User login
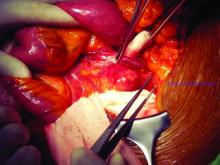
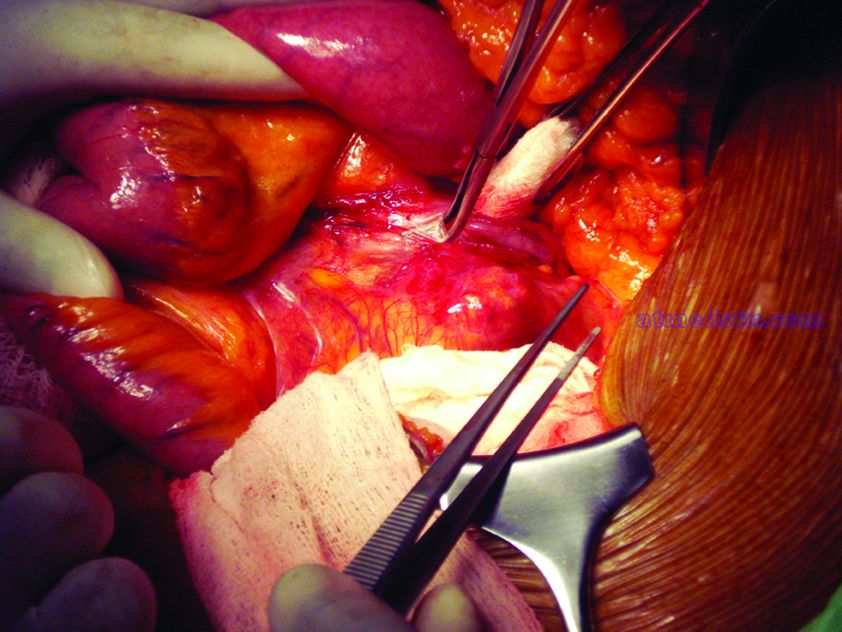
What repair is best for juxtarenal aneurysm?
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
EXPERT ANALYSIS FROM MIDWESTERN VASCULAR 2019
Chronic kidney disease may not be deterrent for B-FEVAR
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
REPORTING FROM MIDWESTERN VASCULAR 2019
Retinal artery blockage doesn’t necessarily portend stroke
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
REPORTING FROM MIDWESTERN VASCULAR 2019
Key clinical point: Retinal artery occlusion may not necessarily increase one’s stroke risk.
Major finding: The risk of stroke in patients with RAO was 2.3%.
Study details: A retrospective, single-institution review of 221 patients from 2004 to 2018.
Disclosures: Dr. Laczynski has no financial relationships to disclose.
Source: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
F-BEVAR safe in patients with one kidney
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
CHICAGO – Patients who have one kidney do as well after fenestrated-branched endovascular aneurysm repair (F-BEVAR) of pararenal or thoracoabdominal aortic aneurysm as patients with both kidneys, according to a study of almost 300 patients presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Despite the worse baseline renal function associated with single functioning kidney patients, F-BEVAR is safe and effective with nearly identical outcomes in patients with a SFK [single functioning kidney] as compared to patients with two functioning kidneys,” said Keouna Pather of Mayo Clinic, Rochester, Minn.
The study evaluated 287 F-BEVAR patients enrolled in a physician-sponsored investigation device exemption study from November 2013 to October 2018. Thirty of those patients had one kidney, the remaining 257 were the control group. Ms. Pather noted that characteristics were similar between both patient groups with the exception that SFK patients were younger (age 70 vs. 74 years; P = .009) and had larger renal artery diameter (6 vs. 5.7 mm; P = .05). “Patients with a SFK had enlargement of their renal artery in a compensatory fashion,” she said.
Survival at 2 years was 92% for SFK patients and 84% for controls.
“The SFK patients did start at a worse baseline of CKD [chronic kidney disease] stages as compared to controls,” she noted. In the SFK group, 63% (n = 19) had Stage III CKD versus 40% (n = 104) of controls (P = .02). Likewise, rates of Stage IV CKD were 10% (n = 3) and 2% (n = 4), respectively (P = .03).
In terms of outcomes, two patients in the control group died within 30 days but none in the SFK group did, Ms. Pather said. Also, a higher percentage of SFK patients had estimated blood loss greater than 1 L, compared with controls (20% vs. 7%; P = .02). All other outcomes, including rates of acute kidney injury (20% vs. 12%; P = .26), were not statistically different, she said.
“Between the groups, there was no significant difference in CKD progression that needed stenting,” she added, with 27% (n = 8) and 26% (n = 67) of the SFK and controls progressing to CKD Stages III to V.
The study also identified predictors of acute kidney injury in SFK patients: total fluoroscopy time (hours), which raised the risk by 78.5%, and estimated blood loss greater than 1 L, which increased risk by 109%.
Predictors of renal function deterioration in SFK patients were renal artery occlusion or reintervention for branch stenosis or kink, which raised the risk threefold; a Crawford extent II, which more than doubled the risk; and acute kidney injury, which raised chances almost fivefold. “Development of postoperative AKI [acute kidney injury] is the most important predictor for renal function deterioration,” Pather said.
When freedom from renal function deterioration at 2 years was compared between the two groups, again the results were similar because of the small sample size of the SFK group: 100% for the SFK group and 84% for controls.
Ms. Pather had no financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Key clinical point: Fenestrated-branched endovascular repair of abdominal aortic aneurysm is safe and effective.
Major finding: Two-year survival rates were 92% for one-kidney patients and 84% for those with two kidneys.
Study details: Retrospective review of a prospectively collected database of 287 patients who had F-BEVAR from 2013 to 2018.
Disclosures: Ms. Pather has no financial relationships to disclose.
Source: Pather K et al. Midwestern Vascular 2019, Abstract 2.
Isolated iliac disease a marker for better health status?
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
CHICAGO – Surgery and endovascular treatment for peripheral artery disease (PAD) among patients with claudication improves health status more in the setting of isolated iliac disease and multilevel disease than in other forms of PAD, which suggests that vascular specialists should give pause before pursuing interventions on superficial femoral and infrapopliteal artery lesions, a researcher of the PORTRAIT registry reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Our analysis demonstrated that interventions for aortoiliac disease and multilevel disease appeared to improve overall health status more over time compared to femoral-popliteal disease and infrapopliteal disease,” said Todd R. Vogel, MD, of the University of Missouri Health System in Columbia.
The study evaluated improvement in Peripheral Artery Questionnaire (PAQ) scores from baseline to post intervention in 623 patients in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry. The patients were selected and combined with anatomic data on the nature of their claudication. Aortoiliac-only (AI) disease represented 20.4% (n = 127) of the study group, femoral-popliteal-only (FP) 35.5% (n = 221), infrapopliteal/distal (IP) 6.3% (n = 39), and multilevel disease (ML) 37.9% (n = 236).
In terms of demographics, patients in the AI group tended to be younger (average age of 61.2 years vs. 66.6 years for the study overall; P less than .001) and had a higher rate of smokers (96.1% former and current smokers vs. 90.7% overall; P less than .001). Otherwise, Dr. Vogel noted, demographics, smoking status, and severity of claudication were similar across the disease groups.
Rates of medical intervention were similar in the AI and ML disease groups, which were primarily endovascular procedures: 26% and 27%, respectively. The AI group had the highest rates of endovascular interventions, at 24%, with the FP group at 15%, IP at 11% and ML at 21%. Those who did not have surgery or endovascular aneurysm repair were treated medically.
“The AI group did significantly better at 3 months than the other groups,” Dr. Vogel pointed out, noting that at 12 months those patients had an average PAQ score of around 78 versus scores of around 75 for FP, 74 for IP, and 70 for ML.
“In the AI group, there’s also an immediate increase in quality of life that is sustained over time,” he said. At 3 months, PAQ scores in AI patients who had endovascular aneurysm repair increased 41 points over baseline, leveling off to a 38.8-point gain at 12 months, the highest gains across all disease groups and all treatment categories.
“However,” Dr. Vogel added, “the group with ML disease probably was the most improved over time on the PAQ scores,” he said, explaining that across the board, this group had lower baseline PAQ scores than all the other groups.
“No significant benefits were found with intervention versus medical management for FP and IP,” he said. “This suggests that intervention should be considered after medical management has been exhausted.”
Dr. Vogel also said the findings support aggressive treatment of AI and ML for symptomatic claudication. “This anatomic region represents the greatest potential benefit for improving overall health status in patients with symptomatic PAD,” he said.
Dr. Vogel had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Study questions preemptive TEVAR for extended type A dissections
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
CHICAGO – The need for additional intervention after repair of the ascending aorta in extended type A aortic dissection has been thought to follow the practice for type B dissection and favor preemptive thoracic endovascular aortic repair. However, preemptive TEVAR may, at least in the midterm, provide no benefit in patients with extended type A dissections, according to results reported at the annual meeting of the Midwestern Vascular Surgery Society.
“TEVAR does not appear to be indicated in patients with extended type A dissections after acute aortic repair,” said Amy B. Reed, MD, of the University of Minnesota.
The study’s hypothesis was that growth rates of dissection and the need for additional intervention in the descending thoracic aorta are similar between extended type A (ExTA) and type B aortic dissection after initial repair of the ascending aorta. Dr. Reed noted that investigators from the INSTEAD-XL trial reported that preemptive TEVAR improved outcomes in patients with type B dissections (Circ Cardiovasc Interv. 2013;6:407-16). “The thinking has been that patients with uncomplicated ExTA would also benefit from early TEVAR,” Dr. Reed said.
The study evaluated 87 consecutive patients from 2011 to 2018, 43 with ExTA and 44 with type B dissections. Characteristics of both groups were similar, except the type B group had a significantly higher rate of coronary artery disease, 16% vs. 0% (P = .01). The distal extent of the dissection was beyond the aortic bifurcation in 75% of the ExTA patients and 52% of the type B group, “so we felt that these groups were really well matched,” Dr. Reed said.
Of the 43 ExTA patients, five had repair and 38 had no intervention. At an average follow-up of 33 months, 23 of the no-intervention patients showed no growth of their dissection, Dr. Reed said. In the type B group, 15 had no repair, and of those nine showed no growth (one patient died early and five did show growth).
“When we look at intervention-free survival, there’s a significant difference between our ExTA patients vs. our type B patients over time, with significantly more type B patients requiring intervention,” she said. At 28 months, 88% of ExTA were intervention free, whereas at 9 months 35% of type B patients were.
“We feel that, following the repair of ascending acute aortic dissection, in those patients with ExTA dissections, there does appear to be a slow progression of distal aortic disease,” Dr. Reed said. “Rarely do these patients develop complications such as dissection needing intervention either in the acute hospital period or delayed.”
Because the findings are based on medium-term follow-up, she said, “We certainly need further follow-up to confirm these midterm findings.”
Dr. Reed had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Hospitalist comanagement reduced odds of MI, shortened vascular surgery stays
CHICAGO – A care model that uses hospitalists to comanage vascular surgery patients cut myocardial infarction rates by more than half and reduced hospital stays by about 12%, according to results of a study of the hospitalist comanagement model from Loyola University Chicago, Maywood, Ill., presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Hospitalist comanagement was associated with decreased length of stay without affecting readmission for patients undergoing amputation, embolectomy, and infected graft,” said Kaavya Adam, a third-year medical student at Loyola University Chicago. “In the overall population, there was a reduction in cases of MI, 30-day readmissions, and overall length of stay.”
In 2014, Loyola implemented a program that used 11 hospitalists to rotate through the vascular surgery service. The hospitalists call on any patient who stays more than 24 hours on the non-ICU floors. Adam said hospitalist duties include evaluating patient comorbidities, adjusting medication, talking with family about medical management, seeing patients on the day of surgery, ordering preoperative labs, and meeting with the anesthesiology and vascular surgery teams.
The study compared outcomes in 866 patients admitted during 2007-2013, before the comanagement model was put into place, and 572 admitted during 2014-2017.
Rates of diabetes, hypertension, chronic kidney disease, coronary artery disease, hyperlipidemia, and malnutrition were similar between the groups. However, the pre-comanagement group had significantly higher rates of ischemic pain (27.8% vs. 10.7%), gangrene (21.3% vs. 13.6%) and ulceration (30.6% vs. 21.9%), while the comanaged group had significantly higher rates of claudication (34.3% vs. 13.2%). The statistical analysis accounted for these variations, Adam said.
“We did find significant results for the reduction in the odds of MI at 30 days; there was a 61% reduction,” he said.
The reduction in hospital stay was even more pronounced for patients with complex cases, Adam said. In amputation, the length of stay was reduced by 3.77 days (P = .01); in embolectomy, by 7.35 (P = .004); and in infected graft, by 8.35 (P = .007).
Continuing research will evaluate the cost effectiveness of the hospitalist model and define a comanagement model that is most beneficial, Mr. Adam said. He had no relevant financial disclosures.
SOURCE: Adam K et al. Midwestern Vascular 2019, Abstract 14.
CHICAGO – A care model that uses hospitalists to comanage vascular surgery patients cut myocardial infarction rates by more than half and reduced hospital stays by about 12%, according to results of a study of the hospitalist comanagement model from Loyola University Chicago, Maywood, Ill., presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Hospitalist comanagement was associated with decreased length of stay without affecting readmission for patients undergoing amputation, embolectomy, and infected graft,” said Kaavya Adam, a third-year medical student at Loyola University Chicago. “In the overall population, there was a reduction in cases of MI, 30-day readmissions, and overall length of stay.”
In 2014, Loyola implemented a program that used 11 hospitalists to rotate through the vascular surgery service. The hospitalists call on any patient who stays more than 24 hours on the non-ICU floors. Adam said hospitalist duties include evaluating patient comorbidities, adjusting medication, talking with family about medical management, seeing patients on the day of surgery, ordering preoperative labs, and meeting with the anesthesiology and vascular surgery teams.
The study compared outcomes in 866 patients admitted during 2007-2013, before the comanagement model was put into place, and 572 admitted during 2014-2017.
Rates of diabetes, hypertension, chronic kidney disease, coronary artery disease, hyperlipidemia, and malnutrition were similar between the groups. However, the pre-comanagement group had significantly higher rates of ischemic pain (27.8% vs. 10.7%), gangrene (21.3% vs. 13.6%) and ulceration (30.6% vs. 21.9%), while the comanaged group had significantly higher rates of claudication (34.3% vs. 13.2%). The statistical analysis accounted for these variations, Adam said.
“We did find significant results for the reduction in the odds of MI at 30 days; there was a 61% reduction,” he said.
The reduction in hospital stay was even more pronounced for patients with complex cases, Adam said. In amputation, the length of stay was reduced by 3.77 days (P = .01); in embolectomy, by 7.35 (P = .004); and in infected graft, by 8.35 (P = .007).
Continuing research will evaluate the cost effectiveness of the hospitalist model and define a comanagement model that is most beneficial, Mr. Adam said. He had no relevant financial disclosures.
SOURCE: Adam K et al. Midwestern Vascular 2019, Abstract 14.
CHICAGO – A care model that uses hospitalists to comanage vascular surgery patients cut myocardial infarction rates by more than half and reduced hospital stays by about 12%, according to results of a study of the hospitalist comanagement model from Loyola University Chicago, Maywood, Ill., presented at the annual meeting of the Midwestern Vascular Surgery Society.
“Hospitalist comanagement was associated with decreased length of stay without affecting readmission for patients undergoing amputation, embolectomy, and infected graft,” said Kaavya Adam, a third-year medical student at Loyola University Chicago. “In the overall population, there was a reduction in cases of MI, 30-day readmissions, and overall length of stay.”
In 2014, Loyola implemented a program that used 11 hospitalists to rotate through the vascular surgery service. The hospitalists call on any patient who stays more than 24 hours on the non-ICU floors. Adam said hospitalist duties include evaluating patient comorbidities, adjusting medication, talking with family about medical management, seeing patients on the day of surgery, ordering preoperative labs, and meeting with the anesthesiology and vascular surgery teams.
The study compared outcomes in 866 patients admitted during 2007-2013, before the comanagement model was put into place, and 572 admitted during 2014-2017.
Rates of diabetes, hypertension, chronic kidney disease, coronary artery disease, hyperlipidemia, and malnutrition were similar between the groups. However, the pre-comanagement group had significantly higher rates of ischemic pain (27.8% vs. 10.7%), gangrene (21.3% vs. 13.6%) and ulceration (30.6% vs. 21.9%), while the comanaged group had significantly higher rates of claudication (34.3% vs. 13.2%). The statistical analysis accounted for these variations, Adam said.
“We did find significant results for the reduction in the odds of MI at 30 days; there was a 61% reduction,” he said.
The reduction in hospital stay was even more pronounced for patients with complex cases, Adam said. In amputation, the length of stay was reduced by 3.77 days (P = .01); in embolectomy, by 7.35 (P = .004); and in infected graft, by 8.35 (P = .007).
Continuing research will evaluate the cost effectiveness of the hospitalist model and define a comanagement model that is most beneficial, Mr. Adam said. He had no relevant financial disclosures.
SOURCE: Adam K et al. Midwestern Vascular 2019, Abstract 14.
REPORTING FROM MIDWESTERN VASCULAR 2019
Key clinical point: Hospitalist comanagement of vascular surgery patients reduced hospital stays.
Major finding: Hospitalist comanagement significantly reduced the odds of MI at 30 days; a 61% reduction.
Study details: Database query of 1,438 vascular surgery admissions during 2007-2017.
Disclosures: Mr. Adam had no relevant financial disclosures.
Source: Adam K et al. Midwestern Vascular 2019, Abstract 14.
Trial: Stem cells may reduce AAA inflammation
CHICAGO – Early results of a trial evaluating allogeneic mesenchymal stem cells to target aortic inflammation in patients with small abdominal aortic aneurysms (AAAs) has reported encouraging early results, according to research presented here at the annual meeting of the Midwestern Vascular Surgery Society.
Katherin Leckie, MD, an integrated vascular surgery resident in the vascular surgery division, Indiana University, Indianapolis, reported on early results from the ARREST (Aneurysm Repression With Mesenchymal Stem Cells) trial. Twenty-one patients have been enrolled so far, and early results showed that treatment with mesenchymal stem cells (MSCs) increased levels of a key anti-inflammatory cell type, the Tr1, in proportion to a key proinflammatory cell type, the Th17.
“In AAA, the Tr1:Th17 ratio is decreased,” Dr. Leckie said. AAA serum stimulates MSC secretion of IL-10. Previously, mouse studies have shown MSCs restore the Tr1:Th17 balance and prevent aneurysms, Dr. Leckie noted.
The ARREST trial is evaluating that finding in humans with small AAAs of 35-50 mm in diameter. When the trial is fully enrolled, the 36 patients are to be evenly divided between three treatment groups: placebo; 1 million MSC/kg; and 3 million MSC/kg. The primary endpoint is change in Tr1:Th17 ratio at 14 days. Secondary endpoints are change in AAA inflammation at 2 weeks and change in AAA diameter and volume at 1-5 years.
At 14 days, the high-dose group had already seen a more than 100% change in Tr1:Th17 ratio (P = .03), versus about a 35% change for the low-dose and no change for the placebo groups. At 28 days, the high-dose group’s change increased to greater than 150%, while that of the low-dose group reached around 50%, with no change in the placebo group.
The study also found a possible trend toward changes in AAA diameter among the three groups after 1 year, Dr. Leckie said. In the placebo group, AAA diameter increased 3.5 mm on average. In the low-dose group, AAA diameter increased almost 1.5 mm. However, in the high-dose group, aortic diameter actually decreased about 0.5 mm (P = .189), Dr. Leckie said.
“MSCs have significantly improved the Tr1:Th17 imbalance at 14 days,” Dr. Leckie said. “There’s also a possible trend toward decreased aortic inflammation at 14 days as well as decreased growth of AAA at 12 months.”
Dr. Leckie had no relevant financial relationships to disclose.
CHICAGO – Early results of a trial evaluating allogeneic mesenchymal stem cells to target aortic inflammation in patients with small abdominal aortic aneurysms (AAAs) has reported encouraging early results, according to research presented here at the annual meeting of the Midwestern Vascular Surgery Society.
Katherin Leckie, MD, an integrated vascular surgery resident in the vascular surgery division, Indiana University, Indianapolis, reported on early results from the ARREST (Aneurysm Repression With Mesenchymal Stem Cells) trial. Twenty-one patients have been enrolled so far, and early results showed that treatment with mesenchymal stem cells (MSCs) increased levels of a key anti-inflammatory cell type, the Tr1, in proportion to a key proinflammatory cell type, the Th17.
“In AAA, the Tr1:Th17 ratio is decreased,” Dr. Leckie said. AAA serum stimulates MSC secretion of IL-10. Previously, mouse studies have shown MSCs restore the Tr1:Th17 balance and prevent aneurysms, Dr. Leckie noted.
The ARREST trial is evaluating that finding in humans with small AAAs of 35-50 mm in diameter. When the trial is fully enrolled, the 36 patients are to be evenly divided between three treatment groups: placebo; 1 million MSC/kg; and 3 million MSC/kg. The primary endpoint is change in Tr1:Th17 ratio at 14 days. Secondary endpoints are change in AAA inflammation at 2 weeks and change in AAA diameter and volume at 1-5 years.
At 14 days, the high-dose group had already seen a more than 100% change in Tr1:Th17 ratio (P = .03), versus about a 35% change for the low-dose and no change for the placebo groups. At 28 days, the high-dose group’s change increased to greater than 150%, while that of the low-dose group reached around 50%, with no change in the placebo group.
The study also found a possible trend toward changes in AAA diameter among the three groups after 1 year, Dr. Leckie said. In the placebo group, AAA diameter increased 3.5 mm on average. In the low-dose group, AAA diameter increased almost 1.5 mm. However, in the high-dose group, aortic diameter actually decreased about 0.5 mm (P = .189), Dr. Leckie said.
“MSCs have significantly improved the Tr1:Th17 imbalance at 14 days,” Dr. Leckie said. “There’s also a possible trend toward decreased aortic inflammation at 14 days as well as decreased growth of AAA at 12 months.”
Dr. Leckie had no relevant financial relationships to disclose.
CHICAGO – Early results of a trial evaluating allogeneic mesenchymal stem cells to target aortic inflammation in patients with small abdominal aortic aneurysms (AAAs) has reported encouraging early results, according to research presented here at the annual meeting of the Midwestern Vascular Surgery Society.
Katherin Leckie, MD, an integrated vascular surgery resident in the vascular surgery division, Indiana University, Indianapolis, reported on early results from the ARREST (Aneurysm Repression With Mesenchymal Stem Cells) trial. Twenty-one patients have been enrolled so far, and early results showed that treatment with mesenchymal stem cells (MSCs) increased levels of a key anti-inflammatory cell type, the Tr1, in proportion to a key proinflammatory cell type, the Th17.
“In AAA, the Tr1:Th17 ratio is decreased,” Dr. Leckie said. AAA serum stimulates MSC secretion of IL-10. Previously, mouse studies have shown MSCs restore the Tr1:Th17 balance and prevent aneurysms, Dr. Leckie noted.
The ARREST trial is evaluating that finding in humans with small AAAs of 35-50 mm in diameter. When the trial is fully enrolled, the 36 patients are to be evenly divided between three treatment groups: placebo; 1 million MSC/kg; and 3 million MSC/kg. The primary endpoint is change in Tr1:Th17 ratio at 14 days. Secondary endpoints are change in AAA inflammation at 2 weeks and change in AAA diameter and volume at 1-5 years.
At 14 days, the high-dose group had already seen a more than 100% change in Tr1:Th17 ratio (P = .03), versus about a 35% change for the low-dose and no change for the placebo groups. At 28 days, the high-dose group’s change increased to greater than 150%, while that of the low-dose group reached around 50%, with no change in the placebo group.
The study also found a possible trend toward changes in AAA diameter among the three groups after 1 year, Dr. Leckie said. In the placebo group, AAA diameter increased 3.5 mm on average. In the low-dose group, AAA diameter increased almost 1.5 mm. However, in the high-dose group, aortic diameter actually decreased about 0.5 mm (P = .189), Dr. Leckie said.
“MSCs have significantly improved the Tr1:Th17 imbalance at 14 days,” Dr. Leckie said. “There’s also a possible trend toward decreased aortic inflammation at 14 days as well as decreased growth of AAA at 12 months.”
Dr. Leckie had no relevant financial relationships to disclose.
REPORTING FROM MIDWESTERN VASCULAR 2019
Is EVAR for ruptured AAA worth revisiting?
CHICAGO – Numerous studies have shown conflicting results for endovascular repair in ruptured abdominal aortic aneurysms (AAA), but an analysis of 4,000-plus cases from a national registry has found a 41% reduction in mortality with endovascular repair vs. open repair, according to a presentation at the annual meeting of the Midwestern Vascular Surgery Society.
“EVAR is becoming an increasingly popular strategy for treatment of AAA,” said Samer Alharthi, MD, MPH, of the University of Toledo in Ohio. “As surgeon experience and endovascular technology have improved, a greater percentage of ruptured AAA are being treated by EVAR.”
Dr. Alharthi reported on a retrospective analysis of 4,133 patients who had repair for ruptured AAA in the American College of Surgeons National Surgical Quality Improvement Program database from 2010 to 2016. Notably, the number of EVAR repairs continue to increase and peaked in 2015, with 53% of ruptured AAA treated by EVAR.
Over the term of the study, the overall mortality rate was 22.6% for EVAR and 33.2% for open repair (P less than .001), Dr. Alharthi said. “After adjusting for cofounders, there was a 41% reduction in the mortality rate with the EVAR approach,” he said.
The only appreciable significant difference in demographics between the two groups was a higher percentage of smokers with chronic obstructive pulmonary disease having open repair – 942 (49.2%) vs. 701 (36.2%) – and a higher percentage of patients with end-stage renal disease having EVAR, Dr. Alharthi said. Other comorbidities had no statistically significant difference.
“Complications – pneumonia, reintubation, and acute renal failure – were higher in the open than the EVAR group,” he said. For example, rates of acute renal failure were 15.4% and 8.2% (P less than.001), respectively. Rates of myocardial infarction were similar between the two groups: 6.3% and 6% (P = .74), respectively.
Dr. Alharthi had no financial relationships to disclose.
SOURCE: Alharthi S et al. Midwestern Vascular 2019, Abstract 13.
CHICAGO – Numerous studies have shown conflicting results for endovascular repair in ruptured abdominal aortic aneurysms (AAA), but an analysis of 4,000-plus cases from a national registry has found a 41% reduction in mortality with endovascular repair vs. open repair, according to a presentation at the annual meeting of the Midwestern Vascular Surgery Society.
“EVAR is becoming an increasingly popular strategy for treatment of AAA,” said Samer Alharthi, MD, MPH, of the University of Toledo in Ohio. “As surgeon experience and endovascular technology have improved, a greater percentage of ruptured AAA are being treated by EVAR.”
Dr. Alharthi reported on a retrospective analysis of 4,133 patients who had repair for ruptured AAA in the American College of Surgeons National Surgical Quality Improvement Program database from 2010 to 2016. Notably, the number of EVAR repairs continue to increase and peaked in 2015, with 53% of ruptured AAA treated by EVAR.
Over the term of the study, the overall mortality rate was 22.6% for EVAR and 33.2% for open repair (P less than .001), Dr. Alharthi said. “After adjusting for cofounders, there was a 41% reduction in the mortality rate with the EVAR approach,” he said.
The only appreciable significant difference in demographics between the two groups was a higher percentage of smokers with chronic obstructive pulmonary disease having open repair – 942 (49.2%) vs. 701 (36.2%) – and a higher percentage of patients with end-stage renal disease having EVAR, Dr. Alharthi said. Other comorbidities had no statistically significant difference.
“Complications – pneumonia, reintubation, and acute renal failure – were higher in the open than the EVAR group,” he said. For example, rates of acute renal failure were 15.4% and 8.2% (P less than.001), respectively. Rates of myocardial infarction were similar between the two groups: 6.3% and 6% (P = .74), respectively.
Dr. Alharthi had no financial relationships to disclose.
SOURCE: Alharthi S et al. Midwestern Vascular 2019, Abstract 13.
CHICAGO – Numerous studies have shown conflicting results for endovascular repair in ruptured abdominal aortic aneurysms (AAA), but an analysis of 4,000-plus cases from a national registry has found a 41% reduction in mortality with endovascular repair vs. open repair, according to a presentation at the annual meeting of the Midwestern Vascular Surgery Society.
“EVAR is becoming an increasingly popular strategy for treatment of AAA,” said Samer Alharthi, MD, MPH, of the University of Toledo in Ohio. “As surgeon experience and endovascular technology have improved, a greater percentage of ruptured AAA are being treated by EVAR.”
Dr. Alharthi reported on a retrospective analysis of 4,133 patients who had repair for ruptured AAA in the American College of Surgeons National Surgical Quality Improvement Program database from 2010 to 2016. Notably, the number of EVAR repairs continue to increase and peaked in 2015, with 53% of ruptured AAA treated by EVAR.
Over the term of the study, the overall mortality rate was 22.6% for EVAR and 33.2% for open repair (P less than .001), Dr. Alharthi said. “After adjusting for cofounders, there was a 41% reduction in the mortality rate with the EVAR approach,” he said.
The only appreciable significant difference in demographics between the two groups was a higher percentage of smokers with chronic obstructive pulmonary disease having open repair – 942 (49.2%) vs. 701 (36.2%) – and a higher percentage of patients with end-stage renal disease having EVAR, Dr. Alharthi said. Other comorbidities had no statistically significant difference.
“Complications – pneumonia, reintubation, and acute renal failure – were higher in the open than the EVAR group,” he said. For example, rates of acute renal failure were 15.4% and 8.2% (P less than.001), respectively. Rates of myocardial infarction were similar between the two groups: 6.3% and 6% (P = .74), respectively.
Dr. Alharthi had no financial relationships to disclose.
SOURCE: Alharthi S et al. Midwestern Vascular 2019, Abstract 13.
REPORTING FROM MIDWESTERN VASCULAR 2019