User login
School avoidance: How to help when a child refuses to go
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; meredith.williamson@tamu.edu
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; meredith.williamson@tamu.edu
THE CASE
Juana*, a 10-year-old who identifies as a cisgender, Hispanic female, was referred to our integrated behavioral health program by her primary care physician. Her mother was concerned because Juana had been refusing to attend school due to complaints of gastrointestinal upset. This concern began when Juana was in first grade but had increased in severity over the past few months.
Upon further questioning, the patient reported that she initially did not want to attend school due to academic difficulties and bullying. However, since COVID-19, her fears of attending school had significantly worsened. Juana’s mother’s primary language was Spanish and she had limited English proficiency; she reported difficulty communicating with school personnel about Juana’s poor attendance.
Juana had recently had a complete medical work-up for her gastrointestinal concerns, with negative results. Since the negative work-up, Juana’s mother had told her daughter that she would be punished if she didn’t go to school.
●
* The patient’s name has been changed to protect her identity.
School avoidance, also referred to as school refusal, is a symptom of an emotional condition that manifests as a child refusing to go to school or having difficulty going to school or remaining in the classroom for the entire day. School avoidance is not a clinical diagnosis but often is related to an underlying disorder.1
School avoidance is common, affecting 5% to 28% of youth sometime in their school career.2 Available data are not specific to school avoidance but focus on chronic absenteeism (missing ≥ 15 days per school year). Rates of chronic absenteeism are high in elementary and middle school (about 14% each) and tend to increase in high school (about 21%).3 Students with disabilities are 1.5 times more likely to be chronically absent than students without disabilities.3 Compared to White students, American Indian and Pacific Islander students are > 50% more likely, Black students 40% more likely, and Hispanic students 17% more likely to miss ≥ 3 weeks of school.3 Rates of chronic absenteeism are similar (about 16%) for males and females.3
Absenteeism can have immediate and long-term negative effects.4 School attendance issues are correlated to negative life outcomes, such as delinquency, teen pregnancy, substance use, and poor academic achievement.5 According to the US Department of Education, individuals who chronically miss school are less likely to achieve educational milestones (particularly in younger years) and may be more likely to drop out of school.3
What school avoidance is (and what it isn’t)
It is important to distinguish school avoidance from truancy. Truancy often is associated with antisocial behavior such as lying and stealing, while school avoidance occurs in the absence of significant antisocial disorders.6 With truancy, the absence usually is hidden from the parent. In contrast, with school avoidance, the parents usually know where their child is; the child often spends the day secluded in their bedroom. Students who engage in truancy do not demonstrate excessive anxiety about attending school but may have decreased interest in schoolwork and academic performance.6 With school avoidance, the child exhibits severe emotional distress about attending school but is willing to complete schoolwork at home.
Why children may avoid school
School avoidance is a biopsychosocial condition with a multitude of underlying causes.4 It is associated most commonly with anxiety disorders and neurodevelopmental disorders, including but not limited to learning disabilities and attention-deficit/hyperactivity disorder.1 Depressive disorders also have been associated with school avoidance.7 Social concerns related to changes with school personnel or classes, academic challenges, bullying, health emergencies, and family stressors also can result in symptoms of school avoidance.1
Continue to: A child seeking to avoid...
A child seeking to avoid school may be motivated by potential negative and/or positive effects of doing so. Kearney and Silverman8 identified 4 primary functions of school refusal behaviors:
- avoiding stimuli at school that lend to negative affect (depression, anxiety)
- escaping the social interactions and/or situations for evaluation that occur at school
- gaining more attention from caregivers, and
- obtaining tangible rewards or benefits outside the school environment.
How school avoidance manifests
School avoidance has attributes of internalizing (depression, anxiety, somatic complaints) and externalizing (aggression, tantrums, running away, clinginess) behaviors. It can cause distress for the student, parents and caregivers, and school personnel.
The avoidance may manifest with behaviors such as crying, hiding, emotional outbursts, and refusing to move prior to the start of the school day. Additionally, the child may beg their parents not to make them go to school or, when at school, they may leave the classroom to go to a safe place such as the nurse’s or counselor’s office.
The avoidance may occur abruptly, such as after a break in the school schedule or a change of school. Or it may be the final result of the student’s gradual inability to cope with the underlying issue.
How to assess for school avoidance
Due to the multifactorial nature of this presenting concern, a comprehensive evaluation is recommended when school avoidance is reported.4 Often the child will present with physical symptoms, such as abdominal pain, nausea, vomiting, diarrhea, headaches, shortness of breath, dizziness, chest pain, and palpitations. A thorough medical examination should be performed to rule out a physiological cause. The medical visit should include clinical interviews with the patient and family members or guardians.
Continue to: To identify school avoidance...
To identify school avoidance in pediatric and adolescent populations, medical history and physical examination—along with social history to better understand familial, social, and academic concerns—should be a regular part of the medical encounter. The School Refusal Assessment Scale-Revised (SRAS-R) for both parents and their children was developed to assess for school avoidance and can be utilized within the primary care setting. Additional psychiatric history for both the family and patient may be beneficial, due to associations between parental mental health concerns and school avoidance in their children.9,10
Assessment for an underlying mental health condition, such as an anxiety or depressive disorder, should be completed when a patient presents with school avoidance.4 More than one-third of children with behavioral problems, such as school avoidance, have been diagnosed with anxiety.11 The 2020 National Survey of Children’s Health found that 7.8% of children and adolescents ages 3 to 17 years had a current anxiety disorder, leading the US Preventive Services Task Force to recommend screening for anxiety in children and adolescents ages 8 to 18 years.12,13 Furthermore, if academic achievement is of concern, then consideration of further assessment for neurodevelopmental disorders is warranted.1
Treatment is multimodal and multidisciplinary
Treatment for school avoidance is often multimodal and may involve interdisciplinary, team-based care including the medical provider, school system (eg, Child Study Team), family, and mental health care provider.1,4
Cognitive behavioral therapy (CBT) is the most-studied intervention for school avoidance, with behavioral, exposure-based interventions often central to therapeutic gains in treatment.1,14,15 The goals of treatment are to increase school attendance while decreasing emotional distress through various strategies, including exposure-based interventions, contingency management with parents and school staff, relaxation training, and/or social skills training.14,16 Collaborative involvement between the medical provider and the school system is key to successful treatment.
Medication may be considered alone or in combination with CBT when comorbid mental health conditions have been identified. Selective serotonin reuptake inhibitors (SSRIs)—including fluoxetine, sertraline, and escitalopram—are considered first-line treatment for anxiety in children and adolescents.17 Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, also have been shown to be effective. Duloxetine is the only medication approved by the US Food and Drug Administration (FDA) for treatment of generalized anxiety disorder in children ages 7 years and older.17
Continue to: SSRIs and SNRIs have a boxed warning...
SSRIs and SNRIs have a boxed warning from the FDA for increased suicidal thoughts and behaviors in children and adolescents. Although this risk is rare, it should be discussed with the patient and parent/guardian in order to obtain informed consent prior to treatment initiation.
Medication should be started at the lowest possible dose and increased gradually. Patients should remain on the medication for 6 to 12 months after symptom resolution and should be tapered during a nonstressful time, such as the summer break.
THE CASE
Based on the concerns of continued school refusal after negative gastrointestinal work-up, Juana’s physician screened her for anxiety and conducted a clinical interview to better understand any psychosocial concerns. Juana’s score of 10 on the General Anxiety Disorder-7 scale indicated moderate anxiety. She reported symptoms consistent with social anxiety disorder contributing to school avoidance.
The physician consulted with the clinic’s behavioral health consultant (BHC) to confirm the multimodal treatment plan, which was then discussed with Juana and her mother. The physician discussed medication options (SSRIs) and provided documentation (in both English and Spanish) from the visit to Juana’s mother so she could initiate a school-based intervention with the Child Study Team at Juana’s school. A plan for CBT—including a collaborative contingency management plan between the patient and her parent (eg, a reward chart for attending school) and exposure interventions (eg, a graduated plan to participate in school-based activities with the end goal to resume full school attendance)—was developed with the BHC. Biweekly follow-up appointments were scheduled with the BHC and monthly appointments were scheduled with the physician to reinforce the interventions.
CORRESPONDENCE
Meredith L. C. Williamson, PhD, 2900 East 29th Street, Suite 100, Bryan, TX 77840; meredith.williamson@tamu.edu
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
1. School Avoidance Alliance. School avoidance facts. Published September 16, 2021. Accessed July 27, 2023. https://schoolavoidance.org/school-avoidance-facts/
2. Kearney CA. School Refusal Behavior in Youth: A Functional Approach to Assessment and Treatment. American Psychological Association; 2001.
3. US Department of Education. Chronic absenteeism in the nation’s schools: a hidden educational crisis. Updated January 2019. Accessed August 3, 2023. www2.ed.gov/datastory/chronicabsenteeism.html
4. Allen CW, Diamond-Myrsten S, Rollins LK. School absenteeism in children and adolescents. Am Fam Physician. 2018;98:738-744.
5. Gonzálvez C, Díaz-Herrero Á, Vicent M, et al. School refusal behavior: latent class analysis approach and its relationship with psychopathological symptoms. Curr Psychology. 2022;41:2078-2088. doi: 10.1007/s12144-020-00711-6
6. Fremont WP. School refusal in children and adolescents. Am Fam Physician. 2003;68:1555-1560.
7. McShane G, Walter G, Rey JM. Characteristics of adolescents with school refusal. Aust N Z J Psychiatry. 2001;35:822-826. doi: 10.1046/j.1440-1614.2001.00955.x
8. Kearney CA, Silverman WK. The evolution and reconciliation of taxonomic strategies for school refusal behavior. Clin Psychology Sci Pract. 1996;3:339-354. doi: 10.1111/j.1468-2850.1996.tb00087.x
9. Kearney CA, Albano AM. School Refusal Assessment Scale-Revised C. Oxford University Press; 2007.
10. Heyne D. School refusal. In: Fisher JE, O’Donohue WT (eds). Practitioner’s Guide to Evidence-based Psychotherapy. Springer Science + Business Media. 2006;600-619. doi: 10.1007/978-0-387-28370-8_60
11. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatrics. 2019;206:256-267.e3. doi: 10.1016/j.jpeds.2018.09.021
12. US Census Bureau. 2020 National Survey of Children’s Health: Topical Frequencies. Published June 2, 2021. Accessed August 4, 2023. www2.census.gov/programs-surveys/nsch/technical-documentation/codebook/NSCH_2020_Topical_Frequencies.pdf
13. USPSTF. Anxiety in children and adolescents: screening. Final Recommendation Statement. Published October 11, 2022. Accessed August 4, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
14. Maynard BR, Brendel KE, Bulanda JJ, et al. Psychosocial interventions for school refusal with primary and secondary school students: a systematic review. Campbell Systematic Rev. 2015;11:1-76. doi: 10.4073/csr.2015.12
15. Kearney CA, Albano AM. When Children Refuse School: Parent Workbook. 3rd ed. Oxford University Press; 2018. doi: 10.1093/med-psych/9780190604080.001.0001
16. Heyne DA, Sauter FM. School refusal. In: Essau CA, Ollendick TH. The Wiley-Blackwell Handbook of the Treatment of Childhood and Adolescent Anxiety. Wiley Blackwell; 2013:471-517.
17. Kowalchuk A, Gonzalez SJ, Zoorob RJ. Anxiety disorders in children and adolescents. Am Fam Physician. 2022;106:657-664.
Caring for the caregiver in dementia
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-
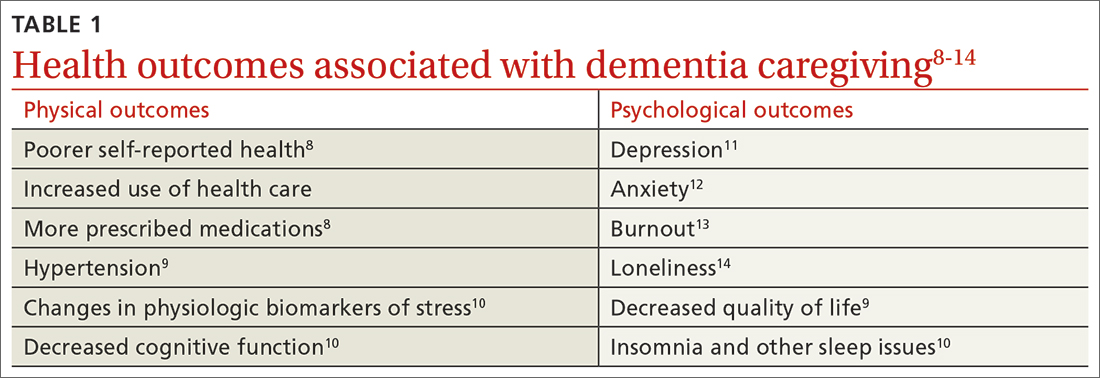
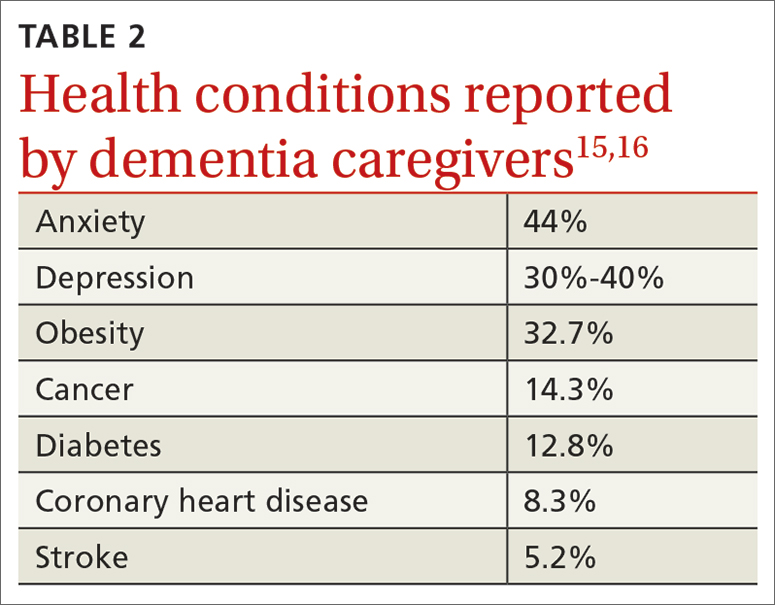
Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17
Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; Kathleen.Young@novanthealth.org
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; Kathleen.Young@novanthealth.org
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; Kathleen.Young@novanthealth.org
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
Conversion disorder: An integrated care approach
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
Tips and tools to help you manage ADHD in children, adolescents
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4
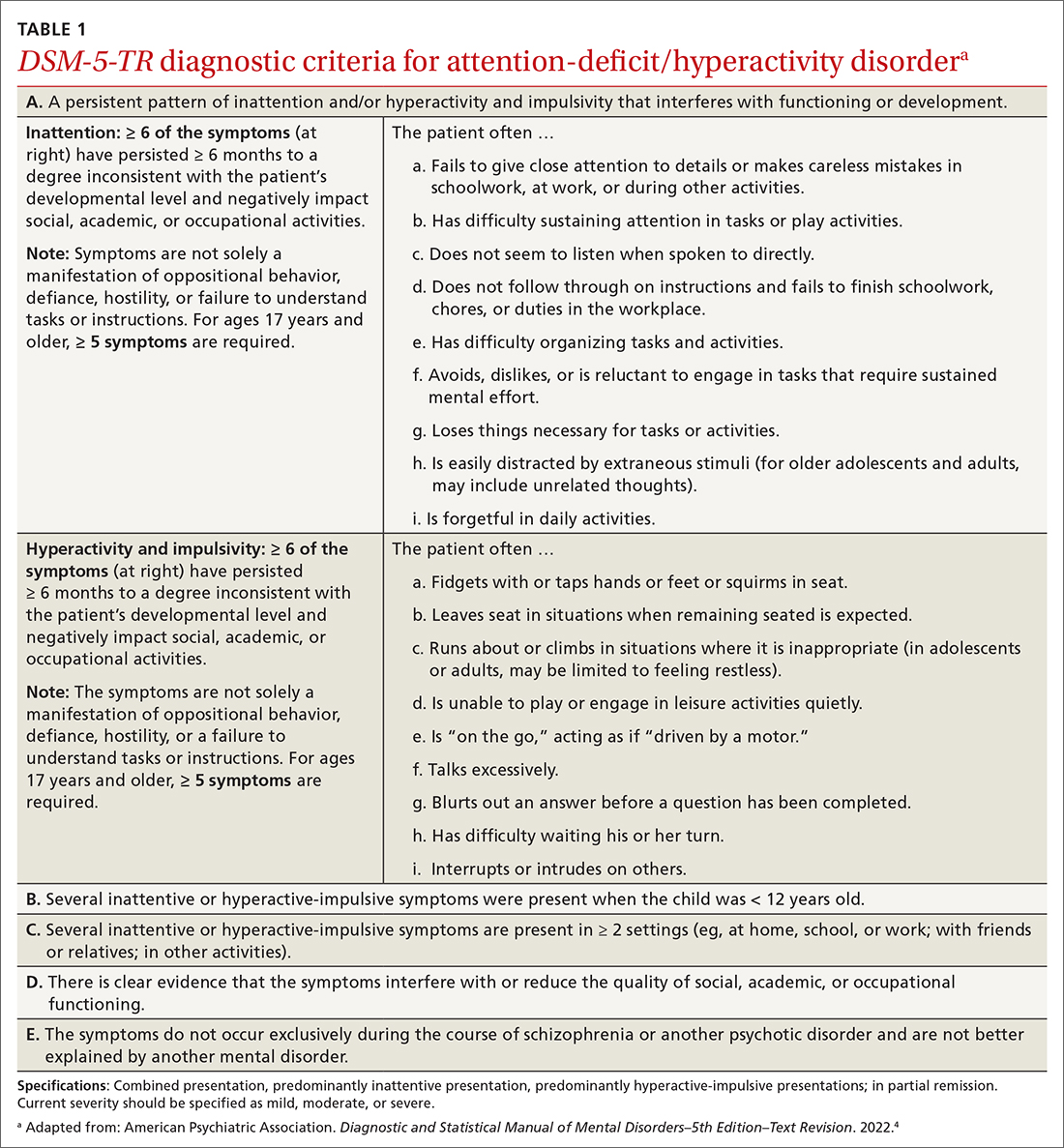
The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.
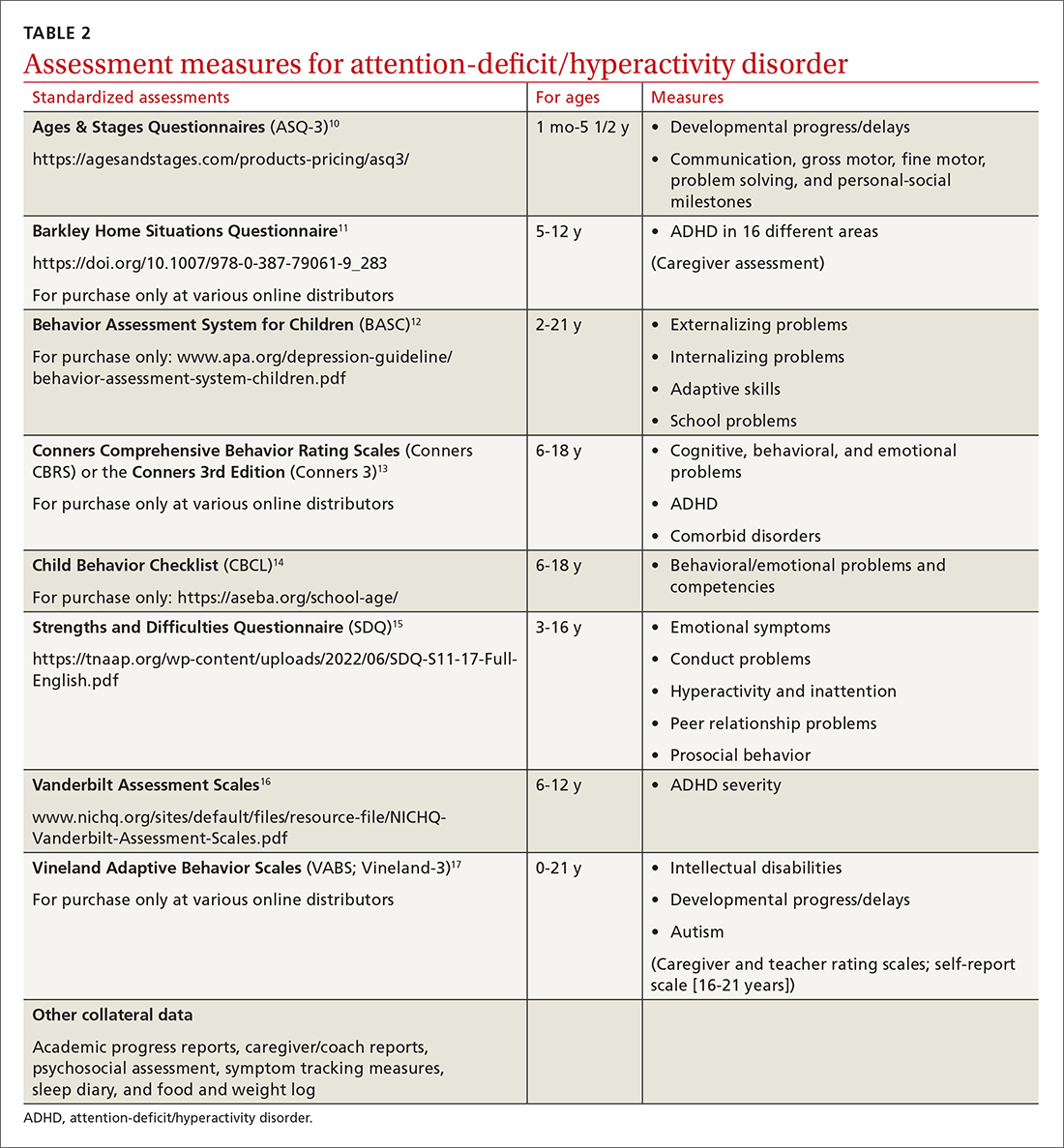
Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).
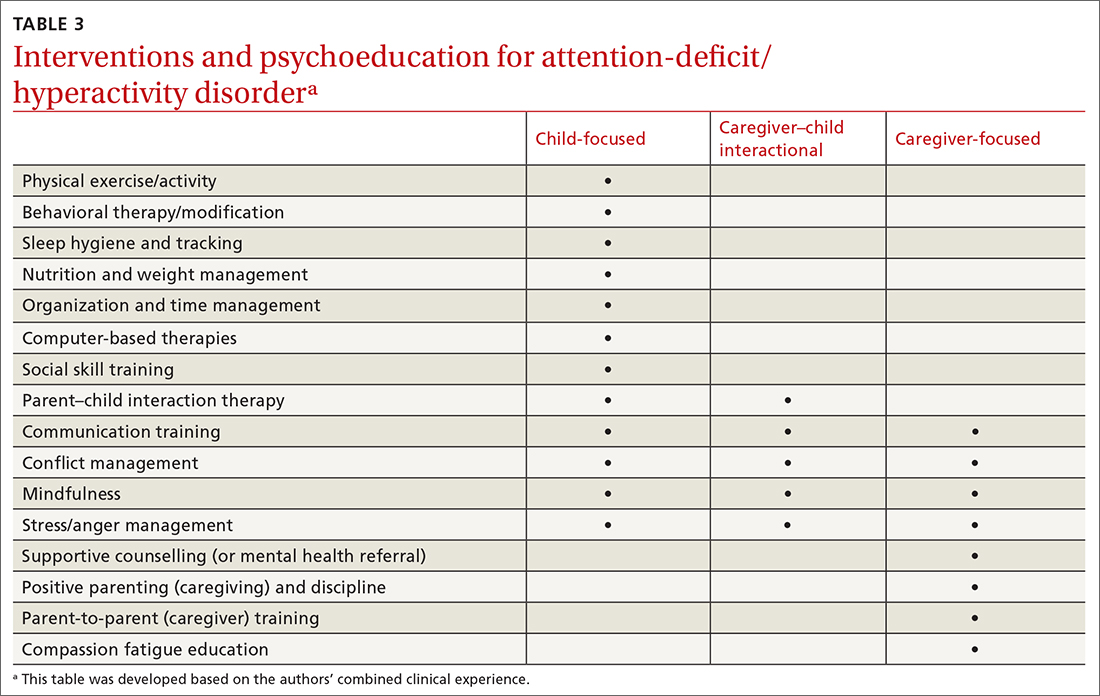
Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
An FP’s guide to identifying—and treating—postpartum depression
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; kbuck@jpshealth.org
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; kbuck@jpshealth.org
THE CASE
Alex T,* a 23-year-old first-time mom, presented to the family medicine office for her baby’s 2-week appointment. When asked how she was doing, she began to cry. She said, “I feel crazy” and indicated that she was feeling down and overwhelmed, and was struggling to bond with the baby. She filled out an Edinburgh Postnatal Depression Scale, a standard postpartum depression (PPD) screen; her score, 15 out of 30, was suggestive of depression. Ms. T had been coming to the practice for the past 3 years and had no significant physical or mental health history. She and the baby did not live with the baby’s father, and his degree of presence in their lives varied.
●
* The patient’s name has been changed to protect her identity.
PPD, traditionally defined as depression in the postpartum period for as long as a year after childbirth, is a common, underdiagnosed outcome of both normal and complicated pregnancies.1 Peripartum depression, which includes PPD and depression during pregnancy, occurs in approximately 10% of pregnancies.2,3 When depression first appears in the postpartum period, most women develop symptoms in the first month after delivery (54% of cases) or in the next 2 to 4 months (40%).4
The most significant risk factor for PPD is previous depression, peripartum or otherwise.1,4-6 Other common risk factors include major life events or stressors during or after pregnancy, domestic violence, poor social support, and preterm birth or an infant admission to the neonatal intensive care unit.1,7 Women with a self-perceived negative birth experience are also likely to experience PPD.8 PPD can be associated with significant morbidity and mortality, with suicide a more common cause of maternal mortality than either hemorrhage or hypertensive disorders of pregnancy.9
Early diagnosis and intervention are crucial to improving patient outcomes. Women with PPD initiate breastfeeding at lower rates and continue for shorter durations.10 PPD also affects maternal–infant bonding; may adversely affect an infant’s social, cognitive, and language development; and may lead to attachment disorders of infancy.11,12 In severe cases, it can lead to failure to thrive or infanticide.11
When to screen. The US Preventive Services Task Force (USPSTF) recommends clinicians screen for depression in pregnant and postpartum women (Grade Ba) and for women at increased risk, provide or refer to counseling interventions (Grade Ba).13,14 The American College of Obstetricians and Gynecologists (ACOG) recommends screening at least once in the postpartum period.15 Repeat screening at follow-up in the later postpartum period increases the likelihood of diagnosis.16 Screening for PPD as part of well-child care improves maternal outcomes, and the American Academy of Pediatrics recommends screening at the 1-, 2-, 4-, and 6-month visits.11,17 These screens are separately billable. Family physicians are uniquely suited to screening at both well-child and postpartum visits, as many women share a medical home with their child, and those who do not are equally willing to receive medical advice from their child’s physician.18
Continue to: Is it "the blues" or something else? Diagnosing PPD
Is it “the blues” or something else? Diagnosing PPD
Many new mothers experience postpartum blues, which manifest as tearfulness, insomnia, irritability, and anxiety. The postpartum blues, however, don’t meet the criteria for major depressive disorder and typically resolve within 14 days of delivery.19-21 On the other end of the spectrum is postpartum psychosis, which is severe and rare, and can also affect new mothers.
Screening for PPD. The most commonly used screening tool for PPD is the Edinburgh Postnatal Depression Scale (EPDS 10), a free 10-item instrument scored out of 30 possible points, with any score ≥ 13 suggesting PPD.22 The EPDS 10 has a sensitivity of 74% and specificity of 97% for the diagnosis of PPD.23 Other screening options include the Beck Depression Inventory II (BDI-II) and the Patient Health Questionnaire 9 (PHQ-9). The 21-item BDI-II takes longer to perform and is less sensitive (57%) than the EPDS.1 The PHQ-9, which asks about some symptoms common to the postpartum period (including sleep changes), is less specific than the EPDS (sensitivity, 75%; specificity, 90%).1 The EPDS also includes screening questions about anxiety.1
A positive depression screen, or any positive response to a question on suicidal ideation, should be followed up for confirmation using the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for major depressive disorder with peripartum onset.24 Women with PPD should also be asked about current or prior symptoms of bipolar disorder or mania.25 Up to 67% of women with bipolar disorder may relapse postpartum, and they also have an elevated risk of postpartum psychosis.26 The Mood Disorder Questionnaire is a useful tool if a concern for bipolar depression arises.27
Refer any woman in whom bipolar depression is a concern to a clinician experienced with its management. The presence of auditory or visual hallucinations should also be assessed as indicators of postpartum psychosis. Active suicidal or homicidal ideation and postpartum psychosis all require emergent psychiatric care.21,22 Intimate partner violence may also exist or escalate in the postpartum period and may exacerbate PPD. Both ACOG and the USPSTF recommend screening postpartum women for intimate partner violence.28,29
Also consider possible medical causes of PPD symptoms. Hypothyroidism in the postpartum period may manifest with some similar symptoms to PPD and is commonly underdiagnosed.22,30 Women with postpartum anemia and low ferritin stores also have a higher likelihood of PPD (odds ratio, 1.7-4.64), and postpartum iron supplementation may reduce this risk (number needed to treat = 4 in at least 1 randomized controlled trial).31 When anemia is present, ensure that it is properly treated.
Continue to: Steps you can take to manage pPD
Steps you can take to manage pPD
Refer any woman who has PPD to a qualified therapist whenever possible. Generally, the psychological recommendations for treatment of PPD are very similar to recommendations for general treatment of depression. Psychotherapy on its own is considered a first-line treatment for mild-to-moderate PPD, and medication plus psychotherapy is considered first-line treatment for severe PPD.32 (Worth noting: It may also be useful to offer counseling to a patient who appears distressed, even if she does not fully meet all DSM-5 criteria.)
Of the psychotherapy options, cognitive behavioral therapy (CBT) is supported by the most evidence. There is also evidence for the use of interpersonal therapy (IPT), especially in higher socioeconomic status populations.33 Key therapeutic targets in IPT are increasing behavioral engagement (eg, reaching out to friends), decreasing negative self-talk (eg, “I am a bad mother”), and negotiating roles and support (eg, both mom’s and family members’ expectations of new motherhood). There is mixed evidence for recommending exercise as a treatment for PPD.32,34 However, as exercise is a low-risk intervention, you may choose to make that recommendation to patients. Additionally, including partners/support people in treatment/visits for PPD has been shown to increase positive outcomes.35
When medication is considered, selective serotonin reuptake inhibitors (SSRIs) are most commonly used. Research indicates that SSRIs are significantly more effective than placebo for treatment of women with PPD.36 Sertraline, in particular, has shown to be both effective in treating PPD and safe in lactation.37,38 Dosing and duration of therapy are equivalent to treatment of major depression outside the perinatal period. Consult a trusted source on medications in lactation before prescribing any antidepressant to a breastfeeding mother. One resource is the National Institutes of Health drugs and lactation database (LactMed; www.ncbi.nlm.nih.gov/books/NBK501922/), which provides detailed information on the levels of medications in breastmilk and their potential effects on an infant.
Women with severe, refractory PPD may require hospitalization. Additional treatment options for women with severe, refractory PPD include electroconvulsive therapy or the new medication brexanolone, which is administered as a 60-hour continuous infusion.39,40
THE CASE
Further conversation with Ms. T revealed that she met the criteria for PPD (major depressive disorder with peripartum onset). She denied suicidal or homicidal ideation and was not experiencing any symptoms of psychosis. A complete blood count was drawn and showed no anemia, and her thyroid-stimulating hormone level was within normal limits. She had a good support network at home, with both her mom and sister taking shifts to help her get some extra rest and allow her to attend medical appointments. She said there was no domestic violence.
Ms. T was introduced to the clinic’s embedded counselor, who scheduled a follow-up appointment within the week to start CBT. After a discussion of risks and benefits, Ms. T also started a low dose of sertraline once daily. At follow-up postpartum visits, she reported significant improvement in her mood. She and her physician decided to taper her SSRI medication at 3 months postpartum. Screens for depression at her infant’s 4- and 6-month well-child visits in the office were reassuringly negative.
a There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.
CORRESPONDENCE
Katherine Buck, PhD, JPS Family Health Center, 1500 South Main Street, 4th Floor, Fort Worth, TX 76110; kbuck@jpshealth.org
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4
1. ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132:e208-e212. doi: 10.1097/AOG.0000000000002927
2. Banti S, Mauri M, Oppo A, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression–Research & Screening Unit study. Compr Psychiatry. 2011;52:343-351. doi: 10.1016/j.comppsych.2010.08.003
3. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164):1515-1520. doi: 10.1176/appi.ajp.2007.06111893
4. Altemus M, Neeb CC, Davis A, et al. Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry. 2012;73:e1485-e1491. doi: 10.4088/JCP.12m07693
5. Wilson LM, Reid AJ, Midmer DK, et al. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ. 1996;154:785-799.
6. Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289-295. doi: 10.1016/j.genhosppsych.2004.02.006
7. Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275-285. doi: 10.1097/00006199-200109000-00004
8. Bell AF, E Andersson. The birth experience and women’s postnatal depression: a systematic review. Midwifery. 2016;39:112-123. doi: 10.1016/j.midw.2016.04.014
9. Palladino CL, Singh V, Campbell J, et al. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol. 2011;118:1056-1063. doi: 10.1097/AOG.0b013e31823294da
10. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158. doi: 10.15585/mmwr.mm6606a1
11. Rafferty J, Mattson G, Earls MF, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143:e20183260. doi: 10.1542/peds.2018-3260
12. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592. doi: 10.1016/s0272-7358(98)00100-7
13. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. doi: 10.1001/jama.2019.0007
14. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:380-387. doi: 10.1001/jama.2015.18392
15. ACOG. Screening for perinatal depression. 2018. Accessed October 5, 2022. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
16. Yawn BP, Bertram S, Kurland M, et al. Repeated depression screening during the first postpartum year. Ann Fam Med. 2015;13:228-234. doi: 10.1370/afm.1777
17. van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, et al. Post-up study: postpartum depression screening in well-child care and maternal outcomes. Pediatrics. 2017;140:e20170110. doi: 10.1542/peds.2017-0110
18. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network study. Ann Fam Med. 2016;14:350-355. doi: 10.1370/afm.1933
19. Nonacs R, Cohen LS. Postpartum mood disorders: diagnosis and treatment guidelines. J Clin Psychiatry. 1998;59(suppl 2):34-40.
20. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097/AOG.0000000000002633
21. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
22. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. 2012;34:436-442. doi: 10.1016/S1701-2163(16)35240-9
23. Owara AH, Carabin H, Reese J, et al. Summary diagnostic validity of commonly used maternal major depression disorder case finding instruments in the United States: a meta-analysis. J Affect Disord. 2016;205:335-343. doi: 10.1016/j.jad.2016.08.014
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington D.C.: 2013:160.
25. Mandelli L, Souery D, Bartova L, et al. Bipolar II disorder as a risk factor for postpartum depression. J Affect Disord. 2016;204:54-58. doi:10.1016/j.jad.2016.06.025
26. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020. doi: 10.1097/AOG.0b013e31816fd910
27. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875. doi: 10.1176/appi.ajp.157.11.1873
28. Curry SJ, Krist AH, Owens DK, et al. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force Final Recommendation Statement. JAMA. 2018;320:1678-1687. doi: 10.1001/jama.2018.14741
29. ACOG Committee Opinion No. 518: Intimate partner violence. Obstet Gynecol. 2012;119:412-417. doi: 10.1097/AOG.0b013e318249ff74
30. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
31. Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstet Gynaecol. 2019;40:19-28. doi: 10.1080/0167482X.2018.1427725
32. Hirst KP, Moutier CY. Postpartum major depression. Am Fam Physician. 2010;82:926-933.
33. Nillni YI, Mehralizade A, Mayer L, et al. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev. 2018;66:136-148. doi: 10.1016/j.cpr.2018.06.004
34. Daley AJ, Macarthur C, Winter H. The role of exercise in treating postpartum depression: a review of the literature. J Midwifery Womens Health. 2007;52:56-62. doi: 10.1016/j.jmwh.2006.08.017
35. Misri S, Kostaras X, Fox D, et al. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry. 2000;45:554-558. doi: 10.1177/070674370004500607
36. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;CD002018. doi: 10.1002/14651858.CD002018.pub2
37. Pinheiro E, Bogen DL, Hoxha D, et al. Sertraline and breastfeeding: review and meta-analysis. Arch Women Ment Health. 2015;18:139-146. doi: 10.1007/s00737-015-0499-y
38. Hantsoo L, Ward-O’Brien D, Czarkowski KA, et al. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231:939-948. doi: 10.1007/s00213-013-3316-1
39. Rundgren S, Brus O, Båve U, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258-264. doi: 10.1016/j.jad.2018.04.043
40. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. doi: 10.1016/S0140-6736(18)31551-4




