User login
Anterior discoid resection using a ‘squeeze’ technique
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
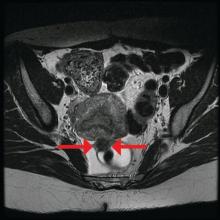
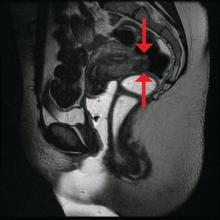
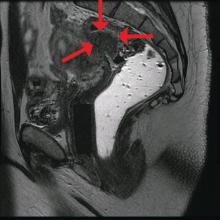
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
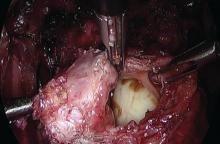
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Discoid resection of rectal endometriotic nodules
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
Is 17-OHPC effective for reducing risk of preterm birth?
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
For preterm birth, we must avoid being too quick to prescribe therapeutic measures
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Implementing enhanced recovery protocols for gynecologic surgery
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.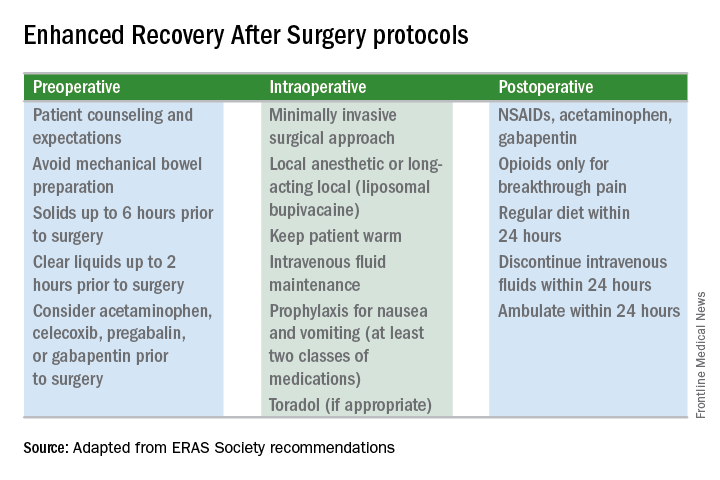
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
Protocols to reduce opioid use and shorten length of stay
While originally pioneered by European anesthesiologists and surgeons in Europe in the 1990s, enhanced recovery after surgery (ERAS) programs, also known as enhanced recovery protocols or fast-track surgery, have now gained popularity across the surgical spectrum within the United States. The goal of these programs is to utilize multidisciplinary and multimodal interventions to minimize the physiologic changes associated with surgery and thereby enhance the perioperative experience – reduced morbidity and mortality, shorter length of stay, less postoperative opioid use, and faster resumption to normal activity, at a decreased cost of care.
1. Enhanced patient education, including managing expectations.
2. Decreased perioperative fasting periods.
3. Blood volume and temperature maintenance intraoperatively.
4. Postoperative mobilization early and often.
5. Multimodal pain relief and nausea/vomiting prophylaxis.
6. Use of postoperative drains and catheters only as long as required.
Today, I have asked Kirsten Sasaki, MD, to discuss some of these ERAS concepts. I have asked Dr. Sasaki to especially focus on decreasing opioid utilization. For a thorough discussion on ERAS recommendations using an evidence-based approach, one can review two excellent papers by Nelson et al. (Gynecol Oncol. 2016 Feb;140[2]:313-22; Gynecol Oncol. 2016 Feb;140[2]:323-32).
Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston. Dr. Sasaki then went on to become our second fellow at the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and Society of Reproductive Surgeons at Advocate Lutheran General Hospital, Park Ridge, Ill. As a Fellow, Dr. Sasaki was recognized for her excellent teaching and research capabilities. Ultimately, however, it was her tremendous surgical skills and surgical sense that led me to invite her to join my practice in 2014.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
While originally pioneered by European anesthesiologists and surgeons in Europe in the 1990s, enhanced recovery after surgery (ERAS) programs, also known as enhanced recovery protocols or fast-track surgery, have now gained popularity across the surgical spectrum within the United States. The goal of these programs is to utilize multidisciplinary and multimodal interventions to minimize the physiologic changes associated with surgery and thereby enhance the perioperative experience – reduced morbidity and mortality, shorter length of stay, less postoperative opioid use, and faster resumption to normal activity, at a decreased cost of care.
1. Enhanced patient education, including managing expectations.
2. Decreased perioperative fasting periods.
3. Blood volume and temperature maintenance intraoperatively.
4. Postoperative mobilization early and often.
5. Multimodal pain relief and nausea/vomiting prophylaxis.
6. Use of postoperative drains and catheters only as long as required.
Today, I have asked Kirsten Sasaki, MD, to discuss some of these ERAS concepts. I have asked Dr. Sasaki to especially focus on decreasing opioid utilization. For a thorough discussion on ERAS recommendations using an evidence-based approach, one can review two excellent papers by Nelson et al. (Gynecol Oncol. 2016 Feb;140[2]:313-22; Gynecol Oncol. 2016 Feb;140[2]:323-32).
Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston. Dr. Sasaki then went on to become our second fellow at the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and Society of Reproductive Surgeons at Advocate Lutheran General Hospital, Park Ridge, Ill. As a Fellow, Dr. Sasaki was recognized for her excellent teaching and research capabilities. Ultimately, however, it was her tremendous surgical skills and surgical sense that led me to invite her to join my practice in 2014.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
While originally pioneered by European anesthesiologists and surgeons in Europe in the 1990s, enhanced recovery after surgery (ERAS) programs, also known as enhanced recovery protocols or fast-track surgery, have now gained popularity across the surgical spectrum within the United States. The goal of these programs is to utilize multidisciplinary and multimodal interventions to minimize the physiologic changes associated with surgery and thereby enhance the perioperative experience – reduced morbidity and mortality, shorter length of stay, less postoperative opioid use, and faster resumption to normal activity, at a decreased cost of care.
1. Enhanced patient education, including managing expectations.
2. Decreased perioperative fasting periods.
3. Blood volume and temperature maintenance intraoperatively.
4. Postoperative mobilization early and often.
5. Multimodal pain relief and nausea/vomiting prophylaxis.
6. Use of postoperative drains and catheters only as long as required.
Today, I have asked Kirsten Sasaki, MD, to discuss some of these ERAS concepts. I have asked Dr. Sasaki to especially focus on decreasing opioid utilization. For a thorough discussion on ERAS recommendations using an evidence-based approach, one can review two excellent papers by Nelson et al. (Gynecol Oncol. 2016 Feb;140[2]:313-22; Gynecol Oncol. 2016 Feb;140[2]:323-32).
Dr. Sasaki completed her internship and residency at Tufts Medical Center, Boston. Dr. Sasaki then went on to become our second fellow at the Fellowship in Minimally Invasive Gynecologic Surgery in affiliation with AAGL and Society of Reproductive Surgeons at Advocate Lutheran General Hospital, Park Ridge, Ill. As a Fellow, Dr. Sasaki was recognized for her excellent teaching and research capabilities. Ultimately, however, it was her tremendous surgical skills and surgical sense that led me to invite her to join my practice in 2014.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
Studies looking at pravastatin for preeclampsia prevention
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
In the United States, preeclampsia affects 3%-5% of all pregnancies and 10%-20% of pregnancies complicated by diabetes. Up to 20% of maternal deaths in the United States – and a much larger percentage of maternal deaths worldwide – occur in women with the condition, as do numerous maternal and fetal comorbidities. These include severe hypertension, pulmonary edema, stroke, and kidney and liver injury in the mother, and stillbirth, placental abruption, growth restriction, and premature delivery of the fetus.
Longer-term complications for the offspring include chronic lung disease, hearing and vision disorders, cerebral palsy and other neurodevelopmental disorders, and – as shown by more recent research – poor cardiovascular and metabolic outcomes.
Preeclampsia predisposes the mother to at least a twofold increased risk of future heart disease, compared with a woman who does not have the condition. In addition, women with preeclampsia who deliver at term are approximately two times more likely to die prematurely from heart disease than women without a history of preeclampsia, and those who deliver before 34 weeks’ gestation have been shown to have a ninefold greater risk of premature death. The American Heart Association, in fact, now includes preeclampsia in its list of heart disease risk factors.
Much attention now is focused on statins (inhibitors of HMG-CoA reductase), which have been used for more than 30 years for the primary and secondary prevention of heart disease. The properties and mechanisms of this class of drugs – and the similarities in the pathophysiology of cardiovascular disease and preeclampsia – make statins a plausible candidate for preeclampsia prevention. Thus far, data from preclinical work and subsequent pilot studies have been encouraging.
The commonalities
Preeclampsia is unique to pregnancy, but its pathophysiology and risk factors largely overlap with those of adult atherosclerotic cardiovascular disease. The exact pathophysiology of preeclampsia is unknown, but it is generally agreed that angiogenic imbalance and endothelial dysfunction play key roles, as do associated inflammation and oxidative stress.
Women with preeclampsia have been shown, for instance, to have had increased levels of antiangiogenic factors (soluble FMS-like tyrosine kinase 1 and soluble endoglin) and decreased levels of angiogenic factors (vascular endothelial growth factor and placental growth factor) prior to developing the condition clinically. Risk factors common to both preeclampsia and heart disease include chronic hypertension, dyslipidemia, diabetes or insulin resistance, obesity, and a family history of the condition.
Statins, meanwhile, have been shown to prevent or reverse angiogenic imbalance by promoting the release of vascular endothelial growth factor and placental growth factor and by suppressing the production of soluble FMS-like tyrosine kinase 1 and soluble endoglin. The drugs also improve vascular relaxation and exhibit anti-inflammatory and antioxidative effects, thereby broadly improving endovascular health. In the cardiovascular arena, notably, men and women who have elevated inflammatory markers even without hypercholesterolemia have been shown to have improved cardiovascular outcomes with statin treatment.
In various mouse models of preeclampsia studied in the past decade, pravastatin, a hydrophilic statin, has had beneficial effects. Mice with the angiogenic imbalance characteristic of preeclampsia that received this statin have shown a reversal of the imbalance, as well as reduced blood pressure, increased levels of nitric oxide synthase production, decreased oxidative stress, improved vascular reactivity, decreased kidney damage and proteinuria, and other positive effects. These effects occurred without detrimental outcomes to the mice or any increase in the rates of anomalies or resorption in offspring (Clin Obstet Gynecol. 2017 Mar;60:161-8).
Moreover, in addition to ameliorating the preeclampsia phenotype, pravastatin use in these animal models has improved pregnancy outcomes and reduced rates of pregnancy losses.
Safety issues
So, can we use statins in pregnancy? When statins were originally marketed in the 1980s, they were labeled pregnancy category X, which means 1) that there is evidence of fetal abnormalities or risk and 2) that these risks clearly outweigh potential benefits.
This designation for statins was based largely on the second half of the definition (no benefit to outweigh any risk). In addition, there were theoretical concerns about the inhibition of cholesterol synthesis during embryologic development and about a small case series of the original lipophilic statins suggesting an increased risk of malformations. While pregnancy category X does not exist anymore, statins are still labeled as contraindicated in pregnancy.
Pravastatin is one of the safest statins to consider in pregnancy for several reasons: It is one of the most hydrophilic statins and is a substrate of the placental efflux transporters, such as P-glycoprotein; both of those properties limit its ability to cross the placenta. It also has a short-elimination half-life, is cleared through both hepatic and renal routes, and is among the most hepatoselective statins available (one of the weakest inhibitors of HMG-CoA reductase). Indeed, in vitro placental transfer studies suggest that pravastatin transfer is limited and slow and that clearance is significantly higher in the fetal-to-maternal direction than in the maternal-to-fetal direction.
Animal studies have demonstrated that pravastatin is not teratogenic and has no effect on placental weight, pup birth weight, and pup adult weight. Moreover, at least six published cohort studies of women with first-trimester exposure to statins (women who had been prescribed the drugs before becoming pregnant and who received the drugs in the first trimester before realizing they were pregnant) showed no patterns or increased rates of congenital anomalies, compared with women without exposure to known teratogens. Additionally, these cohorts did not show any associations with miscarriage or fetal growth restriction (Obstet Gynecol. 2013 Feb;121:349-53).
A more recent cohort study of close to 900,000 women – of which 1,152 women used a statin (pravastatin or other statins) during their first trimester – similarly found no significant increases in any type of congenital malformation, compared with other completed pregnancies in the larger cohort. Notably, the analysis of this cohort was done using propensity score–based methods to control for potential confounders, including prepregnancy conditions that prompted use of a statin (BMJ. 2015;350:h1035).
A drawback to this body of research is that, with the exception of the BMJ study, the cohorts have been generally small; furthermore, in keeping with current recommendations, most of the statin-exposed patients discontinued use of the drugs upon confirmation of their pregnancies, thereby leaving the effects of long-term use unknown.
A promising pilot
Daily pravastatin use in pregnancy, starting in the second trimester, got its first major test of safety and pharmacokinetics in a pilot randomized, controlled trial undertaken by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD’s) Obstetric-Fetal Pharmacology Research Units Network. Women with singleton pregnancies and a history of severe preeclampsia requiring delivery prior to 34 weeks’ gestation were randomized between 12 and 16 weeks’ gestation to receive pravastatin or placebo until delivery.
This pilot is the first of three cohorts of women who were or will be randomized in separate pilot trials to escalating doses of pravastatin: 10 mg, 20 mg, and 40 mg (the last of which is the usual dose for lipid lowering in adults). Results of the first cohort, in which 20 patients were randomized to 10 mg pravastatin or placebo, were reported in 2016, and those from the second cohort will be reported soon. The third pilot is currently enrolling women.
In this first pilot we found no differences in rates of congenital anomalies or other identifiable maternal or fetal/neonatal safety risks, no differences in adverse events, and no maternal, fetal, or neonatal deaths. There were also no reports of myopathy/rhabdomyolysis or liver injury; the most common adverse events were heartburn (reported by four patients in the pravastatin group and three in the placebo group) and musculoskeletal pain (reported by four patients and one patient, respectively).
Although not statistically significant, a 10-mg dose of pravastatin was associated with favorable outcomes. None of the women receiving pravastatin developed preeclampsia, while four in the placebo group developed the disorder (with three of these four having severe preeclampsia).
Women in the pravastatin group also were less likely to have an indicated preterm delivery (one vs. five in the placebo group), and their neonates were less likely to be admitted to intermediate nurseries or the neonatal ICU. In addition, their angiogenic profiles were improved (higher placental growth factor and lower FMS-like tyrosine kinase 1 and soluble endoglin).
Importantly, while pravastatin reduced maternal cholesterol concentrations, there were no differences in birth weight or umbilical cord cholesterol concentrations (total cholesterol or LDL) between the two groups (Am J Obstet Gynecol. 2016;214[6]:720.e1-17).
That cholesterol concentrations were not reduced in fetuses exposed to pravastatin is reassuring and aligns with findings from other studies showing that fetal cholesterol concentrations are largely independent from maternal cholesterol concentrations or diet. For instance, we know from studies of Smith-Lemli-Opitz syndrome, a multiple congenital anomaly/intellectual disability syndrome caused by a defect in cholesterol synthesis, that there is not any significant interaction between cholesterol concentrations of the mother and fetus. Only about 10% of the fetal absolute cholesterol requirement comes from the mother, research has demonstrated.
The future
Infant follow-up in the NICHD study is planned, and a large, randomized clinical trial powered to look at the efficacy of pravastatin for preventing preeclampsia in high-risk women has been approved by the NICHD. Once funded, the study is expected to enroll approximately 1,700 pregnant women who have a history of severe preeclampsia and delivery before 36 weeks’ gestation and who are between 10 and 16 weeks’ gestation.
With continued research, we face the question of whether pravastatin may potentiate the benefit of aspirin in pregnant women. In cardiovascular medicine, there is evidence for additive or synergistic effects of the combined use of aspirin and statins.
Interestingly, a recent prospective cohort study of women with antiphospholipid syndrome and poor outcomes in prior pregnancies showed dramatic improvement in both maternal and fetal/neonatal outcomes when pravastatin was administered after the onset of preeclampsia or intrauterine growth restriction. All 21 women in the cohort were treated with low-dose aspirin and low-molecular-weight heparin; after the development of preeclampsia or intrauterine growth restriction, 10 patients were maintained on aspirin and LMWH, and 11 were started on 20 mg daily pravastatin along with the aspirin and LMWH (J Clin Invest. 2016 Aug;126[8]:2933-40; and J Clin Invest. 2016 Aug;126[8]:2792-4).
Those who received the statin had improved uterine artery Doppler velocimetry, lower systemic blood pressure, and delivered infants with higher birth weights and at a more advanced gestational age (median, 36 weeks’ vs. 26.5 weeks’ gestation). The study did not randomly allocate the women and did not include a placebo arm. Still, it is another impressive proof-of-concept study.
Dr. Costantine is an associate professor of obstetrics and gynecology at the University of Texas Medical Branch in Galveston. He reported that he has no financial disclosures.
Hope for targeted management of preeclampsia
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
In medicine, there are many diseases and conditions that pose significant challenges to health care practitioners. For example, within brain science, there are patients with debilitating neurodegenerative diseases; within emergency medicine, there are patients who have suffered severe and acute trauma; within pediatrics, there are patients with terminal illnesses, such as cancer. In ob.gyn., one of the great obstetrical syndromes is preeclampsia.
Humans have known about preeclampsia for thousands of years, dating back to the 4th and 5th centuries B.C., since the time of Hippocrates. Ancient writings on medical conditions of women reflect a recognition of preeclampsia and eclampsia, although formal classification of the condition as a hypertensive disorder associated specifically with pregnancy did not occur until the late 1800s. Despite this, the pathology of preeclampsia is significantly underdefined, and because the underlying causes of preeclampsia are largely unknown, prevention and management continue to be hindered.
However, recent research indicating an association between statin use and prevention of preeclampsia has given us some hope that targeted management is possible. As ob.gyns. continue to grapple with the intricacies of managing patients with preeclampsia, a condition we increasingly see because of concurrent rises in being overweight, being obese, and having diabetes during pregnancy, so we are eager to see whether the research might confirm an effective role for statins in patient care. Although statins are still quite far from becoming a new standard of care, we are, as a specialty, hungry for a solution to this millennia-old problem.
Therefore, this month we have invited Maged Costantine, MD, an associate professor in the department of obstetrics and gynecology and division of maternal fetal medicine at the University of Texas Medical Branch in Galveston, to discuss this novel and exciting new area of research.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Payment changes drive hysteroscopy to the office
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
The benefits of integrating hysteroscopy into office practice have been compelling for some time. An in-office approach is patient centered, more efficient, and clinically valuable. It also has had the potential to be economically valuable for practices that are able to perform a mix of diagnostic and therapeutic/operative hysteroscopies.
Dramatic shifts within the Centers for Medicare & Medicaid Services fee schedule in 2017 – and commensurate changes in the private insurance market – have now ramped up this value, making it all the more important that physicians consider investing in equipment and adopting an in-office approach.
Central to this increase, in turn, is a significant increase in practice expense reimbursement. CMS has included the costs of equipment, including the costs of the hysteroscopic fluid management system and the hysteroscopic tissue resection system, in recalibrating the practice expense relative value unit. Clearly, physicians are being encouraged to move hysteroscopic procedures into the office.
Weighing an investment
In the Medicare resource-based relative value scale payment system, relative value units (RVUs) are calculated based on three elements: physician work, practice expenses, and malpractice cost. Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
This isn’t the first year that the payment system – a standard for many other payers in determining compensation – allows for higher reimbursement for some hysteroscopic procedures performed in the office. The practice expense relative units have been higher for some time for certain hysteroscopic procedures – such as diagnostic hysteroscopy (code 58555), removal of a foreign body (58562), endometrial ablation (58353), and biopsy/polypectomy (58558) – when these procedures are performed in the office, compared with the hospital or an ambulatory surgical center.
However, the new increase in physician office payment for 58558 changes the equation significantly and ensures a better return on investment. In 2017, CMS offered a 12% increase in the facility fee paid to hospitals and a 2% increase in the facility fee paid to outpatient surgery centers when a hysteroscopic biopsy/polypectomy is performed in these settings, but the physician reimbursement in these cases declined 11%-19%.
On the flip side, an in-office approach to hysteroscopic biopsy/polypectomy has been rewarded in 2017 through a significantly higher practice expense RVU and a “non-facility” total RVU of 38.51 – a 237% increase over the 2016 practice expense RVU of 11.4. Such dramatic differences between the practice RVUs – and total RVUs – for in-office and out-of-office hysteroscopic procedures will continue for 2018.
Private insurers are following suit, and some are increasing their reimbursement even more. As of June 2017 in metropolitan Chicago, Blue Cross Blue Shield has been reimbursing in-office hysteroscopic biopsy/polypectomy at approximately $2,424.00; prior to June, the allowable charge was $742.81.
Equipment costs for in-office hysteroscopy can range from $15,000 to $35,000, based on whether equipment is new or used, the number of trays, and the style of camera and monitor system. Ancillary equipment/disposables cost $10 or less, and $40-$50 or less for diagnostic and many operative procedures, respectively. The prices for handpiece mechanical resection disposables or tissue removal devices vary based on company and blade type, so these costs will need to be accounted for if such equipment is incorporated. Again, the CMS increase in reimbursement for offices accommodates for the inclusion of these disposables as well as fluid management disposable costs.
If diagnostic hysteroscopy (as a separate procedure) is the procedure that you perform most often, the investment will look less favorable. However, if you anticipate performing hysteroscopic biopsies and/or polypectomies as well, the investment will look significantly more favorable now than it has in past years.
Once you have established your in-office system, even those procedures that are weighted equally for the practice setting (non-facility) and hospital/surgery center setting, such as hysteroscopic lysis of adhesions (58559), can be easily incorporated from a financial point of view.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. The setup is relatively simple and requires a dedicated exam room, not a surgical suite. You can perform one or two annual exams while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Hysteroscopy at the hospital, or even at an ambulatory surgical center, involves time driving, changing, and waiting for anesthesia.
For our patients, most importantly, an in-office approach offers less out-of-pocket expense (deductibles), less time away from family/work, avoidance of general anesthesia/intubation, and greater patient comfort from being within a familiar environment. For diagnostic procedures, the patient can be in and out in less than 30 minutes, and for operative procedures, she can be in and out in 1-2 hours, compared with more than 4 hours at the hospital.
Preparing the office
Physicians in Europe have been performing in-office hysteroscopy for years. But in the United States, it is a newer concept, with most gynecologic surgeons having been taught to perform surgical procedures in the operating room. Undoubtedly, our unfamiliarity with in-office surgery has played a role in the slow uptake of hysteroscopy in our practices.
Open communication about everything the patient will see hear and feel before, during and after the procedure is important. Focusing on these details can improve your patient’s experience and your professional relationship with her.
In an earlier edition of Master Class, I addressed instrumentation and technique, elements of pain control and anesthesia, and the value of a vaginoscopic approach to hysteroscopy. Vaginoscopy avoids the use of a vaginal speculum or cervical tenaculum, and is so tolerable to many patients that I use minimal premedication and only rarely use any local anesthetic and/or sedation, even for biopsies and polypectomies.
Preparing your practice for hysteroscopy is a multifaceted process involving not only the purchase and/or rental of equipment but also compliance with guidelines, regulatory considerations, patient rights, hospital transfer arrangements, and other issues. ACOG’s Report of the Presidential Task Force on Patient Safety in the Office Setting is a valuable resource for getting started. The report discusses anesthesia levels and the benefits and risks of a contract anesthesiologist, for instance, as well as the role of and processes for credentialing, privileging, and accreditation.
Checklists and drills are important for ensuring a safe practice, and the report discusses each of these elements and provides templates and examples. A sample “Office Surgical Safety Checklist” to be used for each procedure, for instance, has sections with preoperative steps (before anesthesia/analgesia, and before incision), intraoperative steps, postoperative steps, and discharge steps. Similar in format to checklists used in the aviation industry, each step has a box to be checked off to verify completion.
Mock drills help ensure that staff are knowledgeable about their roles and coordinated in their response to potential complications, such as vasovagal episodes, respiratory arrest caused by laryngospasm, and local anesthetic toxicity reactions. And, while not the focus of drills, we also must be prepared to manage cervical strictures and stenosis, cervical laceration, uterine perforation, and other complications.
Outpatient surgery guidelines from organizations such as the American College of Surgeons, the Joint Commission, state regulatory agencies, and professional liability insurers, can also be useful resources. With the use of ACOG’s report and other such resources, the set-up and the transition to in-office hysteroscopy need not be daunting. For most gynecologic surgeons, it will all feel comfortable after only a few procedures.
Dr. Cholkeri-Singh is with the University of Illinois at Chicago, and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital in Park Ridge, Ill. She is in private practice in Chicago. She is a consultant for Hologic, Bayer HealthCare, Olympus, Caldera Medical, Karl Storz, Medtronic, DYSIS Medical, and Channel Medsystems.
Understanding the new economic benefits of in-office hysteroscopy
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.
As a practicing reproductive endocrinologist and minimally invasive gynecologic surgeon, falling reimbursement has become routine. Furthermore, it was disadvantageous to perform in-office procedures while physician reimbursement was similar whether cases were performed in office, the hospital, or a surgery center. Higher procedural costs in the office, including reusable and disposable instrumentation and staffing, actually discouraged the physician who wanted to perform cases in the office, as it led to an overall reduction in reimbursement. Of course, certain outlying procedures have been reimbursed at a far greater rate in office and, as a result, global endometrial ablation and the Essure procedure now are generally performed in office.
In order to help us all understand the “nuts and bolts” behind the changes in physician compensation for in-office hysteroscopic procedures, I have once again called upon internationally recognized expert in hysteroscopic surgery, Aarathi Cholkeri-Singh, MD. At the AAGL 45th Global Congress on Minimally Invasive Gynecologic Surgery in 2016, Dr. Cholkeri-Singh was the chair and faculty of the postgraduate course, “Hysteroscopy 360° Beyond the Basics: Maximize Treatment, Minimize Failures.” At this year’s Global Congress, Dr. Cholkeri-Singh is a cochair of the postgraduate course “Advanced Operative Hysteroscopy: Expect the Unexpected.”
I am sure after reading Dr. Cholkeri-Singh’s comments, many of our readers of the Master Class in Gynecologic Surgery will add hysteroscopic surgery to their surgical repertoire.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago; director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. He is a consultant for Medtronic.