User login
Valgus Extension Overload in Baseball Players
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
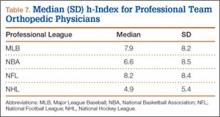
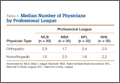
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
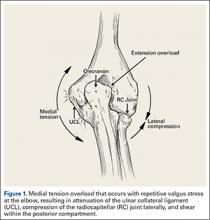
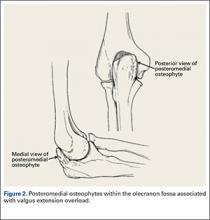
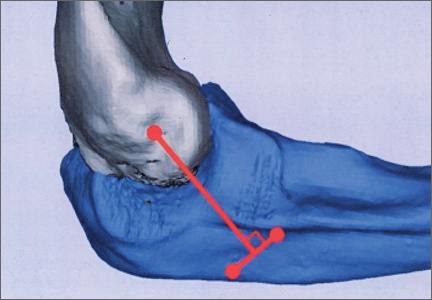
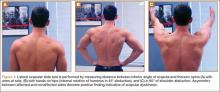
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
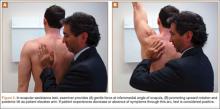
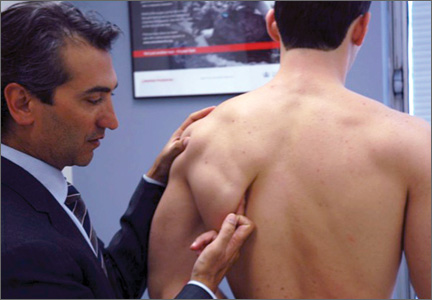
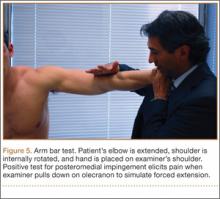
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
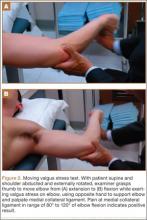
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
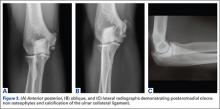
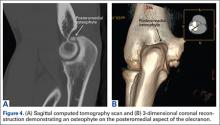
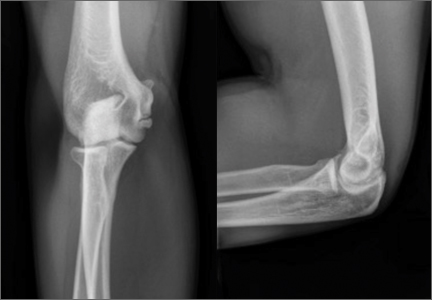
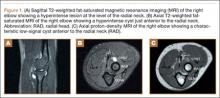
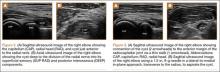
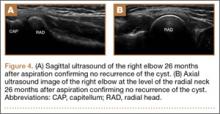
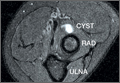
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
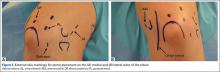
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
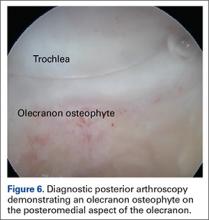
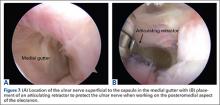
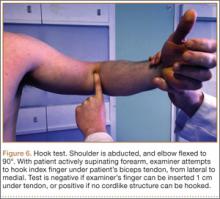
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
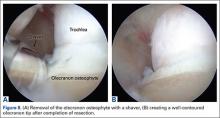
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
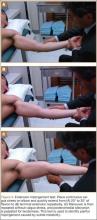
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
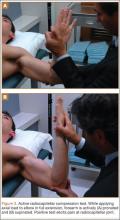
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
The supraphysiological demands imposed on the elbow of a throwing athlete result in predictable patterns of injury. This is especially true of baseball pitchers. Knowledge of elbow anatomy, as well as the biomechanics of throwing, assist in making diagnostic and therapeutic decisions and also influence surgical technique when surgery is required. During the late cocking and early acceleration phases of throwing, valgus torque can reach 65 Nm with angular velocities of the forearm reaching 5000°/sec, which is considered the fasted recorded human movment.1 The valgus torque and rapid extension synergistically create 3 major forces placed on the elbow. The first is a tensile stress along the medial aspect of elbow affecting the ulnar collateral ligament (UCL), flexor pronator mass, and medial epicondyle. Secondly, compression forces affect the lateral aspect of the elbow at the radiocapitellar joint. Finally, a shearing stress occurs in the posterior compartment at the posterior medial tip of the olecranon and the olecranon fossa.
These forces generated on the elbow result in predictable pathology. The recurring tensile forces applied on the medial aspect on the elbow can compromise the integrity of the UCL. It is well known that injury to the UCL leads to valgus instability. Individuals with valgus instability who continue to throw may trigger and/or aggravate injury in the posterior and lateral components of the elbow. Lateral compression forces can often reach 500 N, resulting in radiocapitellar overload syndrome, which occurs in combination with medial ligament instability and valgus extension overload.2 Radiocapitellar compression may cause chondral or osteochondral fracture with resulting intra-articular loose bodes. This compression also contributes to the etiology of osteochondritis dissecans (OCD) in skeletally immature athletes. In the posterior elbow, throwing forcefully and repeatedly pushes the olecranon into the olecranon fossa. Shear stress on the medial olecranon tip and fossa, due to combined valgus and extension forces, lead to the development of osteophytes. This collection of injuries in the medial, lateral, and posterior aspects of the elbow is known as “valgus extension overload syndrome” or VEO. Symptoms in VEO can be the result of chondral lesions, loose bodies, and marginal exostosis.3
The aim of this review is to provide understanding regarding both the relevant anatomy and pathomechanics of VEO, key aspects to clinical evaluation, and effective treatment options.
Functional Anatomy
A functional comprehension of elbow anatomy and biomechanics is essential to understanding the constellation of injuries in VEO. The osseous anatomy of the elbow permits a variety of movements. These include flexion-extension and pronation-supination, which are mediated by the ulnohumeral and radiocapitellar articulations. While in full extension, the elbow has a normal valgus carrying angle of 11° to 16°. It is important to know that 50% of the elbow’s stability is attributed to the configuration of the bones.4-6 This is especially true in varus stress while the elbow is in full extension. The soft tissues, including muscle and ligaments such as the UCL, lateral UCL, and radial UCL complexes, provide the remaining elbow stability.4-6
The UCL complex is composed of 3 main segments known as the anterior, posterior, and oblique bundles (transverse ligament). Collectively, these bundles are responsible for providing medial elbow stability. However, each of these bundles contributes to medial elbow stability in its own way. The first and arguably the most important bundle is the anterior bundle; its most important function is providing stability against valgus stress.4,5,7 It is composed of parallel fibers inserting on the medial coronoid process.4,5,7 Furthermore, its eccentric location with respect to the axis of elbow allows it to provide stability throughout the full range of elbow motion.6 The anterior bundle can be further divided into individual anterior and posterior bands that have reciprocal functionality.5,8,9 The anterior band acts as the chief restraint to valgus stress up to 90° of flexion.9 Any flexion beyond 90° renders the anterior band’s role secondary in resisting valgus stress.9 The posterior band’s function in resisting valgus stress is most important between 60° and full flexion, while having a secondary role in lesser degrees of flexion.8,9 Notably, the posterior band is isometric and is more important in the overhead-throwing athlete due to the fact its primary role in resisting valgus stress occurs at higher degrees of flexion.10
The remaining posterior and oblique bundles of the UCL complex have lesser roles in maintaining elbow stability. The posterior bundle of the UCL complex is fan-shaped, originates from the medial epicondyle, and inserts onto the medial margin the semi-lunar notch. It is more slender and frailer than the anterior bundle. This is reflected in its functionality, as it plays a secondary role in elbow stability during elbow flexion beyond 90°.4,5,8 In contrast to the anterior and posterior bundles, the oblique bundle, also known as the transverse ligament, does not cross the elbow joint. It is a thickening of the caudal most aspect of the joint capsule, which extends from the medial olecranon to the inferior medial coronoid process and as a result functions in expanding the greater sigmoid notch.6
The musculotendinous components of the elbow are essential to providing dynamic functional resistance to valgus stress.11 These components are flexor-pronator musculature that originate from the medial epicondyle. Listed proximally to distally, the flexor-pronator muscles include pronator teres, flexor carpi radialis (FCR), palmaris longus, flexor digitorum superficialis, and the flexor carpi ulnaris (FCU).
Pathomechanics
Once familiarized with the relevant function anatomy, it is crucial to understand the mechanics of throwing in order to understand the pathomechanics of VEO. The action of overhead throwing has been divided into 6 phases.6,12-16 Phase 4, acceleration, is the most relevant when discussing forces on elbow, since the majority of forces are generated during this state. Phase 4 represents a rapid acceleration of the upper extremity with a large forward-directed force on the arm generated by the shoulder muscles. Additionally, there is internal rotation and adduction of the humerus with rapid elbow extension terminating with ball release. The elbow accelerates up to 600,000°/sec2 in a miniscule time frame of 40 to 50 milliseconds.1,5 Immense valgus forces are exerted on the medial aspect of the elbow. The anterior bundle of the UCL bears the majority of the force, with the flexor pronator mass enabling the transmission.11 The majority of injuries occur during stage 4 as a result of the stress load on the medial elbow structures like the UCL. The proceeding phases 5 (deceleration), and 6 (follow-through) involve eventual dissipation of excess kinetic energy as the elbow completely extends. The deceleration during phase 5 is rapid and powerful, occurring at about 500,000°/sec2 in the short span of 50 milliseconds.1,6,12-16 High-velocity throwing, such as baseball pitching, generates forces in the elbow that are opposed by the articular, ligamentous, and muscular portions of the arm. The ulnohumeral articulation stabilizes motion of the arm from 0° to 20° of flexion and beyond 120° of flexion. Static and dynamic soft tissues maintain stability during the remaining of 100° arc of motion.
During deceleration, the elbow undergoes terminal extension resulting in the posteromedial olecranon contacting the trochlea and the olecranon fossa with subsequent dissipation of the combined valgus force and angular moment (Figure 1). This dissipation of force creates pathologic shear and compressive forces in the posterior elbow. Poor muscular control and the traumatic abutment that occurs in the posterior compartment may further add to the pathologic forces. Reactive bone formation is induced by the repetitive compression and shear, resulting in osteophytes on the posteromedial tip of the olecranon (Figure 2). Consequent “kissing lesions” of chondromalacia may occur in the olecranon fossa and posteromedial trochlea. The subsequent development of loose bodies may also occur. The presence of osteophytes and/or loose bodies may result in posteromedial impingement (PMI).
The association between PMI of the olecranon and valgus instability has been elucidated in both clinical and biomechanical investigations.17,18,19,20 Conway18 identified tip exostosis in 24% of lateral radiographs of 135 asymptomatic professional pitchers. Approximately one-fifth (21%) of these pitchers had >1.0 mm increased relative valgus laxity on stress radiographs. Roughly one-third (34%) of players with exostosis had >1.0 mm of increased relative valgus laxity, compared to 16% of players without exostosis formation. These results provide evidence for a probable association between PMI and valgus laxity. In biomechanical research, Ahmad and colleagues17 studied the effect of partial and full thickness UCL injuries on contact forces of the posterior elbow. Posteromedial compartments of cadaver specimens were subjected to physiologic valgus stresses while placed on pressure-senstive film. Contact area and pressure between posteromedial trochlea and olecranon were altered in the setting of UCL insufficiency, helping explain how posteromedial osteophyte formation occurs.
Additional biomechanical studies have also investigated the posteromedial olecranon’s role in functioning as a stabilizing buttress to medial tensile forces. Treating PMI with aggressive bone removal may increase valgus instability as well as strain on the UCL, leading to UCL injury following olecranon resection.19,20 Kamineni and colleagues19 investigated strain on anterior bundle of UCL as a function of increasing applied torque and posteromedial resections of the olecranon. This investigation was done utilizing an electromagnetic tracking placed in cadaver elbows. A nonuniform change in strain was found at 3 mm of resection during flexion and valgus testing. This nonuniform change implied that removal of posteromedial olecranon beyond 3 mm made the UCL more vulnerable to injury. Follow-up investigations looked at kinematic effects of increasing valgus and varus torques and sequential posteromedial olecranon resections.20 Valgus angulation of the elbow increased with all resection levels but no critical amount of olecranon resection was identified. The consensus in the literature indicates that posteromedial articulation of the elbow is a significant stabilizer to valgus stress.17-22 Thus, normal bone should be preserved and only osteophytes should be removed during treatment.
In addition, VEO may lead to injury in the lateral compartment as well. After attenuation and insufficiency of the UCL due to repetitive stress, excessive force transmission to the lateral aspect of the elbow occurs. Compressive and rotatory forces escalate within the radiocapitellar joint, causing synovitis and osteochondral lesions.3,23 These osteochondral lesions include osteochondritis dissecans and osteochondral fractures that may fragment and become loose bodies.
Evaluation of VEO
History
Patients will typically have a history of repetitive throwing or other repetitive overhead activity. VEO is most common in baseball pitchers but may also occur in other sports, such as tennis, football, lacrosse, gymnastics, and javelin throwing. In baseball pitchers, clinical presentation is often preceded by a decrease in pitch velocity, control, and early fatigability. It presents with elbow pain localized to the posteromedial aspect of olecranon after release of the ball, when the elbow reaches terminal extension. Patients also report limited extension, due to impinging posterior osteophytes. Also, locking and catching caused by loose bodies and chondromalacia may be present. VEO may also occur in combination with concomitant valgus instability, as well as in a patient with a prior history of valgus instability. Flexor pronator injury, ulnar neuritis, and subluxation may also be present in a patient with VEO.
Physical Examination
VEO may occur in an isolated fashion or with concomitant pathology. Therefore, a comprehensive physical examination includes evaluating the entire kinetic chain of throwing and a focused examination covering VEO and associated valgus instability. Patients may exhibit crepitus and tenderness over the posteromedial olecranon and a loss of extension with a firm end point. The extension impingement test should be performed where the elbow is snapped into terminal extension. This typically elicits pain in the posterior compartment in a patient with VEO. The arm bar test involves positioning the patient’s shoulder at 90° of forward flexion, full internal rotation, with the patient’s hand placed on the examiner’s shoulder.24 The examiner pulls down on the olecranon, simulating forced extension; pain is indicative of a positive test. It is important to note if there are signs of ulnar neuritis or subluxing ulnar nerve, especially if planning to utilize medial portals during arthroscopic treatment.
Examination maneuvers for valgus instability should also be conducted during evaluation of VEO. The physical examination for valgus instability in the elbow is ideally performed with the patient seated. Secure the patient’s wrist between the examiner’s forearm and trunk, and flex the patient’s elbow between 20° and 30° to unlock the olecranon from its fossa. Proceed to apply valgus stress. This stresses the anterior band of the anterior bundle of the UCL.6,25,26 Palpate the UCL from the medial epicondyle to the proximal ulna as valgus stress is applied. Occasionally, valgus laxity can be appreciated when compared to contralateral side. The milking maneuver is a helpful test to determine UCL injury. Pull on the patient’s thumb while the forearm is supinated, shoulder extended, and the elbow flexed beyond 90°.6 The milking maneuver exerts valgus stress on a flexed elbow. A patient with an injured UCL will experience the subjective feeling of apprehension and instability, with medial elbow pain.
The most sensitive test is the moving valgus stress test. This is performed with the patient in the upright position and the shoulder abducted 90°. Starting with the arm in full flexion, the examiner applies a constant valgus torque to the elbow and then rapidly extends the elbow. Reproduction of pain during range of motion from 120° to 70° represents UCL injury, while pain with extension beyond 70° represents chondral injury to the ulnohumeral joint. Be aware that the absence of increased pain with wrist flexion, along with pain localized slightly posterior to the common flexor origin, differentiates a UCL injury from flexor-pronator muscle injury.6,26,27Examine range of motion in affected and unaffected elbows. Loss of terminal extension may be present, along with secondary to flexion contracture due to repeated attempts at healing and stabilization.25
Imaging
Imaging is essential to the accurate diagnosis of VEO and related conditions. Anterior posterior (AP), lateral, and oblique radiographs of elbow (Figures 3A-3C) may show posteromedial olecranon osteophytes and/or loose bodies. Calcification of ligaments or other soft tissues may also be seen. An AP radiograph with 140° of external rotation may best visualize osteophytes on posteromedial olecranon.18 A computed tomography scan with 2-dimensional sagittal and coronal reconstruction and 3-dimensional surface rendering (Figures 4A, 4B) may best demonstrate morphological abnormalities, loose bodies, and osteophytes. Magnetic resonance imaging (MRI) is essential for assessment of soft tissues and chondral injuries. MRI may detect UCL compromise, synovial plicae, bone edema, olecranon, or stress fractures.
Treatment
Nonoperative Treatment
Treatment consists of both nonoperative and operative modalities. Nonoperative treatment methods are first line in treating VEO. Patients should modify their physical activity and rest from throwing activities. Nonsteroid anti-inflammatory drugs are appropriate to treat pain along with intra-articular corticosteroid injections of the elbow. A wide assessment of pitching mechanics should be performed in an attempt to correct errors in throwing technique and address muscular imbalances. After cessation of the resting period, the patient may initiate a progressive throwing program supervised by an experienced therapist and trainer. A plan for returning to competition should be made upon completion of the throwing program.
Operative Treatment
Surgical treatment is reserved for patients who fail nonoperative treatment. These patients have persistent symptoms of posteromedial impingement and desire to return to pre-injury level of performance. Posteromedial decompression is not recommended when provocative physical examination maneuvers are negative, regardless of presence of olecranon osteophytes on imaging. Osteophytes are an asymptomatic finding typically seen in professional baseball players and do not warrant surgical treatment.18,28 UCL compromise is a relative contraindication to olecranon debridement as UCL injury could become symptomatic following surgery. Surgical options in the appropriate patient to decompress posterior compartment include arthroscopic olecranon debridement or limited incision arthrotomy. Excessive resection of posteromedial osteophytes must be avoided. Arthroscopy has limited morbidity and allows for complete diagnostic assessment. UCL reconstruction should also be considered in combination with posteromedial debridement when the UCL is torn. More challenging indications for UCL reconstruction occur when the UCL is partially torn or torn and asymptomatic. Isolated posteromedial decompression in this setting risks future development of UCL symptoms that would then need to be addressed.
Surgical Technique
As previously mentioned, elbow arthroscopy or limited excision arthrotomy are the preferred operative methods for decompression of the posterior compartment and thus treatment of VEO. Anesthesia and patient positioning should be selected based on the surgeon’s preference. The patient should be positioned supine, prone, or in lateral decubitis. When a UCL reconstruction is expected, supine position is advantageous to avoid repositioning after completing the arthroscopic portion of the procedure. However, arthroscopy can be performed in the lateral position with subsequent repositioning, repeat prepping, and draping for UCL reconstruction (Figures 5A, 5B).
Prepare for elbow arthroscopy by distending the elbow joint with normal saline to aid in protection of neurovascular structures and simplify the insertion of the scope trocar. Perform diagnostic anterior arthroscopy via the proximal anteromedial portal. Assess for presence of loose bodies and osteochondral lesions of the radiocapitellar joint, as well as osteophytes of the coronoid tip and fossa. Utilizing a spinal needle under direct visualization establish a proximal lateral portal with adequate view of the anterior compartment. Proceed to visualize the medial compartment and assess for UCL injury. Apply valgus stress while in 70° of flexion. Visualize the coronoid process and look for medial trochlea gapping of 3 mm or greater, which indicates UCL insufficiency.30
Establish the posterolateral port for visualization of the posterior compartment. A posterior portal is established through the triceps tendon. Proceed to shave and ablate synovitis in order to create an adequate working space. Inspect the posteromedial olecranon, looking for any osteophytes or chondromalacia in the area (Figure 6). Examine the posterior radiocapitellar joint, looking specifically for loose bodies. The presence of loose bodies may require creating an extra mid lateral portal for removal. The ulnar nerve is located superficial to the elbow capsule and can be damaged by instruments utilized in the posteromedial gutter. As a precaution, be sure to remove suction attached to shaver. Place a curved articulating retractor in an accessory posterolateral portal to assist in protecting the ulnar nerve by retracting the capsule away from the surgical field (Figures 7A, 7B).
The osteophyte may be encased in soft tissue. Using a combination of ablation devices and shavers, the osteophyte can be exposed. The olecranon osteophyte can be removed with a small osteotome located at the border of the osteophyte and the normal olecranon. A motorized shaver or burr may also be introduced through the direct posterior portal or the posterolateral portal to complete the contouring of the olecranon (Figures 8A, 8B). Intraoperative lateral radiographs may be obtained for guidance in adequate bone removal and to ensure no bone debris is left in the soft tissues. It is critical that only pathologic osteophyte is removed and that normal olecranon is not compromised. This prevents an increase in UCL strain during valgus loading.19 However, in some non-throwing athletes, more aggressive debridement can be performed due to a smaller risk of UCL injury after posterior decompression.
Often, with the presence of osteophytes on the olecranon, there may be associated chondromalacia of the trochlea. These kissing lesions must be addressed after debridement of osteophytes. Loose flaps or frayed edges are carefully debrided and for any significant lesion the edges are contoured to a stable rim using shavers and curettes. Once altered to a well-shouldered lesion, microfracture is performed. Anterograde drilling of the lesion with perforations separated by 2 to 3 mm allow for the release of marrow elements and induction of a fibrocartilage healing response.
For an isolated posteromedial decompression, early rehabilitation begins with simple elbow flexion and extension exercises. It is important to restore flexor-pronator strength. Six weeks postoperatively, a progressive throwing program that includes plyometric exercises, neuromuscular training, and endurance exercises can be initiated. Patients can typically return to competition 3 to 4 months after surgery, if they have successfully regained preoperative range of motion, preoperative strength in the elbow, and there is no pain or tenderness on stress testing or palpation.
Outcomes
Safety and Advances in Arthroscopy
A clearer understanding of portal placement and proximity to neurovasculature in conjunction with advances in equipment have allowed for continual improvements in elbow arthroscopy techniques. There is plenty of literature indicating that arthroscopic posteromedial decompression is a safe, reliable, effective procedure, with a high rate of patient satisfaction.22,30-34] Andrews and Carson30 published one of the earliest investigations indicating the effectiveness of elbow arthroscopy utilizing objective and subjective outcome scores. They found that preoperative scores indicating patient satisfaction increased from 50% to 83%. Patients who underwent only loose body removal had the best outcomes. Andrews and Timmerman31 later evaluated the results of 72 professional baseball players who underwent either arthroscopic or open elbow surgery. They found that posteromedial olecranon osteophytes and intraarticular loose bodies were the most common diagnoses, present in 65% and 54% of players, respectively. In addition, a 41% reoperation rate was reported after posteromedial olecranon resection, along with 25% a rate of valgus instability necessitating UCL reconstruction. Andrews and Timmerman31 propose that the incidence of UCL injuries is underestimated and that UCL pathology must be treated prior to treating its secondary effects. Recently, Reddy and colleagues32 reviewed the results of 187 arthroscopic procedures. Posterior impingement, loose bodies, and osteoarthritis were the most common problems, occurring in 51%, 31%, and 22% of patients, respectively. Reported results were encouraging, with 87% good to excellent results and 85% of baseball players returning to preinjury levels.
Conclusion
An understanding of the relevant functional anatomy and the biomechanics of throwing is essential to understanding VEO. Potential concomitant valgus instability and UCL injury must be carefully assessed. Only symptomatic patients who have failed conservative treatment should undergo surgery. It is critical to avoid exacerbating and/or causing valgus instability by surgical excessively removing normal bone from the olecranon. Arthroscopy has been shown to be a safe and effective method to treat refractory cases of VEO.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
1. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
2. Fleisig GS, Barrentine SW, Escamilla RF, Andrews JR. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21(6):421-437.
3. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
4. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986,35:59-68.
5. Schwab GH, Bennett JB, Woods GW, Tullos HS. Biomechanics of elbow instability: the role of medial collateral ligament. Clin Orthop Relat Res. 1980;146:42-52.
6. Jobe FW, Kvitne RS. Elbow instability in the athlete. Instr Course Lect. 1991;40:17-23.
7. Søjbjerg JO, Ovesen J, Nielsen S. Experimental elbow instability after transection of the medial collateral ligament. Clin Orthop Relat Res. 1987;218:186-190.
8. Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop Relat Res. 1991;271:170-179.
9. Callaway GH, Field LD, Deng XH, et al. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79(8):1223-1231.
10. Chen FS, Rokito AS, Jobe FW. Medial elbow problems in the overhead-throwing athlete. J Am Acad Orthop Surg. 2001;9(2):99-113.
11. Davidson PA, Pink M, Perry J, Jobe FW. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am J Sports Med. 1995;23(2):245-250.
12. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
13. Hamilton CD, Glousman RE, Jobe FW, Brault J, Pink M, Perry J. Dynamic stability of the elbow: electromyographic analysis of the flexor pronator group and the extensor group in pitchers with valgus instability. J Shoulder Elbow Surg. 1996;5(5):347-354.
14. Glousman RE, Barron J, Jobe FW, Perry J, Pink M. An electromyographic analysis of the elbow in normal and injured pitchers with medial collateral ligament insufficiency. Am J Sports Med. 1992;20(3):311-317.
15. DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1(1):15-25.
16. Sisto DJ, Jobe FW, Moynes DR, Antonelli DJ. An electromyographic analysis of the elbow in pitching. Am J Sports Med. 1987;15(3):260-263.
17. Ahmad CS, Park MC, Elattrache NS. Elbow medial ulnar collateral ligament insufficiency alters posteromedial olecranon contact. Am J Sports Med. 2004;32(7):1607–1612.
18. Ahmad CS, Conway J. Elbow arthroscopy: beginners to advanced: valgus extension overload. In: Egol, ed. Instructional Course Lectures; vol 60. Rosemont, IL: American Academy of Orthopaedic Surgeons; submitted 2009.
19. Kamineni S, ElAttrache NS, O’Driscoll S W, et al. Medial collateral ligament strain with partial posteromedial olecranon resection. A biomechanical study. J Bone Joint Surg Am. 2004;86-A(11):2424–2430.
20. Kamineni S, Hirahara H, Pomianowski S, et al. Partial posteromedial olecranon resection: a kinematic study. J Bone Joint Surg Am. 2003;85-A(6):1005–1011.
21. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315–319.
22. O’Driscoll SW, Morrey BF. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74(1):84–94.
23. Miller CD, Savoie FH 3rd. Valgus extension injuries of the elbow in the throwing athlete. J Am Acad Orthop Surg. 1994;2(5):261-269.
24. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
25. Boatright JR, D’Alessandro DF. Nerve entrapment syndromes at the elbow. In Jobe FW, Pink MM, Glousman RE, Kvitne RE, Zemel NP, eds. Operative Techniques in Upper Extremity Sports Injuries. St. Louis, MO: Mosby-Year Book; 1996:518-537.
26. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
27. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
28. Kooima CL, Anderson K, Craig JV, Teeter DM, van Holsbeeck M. Evidence of subclinical medial collateral ligament injury and posteromedial impingement in professional baseball players. Am J Sports Med. 2004;32(7):1602-1606.
29. Field LD, Altchek DW. Evaluation of the arthroscopic valgus instability test of the elbow. Am J Sports Med. 1996;24(2):177–181.
30. Andrews JR, Carson WG. Arthroscopy of the elbow. Arthroscopy. 1985;1(2):97-107.
31. Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23(4):407-413.
32. Reddy AS, Kvitne RE, Yocum LA, Elattrache NS, Glousman RE, Jobe FW. Arthroscopy of the elbow: a long-term clinical review. Arthroscopy. 2000;16(6):588-594.
33. Rosenwasser MP, Steinmann S. Elbow arthroscopy in the treatment of posterior olecranon impingement. Paper present at: AANA Annual Meeting; 1991; San Diego, CA.
34. Wilson FD, Andrews JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11(2):83-88.
Lateral Ulnar Collateral Ligament Reconstruction: An Analysis of Ulnar Tunnel Locations
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Academic Characteristics of Orthopedic Team Physicians Affiliated With High School, Collegiate, and Professional Teams
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
Shoulder Examination of the Overhead Athlete
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
Supinator Cyst in a Young Female Softball Player Successfully Treated With Aspiration
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Google Glass Distal Biceps Repair
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
GoPro Hero 3 Latarjet Procedure
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Using Wearable Technology to Record Surgical Videos
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Sports Activity After Reverse Total Shoulder Arthroplasty With Minimum 2-Year Follow-Up
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
Physical Examination of the Throwing Athlete’s Elbow
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.