated with particular drugs or having metabolic abnormalities, namely hypomagnesemia, hypocalcemia, and hypokalemia. Most experts think patients who “acquire” LQTS that predisposes to torsades de pointes have underlying structural heart disease or LQTS genetic carrier mutations that combine with transient initiating events (e.g., drugs or metabolic abnormalities) to induce dysrhythmias.1 In addition to certain drugs, cardiac ischemia, and electrolyte abnormalities, cocaine abuse, HIV, and subarachnoid hemorrhage can induce dysrhythmias in susceptible patients.5
Many types of drugs can cause a prolonged QT interval, and others should be avoided in patients with pre-existing prolonged QT (see Table 3, p. 17). Potentially offending drugs that are frequently encountered by inpatient physicians include amiodarone, diltiazem, erythromycin, clarithromycin, ciprofloxacin, fluoxetine, paroxetine, sertraline, haloperidol, ritonavir, and methadone.1 Additionally, drugs that cause electrolyte abnormalities (e.g., diuretics and lithium) should be monitored closely.
Overall, the goals of therapy in LQTS are:
- Decrease the risk of dysrhythmic events;
- Minimize adrenergic response;
- Shorten the QTc;
- Decrease the dispersion of refractoriness; and
- Improve the function of the ion channels.3
Supportive measures should be taken for patients who are acutely symptomatic from LQTS and associated torsades de pointes. In addition to immediate cardioversion for ongoing and hemodynamically significant torsades, intravenous magnesium should be administered, electrolytes corrected, and offending drugs discontinued.5 Temporary transvenous pacing at rates of approximately 100 beats per minute is highly effective in preventing short-term recurrence of torsades in congenital and acquired LQTS, especially in bradycardic patients.5 Isoproterenol infusion increases the heart rate and effectively prevents acute recurrence of torsades in patients with acquired LQTS, but it should be used with caution in patients with structural heart disease.5
Long-term strategies to manage LQTS include:
- Minimizing the risk of triggering cardiac events via adrenergic stimulation;
- Preventing ongoing dysrhythmias;
- Avoiding medications known to prolong the QT interval; and
- Maintaining normal electrolytes and minerals.5
Most patients with congenital long QT should be discouraged from participating in competitive sports, and patients should attempt to eliminate exposures to stress or sudden awakening, though this is not practical in all cases.5 Beta blockers generally are the first-line therapy and are more effective for LQT1 than LQT2 or LQT3.4,5 If patients are still symptomatic despite adequate medical therapy, or have survived cardiac arrest, they should be considered for ICD therapy.4,5 In addition, patients with profound bradycardia benefit from pacemaker implantation.5 Patients who remain symptomatic despite both beta blockade and ICD placement might find cervicothoracic sympathectomy curative.4,5
Perioperative Considerations
Although little data is available to guide physicians in the prevention of torsades de pointes during the course of anesthesia, there are a number of considerations that may reduce the chances of symptomatic dysrhythmias.
First, care should be taken to avoid dysrhythmia triggers in LQTS by providing a calm, quiet environment during induction, monitoring, and treating metabolic abnormalities, and providing an appropriate level of anesthesia.10 Beta-blocker therapy should be continued and potentially measured preoperatively by assessing heart rate response during stress testing.5 An implantable cardioverter-defibrillator (AICD) should be interrogated prior to surgery and inactivated during the operation.5
Finally, Kies et al have recommended general anesthesia with propofol for induction (or throughout), isoflurane as the volatile agent, vecuronium for muscle relaxation, and intravenous fentanyl for analgesia when possible.10
Back to the Case
While the patient had no genetic testing for LQTS, evaluation of previous ECGs demonstrated a prolonged QT interval. The hip fracture repair was considered an urgent procedure, which precluded the ability to undertake genetic testing and consideration for device implantation. The only medication that was known to increase the risk for dysrhythmias in this patient was his diuretic, with the attendant risk of electrolyte abnormalities.
Thus, the patient’s hydrochlorothiazide was discontinued and his pre-existing atenolol continued. The patient’s electrolytes and minerals were monitored closely, and magnesium was administered on the day of surgery. Anesthesia was made aware of the prolonged QT interval, such that they were able to minimize the risk for and anticipate the treatment of dysrhythmias. The patient tolerated the surgery and post-operative period without complication and was scheduled for an outpatient workup and management for his prolonged QT interval.
Bottom Line
Long QT syndrome is frequently genetic in origin, but it can be caused by certain medications and perturbations of electrolytes. Beta blockers are the first-line therapy for the majority of LQTS cases, along with discontinuation of drugs that might induce or aggravate the QT prolongation.
Patients who have had cardiac arrest or who remain symptomatic despite beta-blocker therapy should have an ICD implanted.
In the perioperative period, patients’ electrolytes should be monitored and kept within normal limits. If the patient is on a beta blocker, it should be continued, and the anesthesiologist should be made aware of the diagnosis so that the anesthethic plan can be optimized to prevent arrhythmic complications. TH
Dr. Kamali is a medical resident at the University of Colorado Denver. Dr. Stickrath is a hospitalist at the Veterans Affairs Medical Center in Denver and an instructor of medicine at UC Denver. Dr. Prochazka is director of ambulatory care at the Denver VA and professor of medicine at UC Denver. Dr. Varosy is director of cardiac electrophysiology at the Denver VA and assistant professor of medicine at UC Denver.
References
- Kao LW, Furbee BR. Drug-induced q-T prolongation. Med Clin North Am. 2005;89(6):1125-1144.
- Marchlinski F. Chapter 226, The Tachyarrhythmias; Harrison's Principles of Internal Medicine, 17e. Available at: www.accessmedicine.com/resourceTOC .aspx?resourceID=4. Accessed Nov. 21, 2009.
- Zareba W, Cygankiewicz I. Long QT syndrome and short QT syndrome. Prog Cardiovasc Dis. 2008; 51(3):264-278.
- Booker PD, Whyte SD, Ladusans EJ. Long QT syndrome and anaesthesia. Br J Anaesth. 2003;90(3):349-366.
- Khan IA. Long QT syndrome: diagnosis and management. Am Heart J. 2002;143(1):7-14.
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372(9640):750-763.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88(2):782-784.
- Kapa S, Tester DJ, Salisbury BA, et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752-1760.
- Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: a HuGE review. Genet Med. 2006;8(3):143-155.
- Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005;102(1):204-210.
- Wisely NA, Shipton EA. Long QT syndrome and anaesthesia. Eur J Anaesthesiol. 2002;19(12):853-859.
Case
You are asked to admit a 63-year-old male with a history of hypertension and osteoarthritis. The patient, who fell at home, is scheduled for open repair of his femoral neck fracture the following day. The patient reports tripping over his granddaughter’s toys and denies any associated symptoms around the time of his fall. An electrocardiogram (ECG) reveals a QTc (QT) interval of 480 ms. How should this hospitalized patient’s prolonged QT interval be managed?
Overview
Patients with a prolonged QT interval on routine ECG present an interesting dilemma for clinicians. Although QT prolongation—either congenital or acquired—has been associated with dysrhythmias, the risk of torsades de pointes and sudden cardiac death varies considerably based on myriad underlying factors.1 Therefore, the principle job of the clinician who has recognized QT prolongation is to assess and minimize the risk of the development of clinically significant dysrhythmias, and to be prepared to manage them should they arise.
The QT interval encompasses ventricular depolarization and repolarization. This ventricular action potential proceeds through five phases. The initial upstroke (phase 0) of depolarization occurs with the opening of Na+ channels, triggering the inward Na+ current (INa), and causes the interior of the myocytes to become positively charged. This is followed by initial repolarization (phase 1) when the opening of K+ channels causes an outward K+ current (Ito). Next, the plateau phase (phase 2) of the action potential follows with a balance of inward current through Ca2+channels (Ica-L) and outward current through slow rectifier K+ channels (IKs), and then later through delayed, rapid K+ rectifier channels (IKr). Then, the inward current is deactivated, while the outward current increases through the rapid delayed rectifier (IKr) and opening of inward rectifier channels (IK1) to complete repolarization (phase 3). Finally, the action potential returns to baseline (phase 4) and Na+ begins to enter the cell again (see Figure 1, above).
The long QT syndrome (LQTS) is defined by a defect in these cardiac ion channels, which leads to abnormal repolarization, usually lengthening the QT interval and thus predisposing to ventricular dysrhythmias.2 It is estimated that as many as 85% of these syndromes are inherited, and up to 15% are acquired or sporadic.3 Depending on the underlying etiology of the LQTS, manifestations might first be appreciated at any time from in utero through adulthood.4 Symptoms including palpitations, syncope, seizures, or cardiac arrest bring these patients to medical attention.3 These symptoms frequently elicit physical or emotional stress, but they can occur without obvious inciting triggers.5 A 20% mortality risk exists in patients who are symptomatic and untreated in the first year following diagnosis, and up to 50% within 10 years following diagnosis.4
How is Long QT Syndrome Diagnosed?
The LQTS diagnosis is based on clinical history in combination with ECG abnormalities.6 Important historical elements include symptoms of palpitations, syncope, seizures, or cardiac arrest.3 In addition, a family history of unexplained syncope or sudden death, especially at a young age, should raise LQTS suspicion.5
A variety of ECG findings can be witnessed in LQTS patients.4,5 Although the majority of patients have a QTc >440 ms, approximately one-third have a QTc ≤460 ms, and about 10% have normal QTc intervals.5 Other ECG abnormalities include notched, biphasic, or prolonged T-waves, and the presence of U-waves.4,5 Schwartz et al used these elements to publish criteria (see Table 1, right) that physicians can use to assess the probability that a patient has LQTS.7
Types of Long QT Syndromes
Because the risk of developing significant dysrhythmias with LQTS is dependent on both the actual QT interval, with risk for sudden cardiac death increased two to three times with QT >440 ms compared with QT <440 ms and the specific underlying genotype, it is important to have an understanding of congenital and acquired LQTS and the associated triggers for torsades de pointes.
Congenital LQTS
Congenital LQTS is caused by mutations in cardiac ion channel proteins, primarily sodium, and potassium channels.5,6 These defects either slow depolarization or lengthen repolarization, leading to heterogeneity of repolarization of the membrane.5 This, in turn, predisposes to ventricular dysrhythmias, including torsades de pointes and subsequent ventricular fibrillation and death.2 Currently, 12 genetic defects have been identified in LQTS. Hundreds of mutations have been described to cause these defects (see Table 2, right).8 Approximately 70% of congenital LQTS are caused by mutations in three genes and are classified as LQTS 1, LQTS 2, and LQTS 3.8 The other seven mutation types account for about 5% of cases; a quarter of LQTS cases have no identified genetic mutations.8
LQTS usually can be distinguished by clinical features and some ECG characteristics, but diagnosis of the specific type requires genetic testing.8,9 The most common types of LQTS are discussed below.
- Long QT1 is the most common type, occurring in approximately 40% to 50% of patients diagnosed with LQTS. It is characterized by a defect in the potassium channel alpha subunit leading to IKs reduction.9 These patients typically present with syncope or adrenergic-induced torsades, might have wide, broad-based T-waves on ECG, and respond well to beta-blocker therapy.6 Triggers for these patients include physical exertion or emotional stressors, particularly exercise and swimming. These patients typically present in early childhood.1
- Long QT2 occurs in 35% to 40% of patients and is characterized by a different defect in the alpha subunit of the potassium channel, which leads to reduced IKr.9 ECGs in LQTS2 can demonstrate low-amplitude and notched T-waves. Sudden catecholamine surges related to emotional stress or loud noises and bradycardia can trigger dysrhythmias in Long QT2.6 Thus, beta blockers reduce overall cardiac events in LQTS2 but less effectively than in LQTS1.6 These patients also present in childhood but typically are older than patients with LQTS1.6
- Long QT3 is less common than LQTS1 or LQTS2, at 2% to 8% of LQTS patients, but carries a higher mortality and is not treated effectively with beta blockers. LQTS3 is characterized by a defect in a sodium channel, causing a gain-of-function in the INa.4,9 These patients are more likely to have a fatal dysrhythmia while sleeping, are less susceptible to exercise-induced events, and have increased morbidity and mortality associated with bradycardia.4,9 ECG frequently reveals a relatively long ST segment, followed by a peaked and tall T-wave. Beta-blocker therapy can predispose to dysrhythmias in these patients; therefore, many of these patients will have pacemakers implanted as first-line therapy.6
While less common, Jervell and Lange Nielson syndrome is an autosomal recessive form of LQTS in which affected patients have homozygous mutations in the KCNQ1 or KCNE1 genes. This syndrome occurs in approximately 1% to 7% of LQTS patients, displays a typical QTc >550 ms, can be triggered by exercise and emotional stress, is associated with deafness, and carries a high risk of cardiac events at a young age.6
Acquired Syndromes
In addition to congenital LQTS, certain patients can acquire LQTS after being treated with particular drugs or having metabolic abnormalities, namely hypomagnesemia, hypocalcemia, and hypokalemia. Most experts think patients who “acquire” LQTS that predisposes to torsades de pointes have underlying structural heart disease or LQTS genetic carrier mutations that combine with transient initiating events (e.g., drugs or metabolic abnormalities) to induce dysrhythmias.1 In addition to certain drugs, cardiac ischemia, and electrolyte abnormalities, cocaine abuse, HIV, and subarachnoid hemorrhage can induce dysrhythmias in susceptible patients.5
Many types of drugs can cause a prolonged QT interval, and others should be avoided in patients with pre-existing prolonged QT (see Table 3, p. 17). Potentially offending drugs that are frequently encountered by inpatient physicians include amiodarone, diltiazem, erythromycin, clarithromycin, ciprofloxacin, fluoxetine, paroxetine, sertraline, haloperidol, ritonavir, and methadone.1 Additionally, drugs that cause electrolyte abnormalities (e.g., diuretics and lithium) should be monitored closely.
Overall, the goals of therapy in LQTS are:
- Decrease the risk of dysrhythmic events;
- Minimize adrenergic response;
- Shorten the QTc;
- Decrease the dispersion of refractoriness; and
- Improve the function of the ion channels.3
Supportive measures should be taken for patients who are acutely symptomatic from LQTS and associated torsades de pointes. In addition to immediate cardioversion for ongoing and hemodynamically significant torsades, intravenous magnesium should be administered, electrolytes corrected, and offending drugs discontinued.5 Temporary transvenous pacing at rates of approximately 100 beats per minute is highly effective in preventing short-term recurrence of torsades in congenital and acquired LQTS, especially in bradycardic patients.5 Isoproterenol infusion increases the heart rate and effectively prevents acute recurrence of torsades in patients with acquired LQTS, but it should be used with caution in patients with structural heart disease.5
Long-term strategies to manage LQTS include:
- Minimizing the risk of triggering cardiac events via adrenergic stimulation;
- Preventing ongoing dysrhythmias;
- Avoiding medications known to prolong the QT interval; and
- Maintaining normal electrolytes and minerals.5
Most patients with congenital long QT should be discouraged from participating in competitive sports, and patients should attempt to eliminate exposures to stress or sudden awakening, though this is not practical in all cases.5 Beta blockers generally are the first-line therapy and are more effective for LQT1 than LQT2 or LQT3.4,5 If patients are still symptomatic despite adequate medical therapy, or have survived cardiac arrest, they should be considered for ICD therapy.4,5 In addition, patients with profound bradycardia benefit from pacemaker implantation.5 Patients who remain symptomatic despite both beta blockade and ICD placement might find cervicothoracic sympathectomy curative.4,5
Perioperative Considerations
Although little data is available to guide physicians in the prevention of torsades de pointes during the course of anesthesia, there are a number of considerations that may reduce the chances of symptomatic dysrhythmias.
First, care should be taken to avoid dysrhythmia triggers in LQTS by providing a calm, quiet environment during induction, monitoring, and treating metabolic abnormalities, and providing an appropriate level of anesthesia.10 Beta-blocker therapy should be continued and potentially measured preoperatively by assessing heart rate response during stress testing.5 An implantable cardioverter-defibrillator (AICD) should be interrogated prior to surgery and inactivated during the operation.5
Finally, Kies et al have recommended general anesthesia with propofol for induction (or throughout), isoflurane as the volatile agent, vecuronium for muscle relaxation, and intravenous fentanyl for analgesia when possible.10
Back to the Case
While the patient had no genetic testing for LQTS, evaluation of previous ECGs demonstrated a prolonged QT interval. The hip fracture repair was considered an urgent procedure, which precluded the ability to undertake genetic testing and consideration for device implantation. The only medication that was known to increase the risk for dysrhythmias in this patient was his diuretic, with the attendant risk of electrolyte abnormalities.
Thus, the patient’s hydrochlorothiazide was discontinued and his pre-existing atenolol continued. The patient’s electrolytes and minerals were monitored closely, and magnesium was administered on the day of surgery. Anesthesia was made aware of the prolonged QT interval, such that they were able to minimize the risk for and anticipate the treatment of dysrhythmias. The patient tolerated the surgery and post-operative period without complication and was scheduled for an outpatient workup and management for his prolonged QT interval.
Bottom Line
Long QT syndrome is frequently genetic in origin, but it can be caused by certain medications and perturbations of electrolytes. Beta blockers are the first-line therapy for the majority of LQTS cases, along with discontinuation of drugs that might induce or aggravate the QT prolongation.
Patients who have had cardiac arrest or who remain symptomatic despite beta-blocker therapy should have an ICD implanted.
In the perioperative period, patients’ electrolytes should be monitored and kept within normal limits. If the patient is on a beta blocker, it should be continued, and the anesthesiologist should be made aware of the diagnosis so that the anesthethic plan can be optimized to prevent arrhythmic complications. TH
Dr. Kamali is a medical resident at the University of Colorado Denver. Dr. Stickrath is a hospitalist at the Veterans Affairs Medical Center in Denver and an instructor of medicine at UC Denver. Dr. Prochazka is director of ambulatory care at the Denver VA and professor of medicine at UC Denver. Dr. Varosy is director of cardiac electrophysiology at the Denver VA and assistant professor of medicine at UC Denver.
References
- Kao LW, Furbee BR. Drug-induced q-T prolongation. Med Clin North Am. 2005;89(6):1125-1144.
- Marchlinski F. Chapter 226, The Tachyarrhythmias; Harrison's Principles of Internal Medicine, 17e. Available at: www.accessmedicine.com/resourceTOC .aspx?resourceID=4. Accessed Nov. 21, 2009.
- Zareba W, Cygankiewicz I. Long QT syndrome and short QT syndrome. Prog Cardiovasc Dis. 2008; 51(3):264-278.
- Booker PD, Whyte SD, Ladusans EJ. Long QT syndrome and anaesthesia. Br J Anaesth. 2003;90(3):349-366.
- Khan IA. Long QT syndrome: diagnosis and management. Am Heart J. 2002;143(1):7-14.
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372(9640):750-763.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88(2):782-784.
- Kapa S, Tester DJ, Salisbury BA, et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752-1760.
- Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: a HuGE review. Genet Med. 2006;8(3):143-155.
- Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005;102(1):204-210.
- Wisely NA, Shipton EA. Long QT syndrome and anaesthesia. Eur J Anaesthesiol. 2002;19(12):853-859.
What is the best intervention to help hospitalized patients quit smoking?
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.
Case
A 56-year-old male with a 60-pack-a-year history of cigarette smoking is admitted to the telemetry unit with an initial assessment of acute coronary syndrome. Because there is a no-smoking policy in the hospital, he is willing to comply but is concerned about tobacco withdrawal symptoms.
Overview
As of 2006, approximately 20.8% of U.S. adults smoke cigarettes.1 Responsible for approximately 438,000 deaths annually, cigarette smoking is the most important preventable cause of death and disease in the U.S.2
Smoking cessation reduces the risk of tobacco-related diseases; the potential health benefits are numerous. This is most evident in the reduction of cardiovascular disease events upon tobacco abstinence.3 Yet, it remains a constant struggle for smokers to quit and stay abstinent.
The main barrier to quitting is nicotine addiction, which causes tolerance and physical dependence. Upon cessation of tobacco use, withdrawal symptoms, such as irritability, restlessness, impatience, and depression may occur within a few hours, peak within the first several days, and then wane during the next few months.
The crucial time frame to prevent relapse is the first week of cessation. For smokers to stay off cigarettes, they must break from routines, behaviors, or cues that trigger the urge to smoke.4
Among patients with acute myocardial infarction (AMI) in a study done by Van Spall, et al., 39% of them still smoked.5 Indeed, smoking is associated with 1.5 to three times increased relative risk of AMI, and hospitalists increasingly must manage cardiovascular disease patients’ tobacco dependence during their hospital stay.
Intervention strategies: Methods for smoking cessation need to target two aspects that support tobacco use—physical and psychological factors. High-intensity counseling and systematic behavioral intervention followed by sustained contact—in person or by phone up to one month after discharge—are effective behavioral interventions for sustained tobacco cessation.6 Pharmacotherapy also helps when added to high-intensity counseling of a hospitalized patient. It especially is beneficial for controlling withdrawal symptoms.
In addition, with policies prohibiting smoking in almost all U.S. hospitals, temporary tobacco abstinence promotes smoking cessation for hospitalized patients. Unfortunately, most hospitalized patients go back to smoking soon after discharge. Hospitalization may be the opportune time to help patients try to quit and avoid relapse.
Some hospitals feature inpatient smoking cessation programs in which nurse practitioners and counselors educate and counsel patients. It is highly recommended that a multidisciplinary team be involved in a tobacco cessation program catered to an individual patient’s needs. However, most hospitals have no such program. Nevertheless, the hospitalist can help a patient with brief or low-intensity tobacco cessation counseling, pharmacotherapy for nicotine withdrawal symptom control if clinically indicated, and follow-up upon discharge for relapse prevention.
Counseling: Smoking cessation counseling in the hospital after an AMI has been found to be associated with a relative risk reduction of mortality by 37% in one year. The hospitalist should give a two-minute cessation message as the first step. If tobacco cessation counselors or nurse practitioners are available, their additional counseling also may improve outcomes of smoking cessation therapies.7 However, if no established inpatient tobacco cessation program is available to the hospitalist, the following may be used to aid in physician counseling of the hospitalized cardiac patient:
The first step in treating tobacco dependence is to identify and assess tobacco use status.
Tobacco users willing to quit should be treated using the 5 A’s (Ask, Advise, Assess, Assist, and Arrange) (see Figure 1, p. 30). Tobacco users not willing to quit at the time of interaction should be treated using the 5 R’s for motivational intervention:
- Relevance (indicate why quitting is personally relevant);
- Risks (have patient identify potentially negative consequences of smoking);
- Rewards (have patient identify potential benefits of quitting smoking);
- Roadblocks (have patient identify potential barriers to smoking cessation and provide patient problem-solving techniques and pharmacotherapy to overcome the barriers); and
- Repetition (repeat motivational intervention to unmotivated patient each visit).
Further, former smokers who recently quit using tobacco should be given relapse prevention treatment.8 For the hospitalized smoker with acute cardiovascular disease, providing bedside counseling, enhancing self-coping behavior change, and arranging follow-up after discharge to maintain behavior change can help sustain tobacco abstinence.
Pharmacotherapy: The most important purpose of pharmacotherapy for smoking cessation is to reduce withdrawal symptoms and cigarette cravings. Public Health Service clinical guidelines for smoking cessation mention five first-line agents. These are sustained-release bupropion and four nicotine-replacement therapies (NRT): transdermal patch, gum, nasal spray, and vapor inhaler. Further, there are two second-line agents: clonidine and nortriptyline. Since the clinical guidelines’ release in 2000, the Food and Drug Administration has approved a fifth NRT product, the nicotine lozenge, in 2002, and a partial nicotine agonist, varenicline, in 2006 (see Table 1, right).9,10
Current guidelines recommend that NRT be used with caution in patients with unstable angina, serious arrhythmias, or an MI within the previous two weeks due to limited supportive data on the safety of use in these patients.11 The transdermal patch delivers nicotine at a slow and constant rate in contrast to the other forms of NRT and has been used safely in patients with stable coronary artery disease. However, the use of any NRT, including the patch, in acute cardiovascular disease is not advised due to the nicotine-mediated hemodynamic effects, such as increase heart rate and arterial vasoconstriction, which lead to increased myocardial workload.
Sustained-release bupropion generally is well tolerated by hospitalized patients with cardiovascular disease, but there may be a delay in control of withdrawal symptoms. In addition, blood pressure must be monitored especially if combined with NRT as there have been anecdotal reports of increase in blood pressure with bupropion alone.12 Bupropion must be used cautiously in patients with recent MI. Other contraindications include history of seizure, conditions that potentially can increase risk for convulsions, and use of monoamine oxidase inhibitors (MAOI) within 14 days.
The new drug varenicline has not been studied in hospitalized patients or patients with acute coronary syndrome. However, since it does not have any important hemodynamic effects, it may be useful in this setting and in selected patients with close monitoring for mood changes since there have been anecdotal case reports of psychotic events in patients with underlying psychiatric disorders.13 Its routine use currently is not recommended.
Follow-up after discharge: Pharmacotherapy may be added for withdrawal control, as well as relapse prevention for the hospitalized patient who recently quit smoking. However, inclusion of intensive tobacco cessation counseling during the hospital stay is the most effective intervention given the setting and patient condition, and follow-up support up to at least one month after discharge has been found to be more effective in sustaining tobacco abstinence than pharmacotherapy alone. In order to maximize long-term quit rates among patients who recently abstained from smoking, the hospitalist should arrange access to ongoing outpatient post-discharge support and tobacco cessation treatment.
Back to the Case
After appropriate cardiac testing, the patient was found to have a non-cardiac etiology for his symptoms. From the start of his hospital stay, he was counseled by the hospitalist and started on sustained-release bupropion, but withdrawal symptoms and cravings persisted.
Prior to his discharge home, the patient wanted to discontinue bupropion and be provided an alternative. The patient was given a nicotine patch, and a follow-up appointment at the tobacco cessation clinic within one week of discharge from the hospital was arranged. The patient has been compliant with his quit-smoking treatment and has followed-up for continued tobacco cessation counseling. He hasn’t smoked cigarettes for a year. TH
Dr. Palisoc is a preventive medicine resident at the University of Colorado Denver. Dr. Prochazka works at the Denver VA and is a professor of medicine at the University of Colorado Denver.
References
- CDC. Cigarette Smoking Among Adults, United States, 2006. MMWR Morbidity Mortality Wkly Rep. 2007;56(44):1157-1161. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. Last accessed March 4, 2008.
- CDC. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity, United States, 1997-2001. MMWR Morbidity Mortality Wkly Rep. 2005;54(25):625-628.
- Thomson CC, Rigotti NA. Hospital- and clinic-based smoking cessation interventions for smokers with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):459-479.
- Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346(7):506-512.
- Van Spall HGC, Chong A, Tu JV. Inpatient smoking-cessation counseling and all-cause mortality in patients with acute myocardial infarction. Am Heart J. 2007;154(2):213-220.
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalized patients. Cochrane Database Syst Rev. 2007;Issue 3.
- Ludvig J, Miner B, and Eisenberg, MJ. Smoking cessation in patients with coronary artery disease. Am Heart J. 2005;149(4):565-572.
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service 2000.
- Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;199:1080-1087.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis. 2003;45(6):429-441.
- FDA. Prescribing information of Zyban (bupropion hydrochloride) sustained release tablets. June 2007. Available at www.fda.gov/medwatch/safety/2007/ Aug_PI/Zyban_PI.pdf. Last accessed March 6, 2008.
- FDA. Early Communication About an Ongoing Safety Review: Varenicline (marketed as Chantix). November 2007. Available at www.fda.gov/cder/drug/early_comm/varenicline.htm. Last accessed March 6, 2008.