User login
Automobile Injury: A Common Familiar Risk for Presenting and Comparing Risks in Dermatology
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
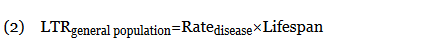
When an odds ratio (OR) was presented, its conversion to RR followed11:
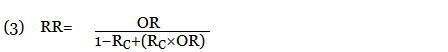
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
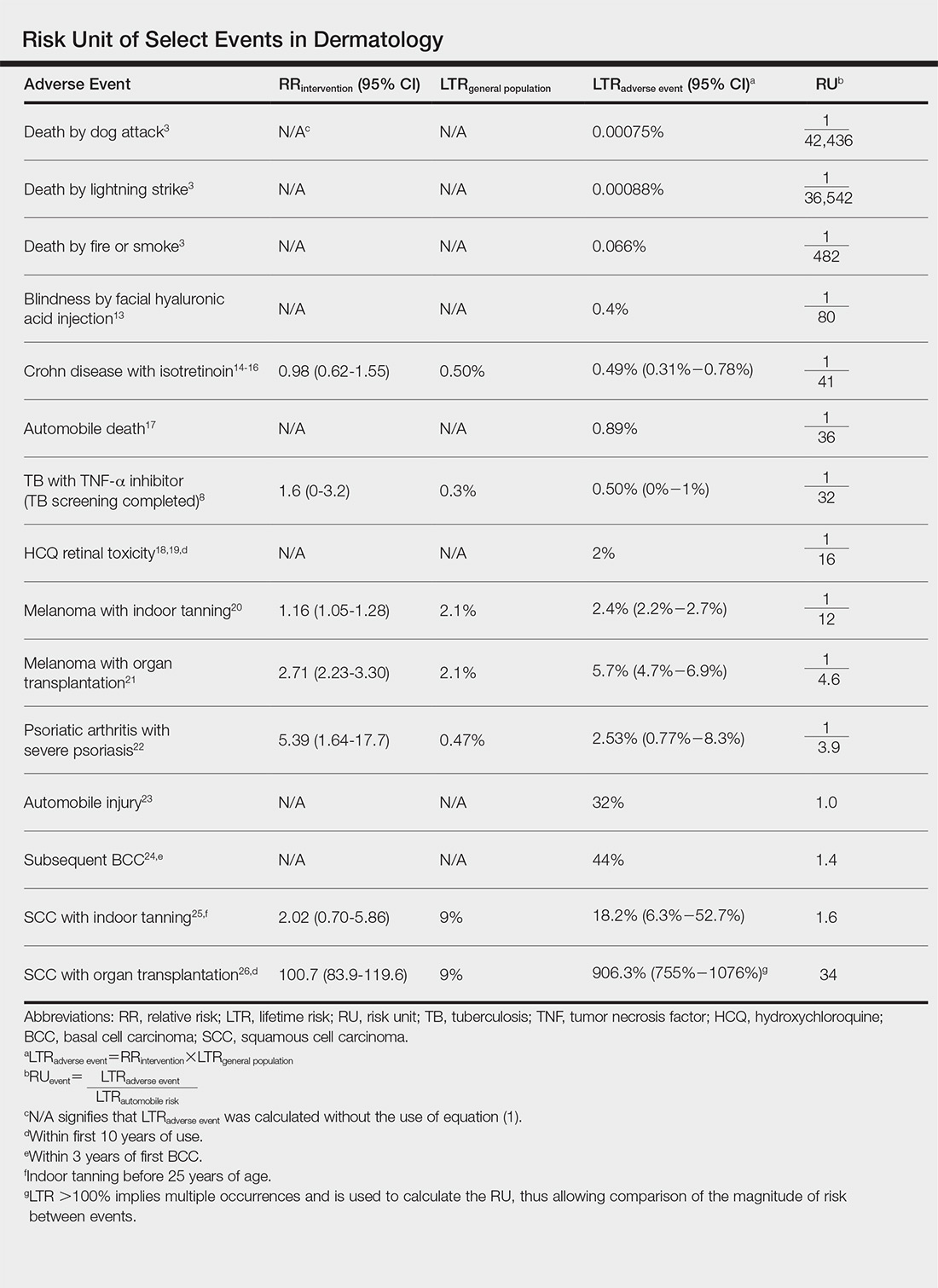
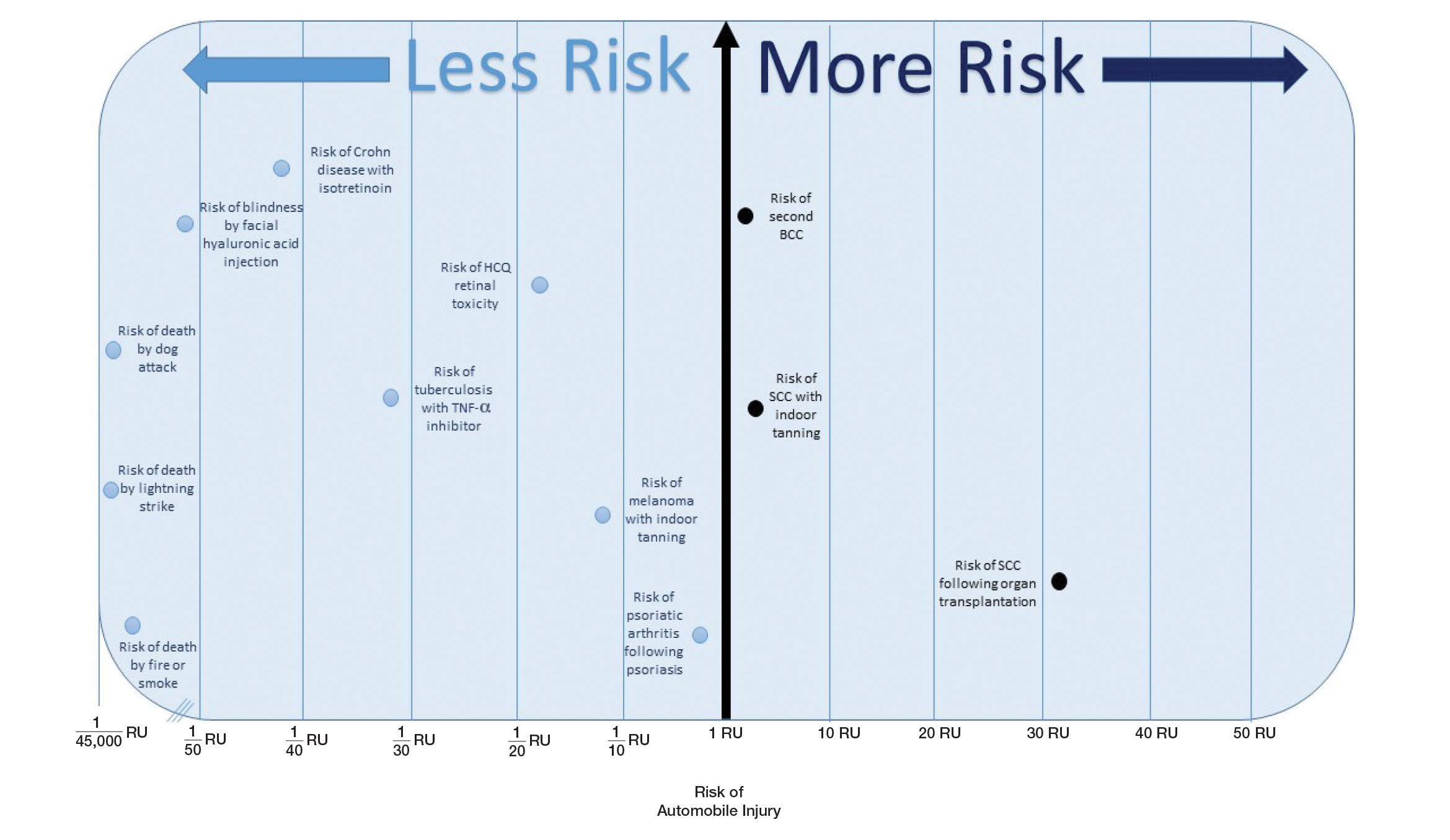
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
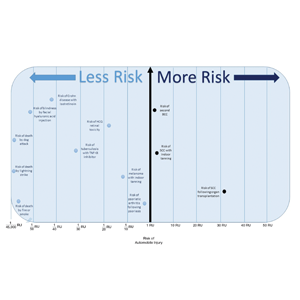
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).
Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
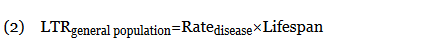
When an odds ratio (OR) was presented, its conversion to RR followed11:
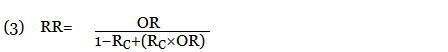
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
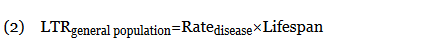
When an odds ratio (OR) was presented, its conversion to RR followed11:
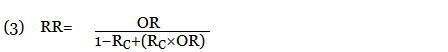
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Practice Points
- Using common identifiable risks may help patients put the risk of certain dermatologic interventions into perspective.
- Numerous interventions in dermatology offer much less risk of an adverse event than the lifetime risk of automobile injury.