User login
Revisiting our approach to behavioral health referrals
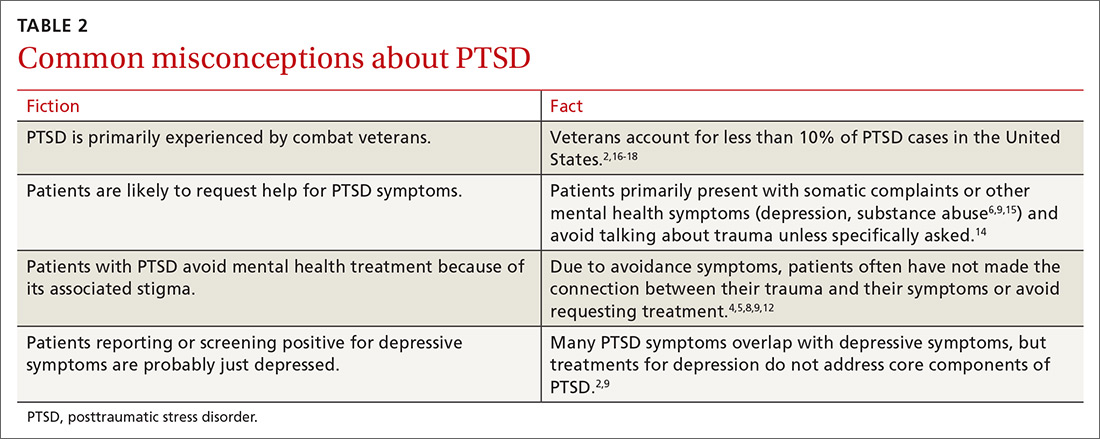
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.
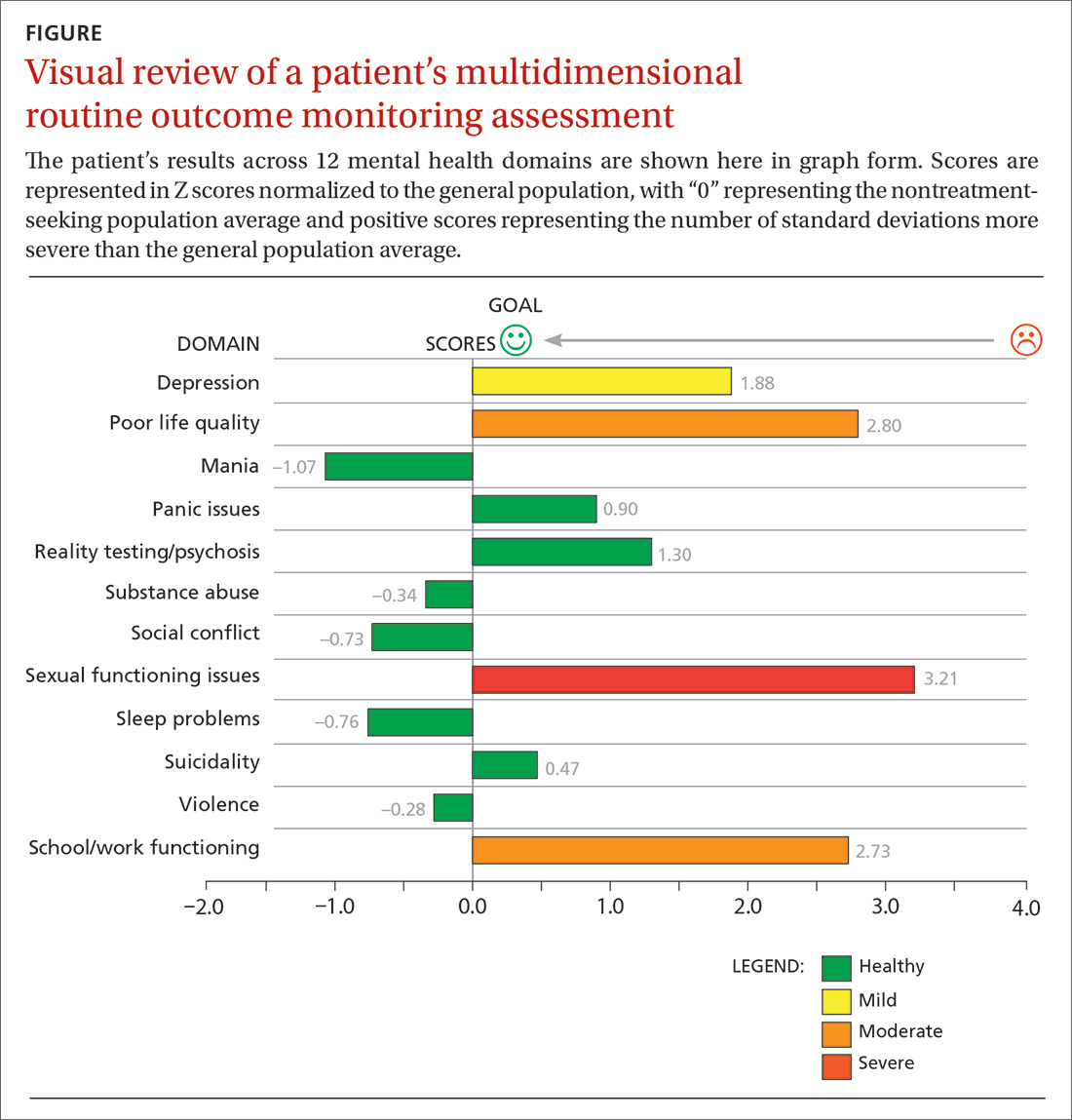
Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.

Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.

Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
Dyspareunia: Keys to biopsychosocial evaluation and treatment planning
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7
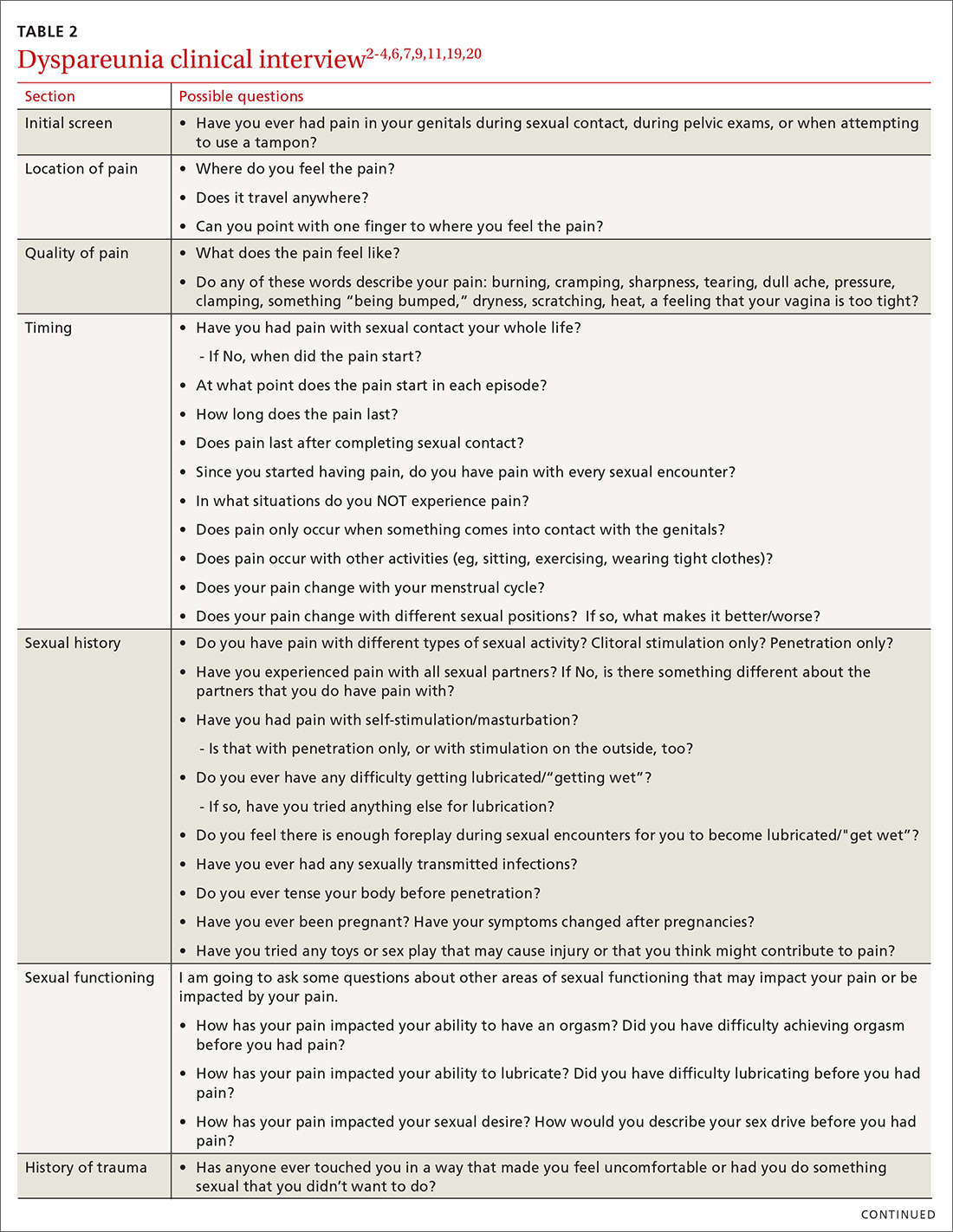
Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
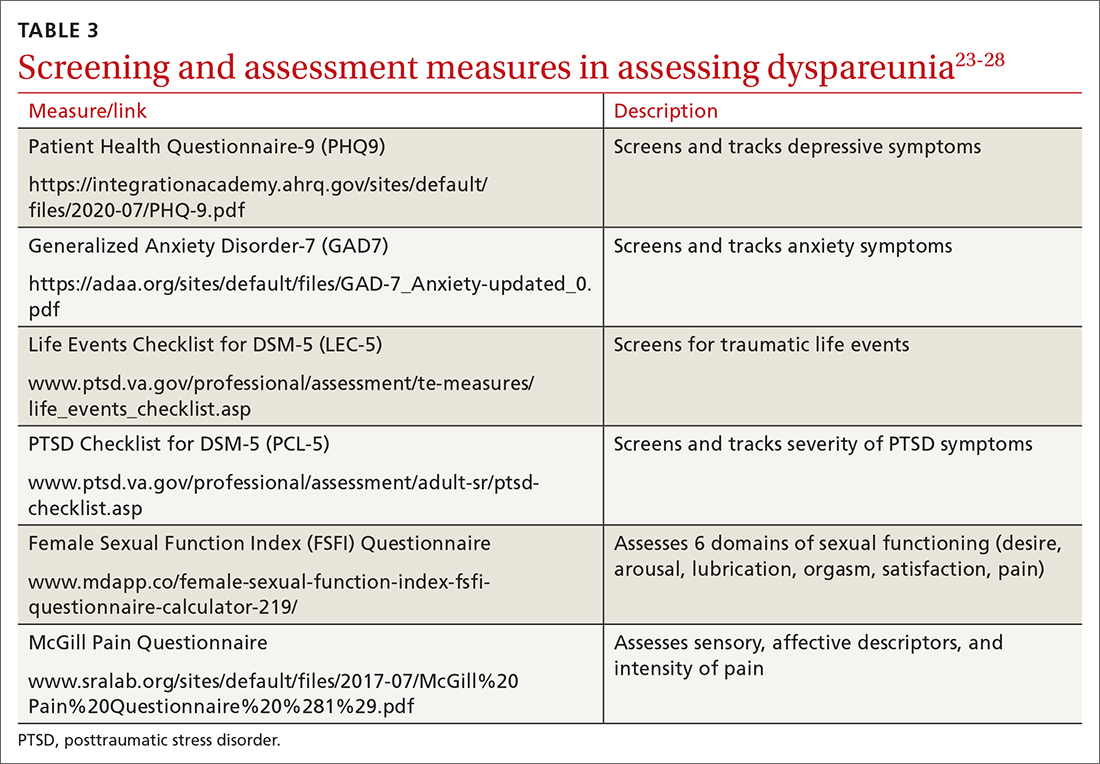
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3
Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
PRACTICE RECOMMENDATIONS
› Screen all patients for sexual dysfunctions, as patients often do not report symptoms on their own. B
› Refer patients with dyspareunia for psychotherapy to address both pain and psychosocial causes and sequela of dyspareunia. A
› Refer patients with dyspareunia for pelvic floor physical therapy to address pain and sexual functioning. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Posttraumatic stress disorder: Often missed in primary care
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.