User login
STEMI: Prereperfusion IV metoprolol shows long-term benefits
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
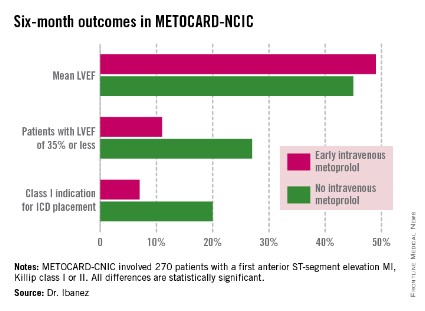
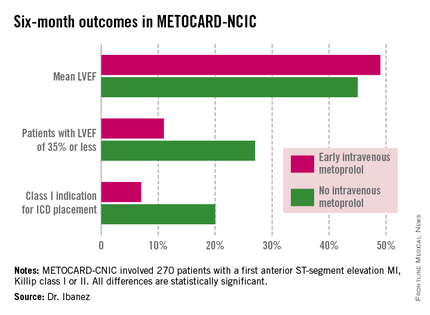
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.
METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
AT ACC 14
Key clinical point: Prereperfusion IV metoprolol may reduce heart failure readmissions.
Major finding: Patients with anterior ST-elevation MI who received intravenous metoprolol prior to primary PCI had a significantly greater left ventricular ejection fraction at 6 months follow-up than those who didn’t. They also were 68% less likely to be hospitalized for heart failure during 2 years of follow-up.
Data source: A six-center prospective trial in which 270 Spanish patients with a first anterior STEMI were randomized to have administration of IV metoprolol or not while being transported for primary PCI.
Disclosures: The METOCARD-CNIC trial was sponsored by the Spanish Ministry of Health and Social Policy and the Carlos III National Center for Cardiovascular Investigations. The presenter reported having no financial conflicts.
Registry renal denervation skews from resistant hypertension
WASHINGTON – Renal denervation may have struck out as antihypertensive therapy in the sham-controlled SYMPLICITY HTN-3 trial reported in March at the American College of Cardiology’s annual meeting, but an independent report at the same meeting from a worldwide renal denervation registry showed that the vast majority of patients who’ve undergone with renal denervation recently were nothing like the patients enrolled in the failed trial.
In fact, patients who met enrollment criteria for the SYMPLICITY HTN-3 trial constituted a bare 8% of "real world" patients treated with renal denervation, based on results from the first 1,000 patients enrolled in the Global SYMPLICITY Registry, said Dr. Michael Böhm, professor and chairman of cardiology at Saarland University Hospital in Homburg/Saar, Germany.
Out of the 751 patients of the first 1,000 in the registry with data available on their office systolic blood pressure 6 months after treatment, 62 patients (8%) fulfilled the entry criteria of the SYMPLICITY HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension) trial by having an office systolic pressure of at least 160 mm Hg, a systolic pressure of at least 135 mm Hg by ambulatory blood pressure monitoring, and on treatment at maximally tolerated dosages with at least three different classes of antihypertensive medications (N. Engl. J. Med. 2014;370:1393-1401).
This low level of patients with clinically confirmed, resistant hypertension occurred against a backdrop in which a scant majority of registry patients even had severe hypertension, let alone a medically uncontrolled form. Among the 751 patients with 6-month follow-up blood pressure data, 433 (58%) received renal denervation with an office systolic blood pressure at baseline of at least 160 mm Hg, said Dr. Böhm.
"SYMPLICITY HTN-3 covered only a few percent of patients" who undergo renal denervation, he said. "The goal of the registry is to include a broad population, including patients without hypertension but other conditions associated with overactivity of the sympathetic nervous system" such as arrhythmia or heart failure.
Among the 433 patients with an office systolic blood pressure of at least 160 mm Hg – the minimum level of hypertension to warrant routine treatment by renal denervation according to several current policies – 244 (56%) were at this high pressure despite treatment with at least three classes of antihypertensive drugs. In this more clearly drug-resistant group, office systolic pressure fell by an average 20.2 mm Hg at 6 months after renal denervation. But only 62 patients on at least three types of drugs were also at maximally tolerated dosages of these drugs, another key element in defining drug resistance. Patients on maximally tolerated dosages represented only a quarter of all patients on multiple drugs in the Registry, and just 14% of the 433 patients with severe hypertension.
Among the 62 patients who matched the SYMPLICITY HTN-3 enrollment criteria, the average drop in office, systolic blood pressure at 6 months after treatment was 17.3 mm Hg, not too different from the average drop of 14.1 mm Hg seen in the renal denervation arm of SYMPLICITY HTN-3, Dr. Böhm noted.
The registry results showed that in patients with an office systolic pressure of less than 160 mm Hg, renal denervation was lousy at blood pressure reduction. Among the 222 patients who had pretreatment systolic pressures of 140-159 mm Hg and 6-month follow-up, office systolic blood pressure dropped by an average of 4.6 mm Hg. The procedure was even less effective in the 96 registry patients with an office systolic pressure of less than 140 mm Hg prior to treatment. In this subgroup systolic pressures averaged a 14.2-mm Hg increase 6 months after treatment.
"The only significant interaction between blood pressure lowering and no lowering was blood pressure at baseline," Dr. Böhm said. He gave no details on how many patients in the registry underwent renal denervation to treat hypertension and how many for other reasons.
The Global SYMPLICITY Register involves more than 200 centers in many European countries as well as in Canada, Australia, Korea, and elsewhere. A large number of registry centers are in Germany, and many centers there and elsewhere participated in the first two SYMPLICITY HTN trials. Participating centers entered the registry based on their experience with renal denervation and had to have performed at least 30 procedures prior to entry into the registry. The 6-month data also showed that renal denervation was "very safe," with "very low" rates of adverse events, Dr. Böhm said.
The Global SYMPLICITY Registry is sponsored by Medtronic, the company that markets Symplicity renal denervation devices. Dr, Böhm said that he has served on an advisory board to, been a speaker for, and received research support from Medtronic as well as from several other drug and device companies.
On Twitter @mitchelzoler
WASHINGTON – Renal denervation may have struck out as antihypertensive therapy in the sham-controlled SYMPLICITY HTN-3 trial reported in March at the American College of Cardiology’s annual meeting, but an independent report at the same meeting from a worldwide renal denervation registry showed that the vast majority of patients who’ve undergone with renal denervation recently were nothing like the patients enrolled in the failed trial.
In fact, patients who met enrollment criteria for the SYMPLICITY HTN-3 trial constituted a bare 8% of "real world" patients treated with renal denervation, based on results from the first 1,000 patients enrolled in the Global SYMPLICITY Registry, said Dr. Michael Böhm, professor and chairman of cardiology at Saarland University Hospital in Homburg/Saar, Germany.
Out of the 751 patients of the first 1,000 in the registry with data available on their office systolic blood pressure 6 months after treatment, 62 patients (8%) fulfilled the entry criteria of the SYMPLICITY HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension) trial by having an office systolic pressure of at least 160 mm Hg, a systolic pressure of at least 135 mm Hg by ambulatory blood pressure monitoring, and on treatment at maximally tolerated dosages with at least three different classes of antihypertensive medications (N. Engl. J. Med. 2014;370:1393-1401).
This low level of patients with clinically confirmed, resistant hypertension occurred against a backdrop in which a scant majority of registry patients even had severe hypertension, let alone a medically uncontrolled form. Among the 751 patients with 6-month follow-up blood pressure data, 433 (58%) received renal denervation with an office systolic blood pressure at baseline of at least 160 mm Hg, said Dr. Böhm.
"SYMPLICITY HTN-3 covered only a few percent of patients" who undergo renal denervation, he said. "The goal of the registry is to include a broad population, including patients without hypertension but other conditions associated with overactivity of the sympathetic nervous system" such as arrhythmia or heart failure.
Among the 433 patients with an office systolic blood pressure of at least 160 mm Hg – the minimum level of hypertension to warrant routine treatment by renal denervation according to several current policies – 244 (56%) were at this high pressure despite treatment with at least three classes of antihypertensive drugs. In this more clearly drug-resistant group, office systolic pressure fell by an average 20.2 mm Hg at 6 months after renal denervation. But only 62 patients on at least three types of drugs were also at maximally tolerated dosages of these drugs, another key element in defining drug resistance. Patients on maximally tolerated dosages represented only a quarter of all patients on multiple drugs in the Registry, and just 14% of the 433 patients with severe hypertension.
Among the 62 patients who matched the SYMPLICITY HTN-3 enrollment criteria, the average drop in office, systolic blood pressure at 6 months after treatment was 17.3 mm Hg, not too different from the average drop of 14.1 mm Hg seen in the renal denervation arm of SYMPLICITY HTN-3, Dr. Böhm noted.
The registry results showed that in patients with an office systolic pressure of less than 160 mm Hg, renal denervation was lousy at blood pressure reduction. Among the 222 patients who had pretreatment systolic pressures of 140-159 mm Hg and 6-month follow-up, office systolic blood pressure dropped by an average of 4.6 mm Hg. The procedure was even less effective in the 96 registry patients with an office systolic pressure of less than 140 mm Hg prior to treatment. In this subgroup systolic pressures averaged a 14.2-mm Hg increase 6 months after treatment.
"The only significant interaction between blood pressure lowering and no lowering was blood pressure at baseline," Dr. Böhm said. He gave no details on how many patients in the registry underwent renal denervation to treat hypertension and how many for other reasons.
The Global SYMPLICITY Register involves more than 200 centers in many European countries as well as in Canada, Australia, Korea, and elsewhere. A large number of registry centers are in Germany, and many centers there and elsewhere participated in the first two SYMPLICITY HTN trials. Participating centers entered the registry based on their experience with renal denervation and had to have performed at least 30 procedures prior to entry into the registry. The 6-month data also showed that renal denervation was "very safe," with "very low" rates of adverse events, Dr. Böhm said.
The Global SYMPLICITY Registry is sponsored by Medtronic, the company that markets Symplicity renal denervation devices. Dr, Böhm said that he has served on an advisory board to, been a speaker for, and received research support from Medtronic as well as from several other drug and device companies.
On Twitter @mitchelzoler
WASHINGTON – Renal denervation may have struck out as antihypertensive therapy in the sham-controlled SYMPLICITY HTN-3 trial reported in March at the American College of Cardiology’s annual meeting, but an independent report at the same meeting from a worldwide renal denervation registry showed that the vast majority of patients who’ve undergone with renal denervation recently were nothing like the patients enrolled in the failed trial.
In fact, patients who met enrollment criteria for the SYMPLICITY HTN-3 trial constituted a bare 8% of "real world" patients treated with renal denervation, based on results from the first 1,000 patients enrolled in the Global SYMPLICITY Registry, said Dr. Michael Böhm, professor and chairman of cardiology at Saarland University Hospital in Homburg/Saar, Germany.
Out of the 751 patients of the first 1,000 in the registry with data available on their office systolic blood pressure 6 months after treatment, 62 patients (8%) fulfilled the entry criteria of the SYMPLICITY HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension) trial by having an office systolic pressure of at least 160 mm Hg, a systolic pressure of at least 135 mm Hg by ambulatory blood pressure monitoring, and on treatment at maximally tolerated dosages with at least three different classes of antihypertensive medications (N. Engl. J. Med. 2014;370:1393-1401).
This low level of patients with clinically confirmed, resistant hypertension occurred against a backdrop in which a scant majority of registry patients even had severe hypertension, let alone a medically uncontrolled form. Among the 751 patients with 6-month follow-up blood pressure data, 433 (58%) received renal denervation with an office systolic blood pressure at baseline of at least 160 mm Hg, said Dr. Böhm.
"SYMPLICITY HTN-3 covered only a few percent of patients" who undergo renal denervation, he said. "The goal of the registry is to include a broad population, including patients without hypertension but other conditions associated with overactivity of the sympathetic nervous system" such as arrhythmia or heart failure.
Among the 433 patients with an office systolic blood pressure of at least 160 mm Hg – the minimum level of hypertension to warrant routine treatment by renal denervation according to several current policies – 244 (56%) were at this high pressure despite treatment with at least three classes of antihypertensive drugs. In this more clearly drug-resistant group, office systolic pressure fell by an average 20.2 mm Hg at 6 months after renal denervation. But only 62 patients on at least three types of drugs were also at maximally tolerated dosages of these drugs, another key element in defining drug resistance. Patients on maximally tolerated dosages represented only a quarter of all patients on multiple drugs in the Registry, and just 14% of the 433 patients with severe hypertension.
Among the 62 patients who matched the SYMPLICITY HTN-3 enrollment criteria, the average drop in office, systolic blood pressure at 6 months after treatment was 17.3 mm Hg, not too different from the average drop of 14.1 mm Hg seen in the renal denervation arm of SYMPLICITY HTN-3, Dr. Böhm noted.
The registry results showed that in patients with an office systolic pressure of less than 160 mm Hg, renal denervation was lousy at blood pressure reduction. Among the 222 patients who had pretreatment systolic pressures of 140-159 mm Hg and 6-month follow-up, office systolic blood pressure dropped by an average of 4.6 mm Hg. The procedure was even less effective in the 96 registry patients with an office systolic pressure of less than 140 mm Hg prior to treatment. In this subgroup systolic pressures averaged a 14.2-mm Hg increase 6 months after treatment.
"The only significant interaction between blood pressure lowering and no lowering was blood pressure at baseline," Dr. Böhm said. He gave no details on how many patients in the registry underwent renal denervation to treat hypertension and how many for other reasons.
The Global SYMPLICITY Register involves more than 200 centers in many European countries as well as in Canada, Australia, Korea, and elsewhere. A large number of registry centers are in Germany, and many centers there and elsewhere participated in the first two SYMPLICITY HTN trials. Participating centers entered the registry based on their experience with renal denervation and had to have performed at least 30 procedures prior to entry into the registry. The 6-month data also showed that renal denervation was "very safe," with "very low" rates of adverse events, Dr. Böhm said.
The Global SYMPLICITY Registry is sponsored by Medtronic, the company that markets Symplicity renal denervation devices. Dr, Böhm said that he has served on an advisory board to, been a speaker for, and received research support from Medtronic as well as from several other drug and device companies.
On Twitter @mitchelzoler
AT ACC 2014
Key clinical point: Real-world use of renal denervation has not targeted patients with drug-resistant hypertension.
Major finding: Only 8% of patient in the Global SYMPLICITY Registry for renal denervation matched the patients enrolled in the SYMPLICITY HTN-3 trial.
Data source: The Global SYMPLICITY Registry, which enrolled 1,000 patients who underwent renal denervation at more than 200 centers worldwide.
Disclosures: The registry is sponsored by Medtronic, the company that markets Symplicity renal denervation devices. Dr, Böhm said that he has served on an advisory board to, been a speaker for, and received research support from Medtronic as well as from several other drug and device companies.
Heart failure: Quality of life, diastolic function rose with intensity-interval exercise
WASHINGTON – A high-intensity cardiac rehabilitation program safely improved quality of life, diastolic function, depressive symptoms, and physical fitness in patients with systolic heart failure and reduced ejection fraction in a randomized controlled trial.
In the subgroup of study participants over age 65, however, the benefits were limited and the dropout rate high, Dr. Christina Chrysohoou said at the annual meeting of the American College of Cardiology. "I think patients over age 65 may benefit more from a less-intensive exercise time and rest periods."
Her study included 100 consecutive patients with a left ventricular ejection fraction (LVEF) below 30%; heart failure of ischemic etiology was present in 70%. One-third of subjects had an implantable cardioverter-defibrillator at study entry. All participants underwent pre-enrollment Holter monitoring with electrophysiologic follow-up as warranted. Participants were randomized to a high-intensity exercise program or to a control arm of standard dietary advice and a recommendation to walk for up to 2 miles daily, said Dr. Chrysohoou, a cardiologist at the University of Athens.
The exercise program consisted of a warm-up followed a 30-minute session of alternating 30-second bursts of ergometric exercise at 100% of a patient’s maximum workload followed by 30 seconds of recovery at 40%-60% of maximum workload. Maximum workload was determined from a baseline treadmill exercise test. The 12-week study was completed by 33 of 50 patients in the high-intensity exercise group and 39 of 50 controls.
Quality of life scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean score of 21 at baseline to 7 in the intensive-exercise group, and declined slightly from 19 to 21 in the control group.
At baseline, the mean score on the Zung Depression Scale was 37 out of a possible 80; at 12 weeks, scores improved to 30 in the high-intensity exercise group and increased to 41 in the controls.
Maximal oxygen consumption, or VO2max, improved from 16 to 21 mL/kg per minute in the interval-exercise group while remaining unchanged in controls. Similarly, peak power output increased from 84 to 105 W in the exercise program participants while remaining unchanged in controls. Six-minute walk time improved from 422 m to 476 m in the intensive exercisers, a 13% better result than that seen in controls.
Diastolic function on Doppler imaging significantly improved in the exercise group but not in the controls. The E/A wave ratio, which represents the relationship between early passive left ventricular filling and atrial contraction in late diastole, decreased by 24%. Also, the left ventricular outflow velocity integral increased by 4%.
There were no adverse events in either study arm.
LVEF did not significantly improve in either study arm, but that was not surprising, said Dr. Chrysohoou. "You may have an LVEF of 20% and be able to run a marathon, or an LVEF of 30%-35% and not even be able to walk around the house."
This was an unfunded study, and Dr. Chrysohoou reported having no financial conflicts.
WASHINGTON – A high-intensity cardiac rehabilitation program safely improved quality of life, diastolic function, depressive symptoms, and physical fitness in patients with systolic heart failure and reduced ejection fraction in a randomized controlled trial.
In the subgroup of study participants over age 65, however, the benefits were limited and the dropout rate high, Dr. Christina Chrysohoou said at the annual meeting of the American College of Cardiology. "I think patients over age 65 may benefit more from a less-intensive exercise time and rest periods."
Her study included 100 consecutive patients with a left ventricular ejection fraction (LVEF) below 30%; heart failure of ischemic etiology was present in 70%. One-third of subjects had an implantable cardioverter-defibrillator at study entry. All participants underwent pre-enrollment Holter monitoring with electrophysiologic follow-up as warranted. Participants were randomized to a high-intensity exercise program or to a control arm of standard dietary advice and a recommendation to walk for up to 2 miles daily, said Dr. Chrysohoou, a cardiologist at the University of Athens.
The exercise program consisted of a warm-up followed a 30-minute session of alternating 30-second bursts of ergometric exercise at 100% of a patient’s maximum workload followed by 30 seconds of recovery at 40%-60% of maximum workload. Maximum workload was determined from a baseline treadmill exercise test. The 12-week study was completed by 33 of 50 patients in the high-intensity exercise group and 39 of 50 controls.
Quality of life scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean score of 21 at baseline to 7 in the intensive-exercise group, and declined slightly from 19 to 21 in the control group.
At baseline, the mean score on the Zung Depression Scale was 37 out of a possible 80; at 12 weeks, scores improved to 30 in the high-intensity exercise group and increased to 41 in the controls.
Maximal oxygen consumption, or VO2max, improved from 16 to 21 mL/kg per minute in the interval-exercise group while remaining unchanged in controls. Similarly, peak power output increased from 84 to 105 W in the exercise program participants while remaining unchanged in controls. Six-minute walk time improved from 422 m to 476 m in the intensive exercisers, a 13% better result than that seen in controls.
Diastolic function on Doppler imaging significantly improved in the exercise group but not in the controls. The E/A wave ratio, which represents the relationship between early passive left ventricular filling and atrial contraction in late diastole, decreased by 24%. Also, the left ventricular outflow velocity integral increased by 4%.
There were no adverse events in either study arm.
LVEF did not significantly improve in either study arm, but that was not surprising, said Dr. Chrysohoou. "You may have an LVEF of 20% and be able to run a marathon, or an LVEF of 30%-35% and not even be able to walk around the house."
This was an unfunded study, and Dr. Chrysohoou reported having no financial conflicts.
WASHINGTON – A high-intensity cardiac rehabilitation program safely improved quality of life, diastolic function, depressive symptoms, and physical fitness in patients with systolic heart failure and reduced ejection fraction in a randomized controlled trial.
In the subgroup of study participants over age 65, however, the benefits were limited and the dropout rate high, Dr. Christina Chrysohoou said at the annual meeting of the American College of Cardiology. "I think patients over age 65 may benefit more from a less-intensive exercise time and rest periods."
Her study included 100 consecutive patients with a left ventricular ejection fraction (LVEF) below 30%; heart failure of ischemic etiology was present in 70%. One-third of subjects had an implantable cardioverter-defibrillator at study entry. All participants underwent pre-enrollment Holter monitoring with electrophysiologic follow-up as warranted. Participants were randomized to a high-intensity exercise program or to a control arm of standard dietary advice and a recommendation to walk for up to 2 miles daily, said Dr. Chrysohoou, a cardiologist at the University of Athens.
The exercise program consisted of a warm-up followed a 30-minute session of alternating 30-second bursts of ergometric exercise at 100% of a patient’s maximum workload followed by 30 seconds of recovery at 40%-60% of maximum workload. Maximum workload was determined from a baseline treadmill exercise test. The 12-week study was completed by 33 of 50 patients in the high-intensity exercise group and 39 of 50 controls.
Quality of life scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean score of 21 at baseline to 7 in the intensive-exercise group, and declined slightly from 19 to 21 in the control group.
At baseline, the mean score on the Zung Depression Scale was 37 out of a possible 80; at 12 weeks, scores improved to 30 in the high-intensity exercise group and increased to 41 in the controls.
Maximal oxygen consumption, or VO2max, improved from 16 to 21 mL/kg per minute in the interval-exercise group while remaining unchanged in controls. Similarly, peak power output increased from 84 to 105 W in the exercise program participants while remaining unchanged in controls. Six-minute walk time improved from 422 m to 476 m in the intensive exercisers, a 13% better result than that seen in controls.
Diastolic function on Doppler imaging significantly improved in the exercise group but not in the controls. The E/A wave ratio, which represents the relationship between early passive left ventricular filling and atrial contraction in late diastole, decreased by 24%. Also, the left ventricular outflow velocity integral increased by 4%.
There were no adverse events in either study arm.
LVEF did not significantly improve in either study arm, but that was not surprising, said Dr. Chrysohoou. "You may have an LVEF of 20% and be able to run a marathon, or an LVEF of 30%-35% and not even be able to walk around the house."
This was an unfunded study, and Dr. Chrysohoou reported having no financial conflicts.
AT ACC 14
Key clinical point: Cardiac rehabilitation benefits systolic heart failure patients, especially those under age 65.
Major finding: Quality of life scores on the Minnesota Living with Heart Failure Questionnaire improved from a mean of 21 to 7 over the course of a 12-week structured program of high-intensity interval exercise.
Data source: A 12-week, randomized, prospective study of 100 consecutive patients with heart failure and an LVEF below 30%.
Disclosures: This study was unfunded, and Dr. Chrysohoou reported having no financial conflicts.
Eccentric exercise tamed elevated liver enzymes
WASHINGTON – Eccentric exercise in the form of downhill walking is a promising new means of reducing elevated liver enzyme levels in overweight and obese sedentary individuals.
This finding suggests this form of exercise may have a role as a therapeutic intervention in patients with nonalcoholic fatty liver disease, a condition which is rapidly growing in prevalence and which is associated with increased risks of diabetes and cardiovascular events, Dr. Christoph Saely observed at the annual meeting of the American College of Cardiology.
It’s known that physical exercise can be effective in lowering elevated hepatic enzymes, but it’s equally clear that conventional forms of exercise such as stair-climbing, jogging, and brisk walking are too vigorous and demanding for many obese sedentary patients. They’re unable to get with the program.
That’s where eccentric training holds particular value, according to Dr. Saely of the Academic Teaching Hospital of Feldkirch, Austria.
Eccentric exercise occurs when active muscle contraction occurs simultaneously with the muscle’s lengthening. Thus, it involves active resistance to stretching. During a biceps curl, for example, the eccentric muscle work occurs as the weight is being lowered, while concentric muscle training happens as the weight is being raised. Similarly, walking uphill involves concentric muscle contraction, while walking downhill emphasizes eccentric muscle action.
Eccentric exercise training creates more force yet uses less energy. It protects joints from damage. Physical therapists and fitness trainers find eccentric exercise regimens are particularly useful in the elderly, infirm, and sedentary because such individuals find it less exhausting and they are more willing to participate.
Dr. Saely and his coinvestigators have an ideal outdoor laboratory in which to conduct a pilot study of the effects of eccentric exercise because their hospital is located in the Austrian Alps. Subjects can hike downhill, then take a cable car back up, with adherence to the program monitored electronically at the vehicle entrance.
He reported on 42 overweight or obese sedentary patients with elevated liver enzymes and 12 matched controls who were assigned to hike down a predefined route in the Alps three to five times per week for 8 weeks. The course featured a 540-meter drop in altitude.
Mean serum alanine aminotransferase levels in the overweight group dropped from 36 IU/L at baseline to 31 IU/L at 8 weeks. Serum gamma-glutamyl transferase fell from 56 IU/L to 44 IU/L. And the alanine aminotransferase/aspartate aminotransferase ratio improved from 1.22 IU/L to 1.02 IU/L. Meanwhile, liver enzyme levels remained unchanged over time in the controls.
The eccentric exercise regimen was well tolerated, participation was high, and there were no serious adverse events, according to Dr. Saely.
While not everyone is fortunate enough to have a mountain in the backyard with a gondola for the return trip, knowledgeable fitness instructors can demonstrate eccentric exercises using weights and machines that target large muscle groups.
Dr. Saely reported having no conflicts of interest regarding this study.
WASHINGTON – Eccentric exercise in the form of downhill walking is a promising new means of reducing elevated liver enzyme levels in overweight and obese sedentary individuals.
This finding suggests this form of exercise may have a role as a therapeutic intervention in patients with nonalcoholic fatty liver disease, a condition which is rapidly growing in prevalence and which is associated with increased risks of diabetes and cardiovascular events, Dr. Christoph Saely observed at the annual meeting of the American College of Cardiology.
It’s known that physical exercise can be effective in lowering elevated hepatic enzymes, but it’s equally clear that conventional forms of exercise such as stair-climbing, jogging, and brisk walking are too vigorous and demanding for many obese sedentary patients. They’re unable to get with the program.
That’s where eccentric training holds particular value, according to Dr. Saely of the Academic Teaching Hospital of Feldkirch, Austria.
Eccentric exercise occurs when active muscle contraction occurs simultaneously with the muscle’s lengthening. Thus, it involves active resistance to stretching. During a biceps curl, for example, the eccentric muscle work occurs as the weight is being lowered, while concentric muscle training happens as the weight is being raised. Similarly, walking uphill involves concentric muscle contraction, while walking downhill emphasizes eccentric muscle action.
Eccentric exercise training creates more force yet uses less energy. It protects joints from damage. Physical therapists and fitness trainers find eccentric exercise regimens are particularly useful in the elderly, infirm, and sedentary because such individuals find it less exhausting and they are more willing to participate.
Dr. Saely and his coinvestigators have an ideal outdoor laboratory in which to conduct a pilot study of the effects of eccentric exercise because their hospital is located in the Austrian Alps. Subjects can hike downhill, then take a cable car back up, with adherence to the program monitored electronically at the vehicle entrance.
He reported on 42 overweight or obese sedentary patients with elevated liver enzymes and 12 matched controls who were assigned to hike down a predefined route in the Alps three to five times per week for 8 weeks. The course featured a 540-meter drop in altitude.
Mean serum alanine aminotransferase levels in the overweight group dropped from 36 IU/L at baseline to 31 IU/L at 8 weeks. Serum gamma-glutamyl transferase fell from 56 IU/L to 44 IU/L. And the alanine aminotransferase/aspartate aminotransferase ratio improved from 1.22 IU/L to 1.02 IU/L. Meanwhile, liver enzyme levels remained unchanged over time in the controls.
The eccentric exercise regimen was well tolerated, participation was high, and there were no serious adverse events, according to Dr. Saely.
While not everyone is fortunate enough to have a mountain in the backyard with a gondola for the return trip, knowledgeable fitness instructors can demonstrate eccentric exercises using weights and machines that target large muscle groups.
Dr. Saely reported having no conflicts of interest regarding this study.
WASHINGTON – Eccentric exercise in the form of downhill walking is a promising new means of reducing elevated liver enzyme levels in overweight and obese sedentary individuals.
This finding suggests this form of exercise may have a role as a therapeutic intervention in patients with nonalcoholic fatty liver disease, a condition which is rapidly growing in prevalence and which is associated with increased risks of diabetes and cardiovascular events, Dr. Christoph Saely observed at the annual meeting of the American College of Cardiology.
It’s known that physical exercise can be effective in lowering elevated hepatic enzymes, but it’s equally clear that conventional forms of exercise such as stair-climbing, jogging, and brisk walking are too vigorous and demanding for many obese sedentary patients. They’re unable to get with the program.
That’s where eccentric training holds particular value, according to Dr. Saely of the Academic Teaching Hospital of Feldkirch, Austria.
Eccentric exercise occurs when active muscle contraction occurs simultaneously with the muscle’s lengthening. Thus, it involves active resistance to stretching. During a biceps curl, for example, the eccentric muscle work occurs as the weight is being lowered, while concentric muscle training happens as the weight is being raised. Similarly, walking uphill involves concentric muscle contraction, while walking downhill emphasizes eccentric muscle action.
Eccentric exercise training creates more force yet uses less energy. It protects joints from damage. Physical therapists and fitness trainers find eccentric exercise regimens are particularly useful in the elderly, infirm, and sedentary because such individuals find it less exhausting and they are more willing to participate.
Dr. Saely and his coinvestigators have an ideal outdoor laboratory in which to conduct a pilot study of the effects of eccentric exercise because their hospital is located in the Austrian Alps. Subjects can hike downhill, then take a cable car back up, with adherence to the program monitored electronically at the vehicle entrance.
He reported on 42 overweight or obese sedentary patients with elevated liver enzymes and 12 matched controls who were assigned to hike down a predefined route in the Alps three to five times per week for 8 weeks. The course featured a 540-meter drop in altitude.
Mean serum alanine aminotransferase levels in the overweight group dropped from 36 IU/L at baseline to 31 IU/L at 8 weeks. Serum gamma-glutamyl transferase fell from 56 IU/L to 44 IU/L. And the alanine aminotransferase/aspartate aminotransferase ratio improved from 1.22 IU/L to 1.02 IU/L. Meanwhile, liver enzyme levels remained unchanged over time in the controls.
The eccentric exercise regimen was well tolerated, participation was high, and there were no serious adverse events, according to Dr. Saely.
While not everyone is fortunate enough to have a mountain in the backyard with a gondola for the return trip, knowledgeable fitness instructors can demonstrate eccentric exercises using weights and machines that target large muscle groups.
Dr. Saely reported having no conflicts of interest regarding this study.
AT ACC 14
Major finding: An exercise regimen of regular downhill walking for 8 weeks lowered serum alanine aminotransferase levels in overweight and obese sedentary patients from a mean baseline of 36 IU/L to 31 IU/L.
Data source: Pilot study of 42 overweight or obese sedentary patients with elevated liver enzyme levels and 12 matched controls who participated in an eccentric muscle contraction regimen involving downhill hiking 3-5 times per week for 8 weeks.
Disclosures: The presenter reported having no financial conflicts. The study was conducted free of commercial support.
Six factors predict 1-year survival after TAVR
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
WASHINGTON – Patient gender was the only factor that significantly affected stroke risk during the first year following transcatheter aortic valve replacement, according to an analysis of data from nearly 6,000 patients in the U.S. registry for these procedures.
The 1-year outcome results also showed six factors that significantly affected 1-year mortality following transcatheter aortic valve replacement (TAVR): age, gender, severe chronic obstructive pulmonary disease (COPD), end-stage renal disease, TAVR access route, and risk score.
"Identification of these associations is essential for developing risk-prediction models, and will aid in patient-selection criteria for TAVR," said Dr. David R. Holmes Jr., who presented the results at the annual meeting of the American College of Cardiology. "The factors we have identified will be used to develop models that patients and physicians can use for deciding on appropriate treatment for aortic stenosis."
One-year outcomes for 5,980 of the first U.S. Medicare patients to undergo TAVR since it arrived on the U.S. market in November 2011 came from records of the Centers for Medicare & Medicaid Services (CMS) for patients enrolled during November 2011–July 2013 in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy Registry.
The registry’s findings also continued to generate interest in the risk level of the first wave of U.S. patients undergoing routine TAVR. The 5,980 registry patients with 1-year outcome data had a median PROM (STS Predicted Risk of Operative Mortality) score of 7% prior to treatment, showing that a substantial number of U.S. TAVR patients had STS scores below the device’s labeled minimum risk score of 8%.
"Sometimes you look at a patient and say, ‘I don’t care what the STS score is, I think this patient will have problems’ " with surgical valve replacement, said Dr. Holmes, an interventional cardiologist and professor of medicine at the Mayo Clinic in Rochester, Minn. "You need two thoracic surgeons who say that the patient is high risk" and should undergo TAVR regardless of a low STS PROM score, "and that is what carries the day" in terms of securing Medicare coverage for the procedure, he said.
The STS and ACC began the registry at the request of the Food and Drug Administration and to satisfy a CMS mandate to track all Medicare TAVR patients. The first wave of patients entered the registry when the Sapien valve system marketed by Edwards became the first TAVR device approved for routine U.S. use in patients with severe aortic stenosis judged ineligible for surgical valve replacement. In October 2012, the FDA approved the same device as an alternative to surgery for "high-risk" but operable patients, generally defined as those with an STS PROM of at least 8%. The PARTNER trial that led to FDA approval of the Sapien system for operable patients had a minimum STS PROM score threshold for enrollment of 10%, and enrolled patients had an average score of 11.8% (N. Engl. J. Med. 2011;364:2187-98).
Last November, researchers running the registry reported in-hospital and 30-day outcomes for 7,710 of the first TAVR patients who entered during November 2011–May 2013 (JAMA 2013;310:2069-77). They collected 1-year outcomes by linking the registry to data from the CMS Administrative Claims Center.
The results showed a 1-year mortality of 26%, and a 1-year stroke rate of 4%.
"Mortality was fairly consistent with results from trials; however, the stroke rate was lower, which might result from how strokes are identified" in a registry compared with more structured follow-up in trials, commented Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at the Piedmont Heart Institute in Atlanta. "It’s also important that the mortality rate [in the registry] occurred with a high, 36% rate of nontransfemoral access. The higher mortality seen in the registry with nontransfemoral access cannot be discounted. This is probably the highest event rate we will see" with TAVR, Dr. Kandzari predicted. "We can expect outcomes will continue to improve" with advances in TAVR technique and technology.
In multivariate modeling, gender was the only analyzed factor that significantly linked with the 1-year stroke rate. Men had a relative 34% lower stroke rate than women. Women are known to have higher stroke rates than men following other catheter procedures, such as arrhythmia ablations, though the reasons why remain unclear, Dr. Holmes noted.
The relative rates for the six factors that each had a significant link to mortality were a 36% increased mortality for patients aged 85-94 years, compared with those younger than 75 years; a 19% increased rate in men, compared with women; a 41% increased rate in patients with severe COPD, compared with patients with no or mild COPD; an 81% increased rate in dialysis patients, compared with patients with normal creatinine levels; a 42% higher rate with nontransfemoral access, compared with transfemoral access; and a 44% higher mortality among patients with an STS PROM score of 8%-15%, compared with patients with a score of less than 8%.
The substantial number of U.S. patients who have undergone TAVR despite relatively low STS PROM scores, a pattern first seen in baseline data from the entire group of 7,710 patients with in-hospital data in the registry reported last November, also stuck out in the subgroup of 5,980 patients with 1-year data in the new report. Some experts at the meeting commented on the low PROM scores, a phenomenon that last year’s JAMA report dubbed "risk creep," as well as on the rapid uptake of TAVR in routine U.S. practice, with some 8,000 patients undergoing the procedure at about 230 U.S. sites during the first 21 months it was available.
"I’m amazed that we brought 200 centers online [performing TAVR] in just 20 months," commented Dr. David L. Brown, an interventional cardiologist and professor of medicine at Stony Brook (N.Y.) University.
"It’s very striking how quickly the technology was adopted in the United States; 5,980 patients is an enormous number compared with the numbers in the randomized trials," commented Dr. Cindy L. Grines, an interventional cardiologist and vice president at Detroit Medical Center.
"There was incredible, pent-up interest" in TAVR, Dr. Holmes explained.
Dr. Brown characterized the low STS PROM scores of many of the TAVR patients in the registry as "real-world risk creep." Dr. Grines provided a rationale for why patients with relatively low STS PROM scores may undergo TAVR. "There is something to be said for the eyeball estimate of how sick a patient is, and no registry can collect all that information," she said.
"It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high," commented Dr. James B. Hermiller, an interventional cardiologist at St. Vincent Heart Center in Indianapolis.
The registry does not receive commercial support. Dr. Holmes had no relevant disclosures. Dr. Kandzari has been a consultant to or received honoraria from Micell Technologies, Biotronik, and other companies. Dr. Brown had no disclosures. Dr. Grines has been a consultant to or received honoraria from Bristol-Meyers Squibb, Merck, and other companies. Dr. Hermiller has been a consultant to or received honoraria from St. Jude, Abbott Vascular, Boston Scientific, and Medtronic.
On Twitter @mitchelzoler
AT ACC 2014
Key clinical point: One-year outcome data from almost 6,000 U.S. TAVR patients give potential insight into the procedure’s best targets.
Major finding: Six factors significantly influenced 1-year survival following transcatheter aortic valve replacement.
Data source: The STS/ACC Transcatheter Valve Therapy Registry, which included 1-year outcomes data for the first 5,980 U.S. patients who underwent TAVR since November 2011.
Disclosures: The registry does not receive commercial support. Dr. Holmes had no relevant disclosures.
Apixaban for VTE reduced subsequent hospitalizations
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
Major finding: Patients whose acute venous thromboembolism was treated with 6 months of apixaban were 21% less likely to have another hospitalization than were those who received enoxaparin followed by warfarin.
Data source: The double-blind AMPLIFY trial, which randomized 5,365 patients with acute VTE to 6 months of fixed-dose apixaban or conventional therapy with enoxaparin followed by warfarin.
Disclosures: The study was sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported having no financial conflicts.
Apixaban for VTE reduced subsequent hospitalizations
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
Dr. Vera DePalo, FCCP, comments: This large, industry-sponsored trial provides an alternative to the standard treatments for VTE. Patients had a less likelihood of hospitalization for recurrence or major bleeding. If hospitalization did occur, there was a much longer median time to hospitalization and a shorter hospital length of stay.
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
WASHINGTON – Treating acute venous thromboembolism with the fixed-dose oral factor Xa inhibitor apixaban significantly reduces subsequent all-cause hospitalizations, compared with conventional therapy with enoxaparin followed by warfarin, according to a secondary analysis of the landmark AMPLIFY trial.
The 21% reduction in the risk of hospitalization in the apixaban group during the 6 months following the initial VTE was driven mainly by fewer hospitalizations for recurrent VTE or major bleeding. There were also significantly fewer physician office visits by patients on apixaban than for those on enoxaparin/warfarin, Dr. Margot Johnson reported at the annual meeting of the American College of Cardiology.
An analysis of the cost savings associated with this reduction in hospitalizations seen in AMPLIFY is underway and will be reported later this year, added Dr. Johnson of King’s College Hospital in London.
AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis) was an industry-sponsored randomized double-blind study of 5,365 patients with acute symptomatic VTE who were assigned to 6 months of treatment with apixaban (Eliquis) at 10 mg b.i.d. for 7 days followed by 5 mg b.i.d. or to enoxaparin followed by warfarin. In the previously reported primary outcomes (N. Engl. J. Med. 2013;369:799-808, apixaban showed noninferiority to the conventional regimen in terms of the rate of recurrent VTE or VTE-related death, and a highly significant superiority in terms of major bleeding, with a 69% risk reduction.
Dr. Johnson reported that during the 6-month study period, 5.72% of the apixaban group had one or more hospitalizations after the initial event, compared with 7.07% of the control group. This translated to a highly significant 21% relative risk reduction. For every 74 patients treated with apixaban instead of enoxaparin/warfarin, one hospitalization was avoided. Moreover, when a hospitalization occurred in apixaban-treated patients, the mean length of stay was shorter: 10.2 vs. 11.7 days in the enoxaparin-warfarin group.
The median time to a first hospitalization was 63 days in the apixaban group, compared with 34.5 days in controls. The apixaban group’s advantage in terms of hospitalization risk was consistent across subgroups on the basis of age, body weight, sex, and renal function.
The number of emergency department visits during the 6-month follow-up period was similar in the two study arms. However, only 5.8% of the apixaban group visited a physician’s office, compared with 7.3% of controls. The reasons for these office visits were basically the same as for the hospitalizations: mostly recurrent VTEs and bleeding episodes. In the apixaban group, 35 patients had an office visit for recurrent VTE, compared with 61 controls. And 71 apixaban-treated patients made an office visit for bleeding episodes, compared with 130 controls.
Session cochair Dr. Emile R. Mohler commented on the finding that 37 patients in the apixaban group and 48 on enoxaparin/warfarin required hospitalization for recurrent VTE.
"It seems like we’re not doing a good enough job there. Either both of these anticoagulants don’t work well, or the patients aren’t taking the medication, or we’re not following up with them enough. I can’t remember the last time in my own clinical practice that somebody who took their medication came back within 6 months of having a VTE. It seems strange. I think there’s a lot of room for improvement," commented Dr. Mohler, professor of medicine and director of vascular medicine at the University of Pennsylvania, Philadelphia.
Dr. Johnson agreed about the room for improvement. But she added that, although the data she presented were based upon an intention-to-treat analysis, the results were the same – significantly fewer hospitalizations in the apixaban group – in a per-protocol analysis that excluded patients with less than 80% adherence to their study medication.
"One of the big things we saw in AMPLIFY is that whenever you stop an anticoagulant, you see the recurrence rate go up by about 10% per year. So it’s very important that these people continue their anticoagulation – and the more we can reduce their bleeding events, the more likely they are to comply with that therapy and be protected from having another VTE," she added.
Session cochair Dr. John P. Cooke commented that one underutilized aspect of treatment for acute VTE is compressive support, which he said has been given short shrift in the major practice guidelines.
Often as physicians, we give patients a pill and we think that we’ve treated them. Compressive support is important in VTE," stressed Dr. Cooke, chair of the department of cardiovascular sciences at the Houston Methodist Research Institute and director of the Center for Cardiovascular Regeneration at the Houston Methodist DeBakey Heart and Vascular Center.
The AMPLIFY trial was sponsored by Bristol-Myers Squibb and Pfizer. Dr. Johnson reported having no financial conflicts.
Major finding: Patients whose acute venous thromboembolism was treated with 6 months of apixaban were 21% less likely to have another hospitalization than were those who received enoxaparin followed by warfarin.
Data source: The double-blind AMPLIFY trial, which randomized 5,365 patients with acute VTE to 6 months of fixed-dose apixaban or conventional therapy with enoxaparin followed by warfarin.
Disclosures: The study was sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported having no financial conflicts.
Off-label use of novel anticoagulants accelerates
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, Further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
Dr. Jun Chiong, FCCP, comments: Direct thrombin inhibitor (DTI) use has definitely gained wide acceptance in a short period of time, mainly because of its efficacy and its stable drug level, which eliminated regular prothrombin time testing requirements that are inherent with warfarin.
Marketing including print advertisements played a significant role in its widespread use. However, it is important for prescribers to remember that it's our role to read package inserts before prescribing any drugs as well as manufacturers role to educate the medical providers about its use, contraindications, and misuse of these agents.
To date, studies on DTI for valvular atrial fibrillation failed to demonstrate benefit over warfarin.
Dr. Jun Chiong, FCCP, comments: Direct thrombin inhibitor (DTI) use has definitely gained wide acceptance in a short period of time, mainly because of its efficacy and its stable drug level, which eliminated regular prothrombin time testing requirements that are inherent with warfarin.
Marketing including print advertisements played a significant role in its widespread use. However, it is important for prescribers to remember that it's our role to read package inserts before prescribing any drugs as well as manufacturers role to educate the medical providers about its use, contraindications, and misuse of these agents.
To date, studies on DTI for valvular atrial fibrillation failed to demonstrate benefit over warfarin.
Dr. Jun Chiong, FCCP, comments: Direct thrombin inhibitor (DTI) use has definitely gained wide acceptance in a short period of time, mainly because of its efficacy and its stable drug level, which eliminated regular prothrombin time testing requirements that are inherent with warfarin.
Marketing including print advertisements played a significant role in its widespread use. However, it is important for prescribers to remember that it's our role to read package inserts before prescribing any drugs as well as manufacturers role to educate the medical providers about its use, contraindications, and misuse of these agents.
To date, studies on DTI for valvular atrial fibrillation failed to demonstrate benefit over warfarin.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, Further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, Further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
Key clinical point: Off-label use of novel anticoagulants is growing.
Major finding: By the fourth quarter of 2012, about 15% of patients with nonvalvular AF and about 14% of those with valvular AF were on a novel anticoagulant.
Data source: A study of more than 190,000 patients participating in the PINNACLE Registry.
Disclosures: The PINNACLE Registry is funded by the ACC’s National Cardiovascular Data Registry. The presenter reported having no financial conflicts.
Even mild preop sepsis boosts postop clot risk
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.
Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
Dr. Marcos I. Restrepo, FCCP, comments: This interesting observation links the associated risk of preoperative sepsis and any degree of sepsis severity with a higher risk of developing postoperative arterial and venous thrombosis. Therefore, it is critical to recognize and identify preoperative patients with systemic inflammatory response syndrome with a suspected or confirmed source of infection ("sepsis") in order to initiate early and appropriate thromboprophylactic measures.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donzé said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donzé of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donzé presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis (see graphic).
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
Major finding: Preoperative sepsis is a strong independent risk factor for postoperative arterial and venous thrombosis; the more severe the sepsis, the greater the thrombosis risk.
Data source: This was an analysis of nearly 1.75 million surgical procedures at 314 U.S. hospitals detailed in the American College of Surgeons National Quality Improvement Program registry.
Disclosures: The presenter reported having no financial conflicts.
Efficacy, safety confirmed for transcatheter pulmonary valve
WASHINGTON – A transcatheter pulmonary valve system that provides a new right ventricle to pulmonary artery conduit to congenital heart disease patients without the need for open heart surgery performed a little better in a real world registry at 10 U.S. centers than it had in the pivotal trial that led to the system’s 2010 FDA approval.
The new results "confirm the strong performance of the Melody transcatheter pulmonary valve achieved by real world providers with results comparable to the U.S. investigational device exemption [IDE] trial," Dr. Aimee K. Armstrong said at the annual meeting of the American College of Cardiology. The "high level" of 97% freedom from transcatheter pulmonary valve (TPV) dysfunction at 1 year "was better than in the IDE trial," where the level reached 94%, noted Dr. Armstrong, an interventional cardiologist in the pediatric cardiac catheterization laboratory at the University of Michigan in Ann Arbor.
The registry study, which the Food and Drug Administration mandated when it approved the Melody valve in 2010, ran during July 2010 to July 2012 at 10 U.S. centers that had not participated in the pivotal trial. The 99 patients who received an implant that stayed in place for at least 1 day ranged from 5 to 45 years old, with an average age of 20 years.
Although patient follow-up averaged 22 months, the study’s primary endpoint was acceptable hemodynamic function within the conduit at 6 months, with a prespecified performance goal of 75% of patients achieving this outcome. The outcome actually occurred in 97% of the 90 evaluable patients at 6 months, and in 88% of all 99 patients who received a conduit. The difference between each of these rates and the performance goal was statistically significant, Dr. Armstrong said.
The transcatheter valve showed excellent performance by other criteria as well. Acceptable hemodynamic function continued through 1 year in 94% of the 87 implanted patients with evaluable data at 12 months, which translated to 83% of the entire 99 patients in the implanted group. Severe or moderate pulmonary valve regurgitation existed in 85% of the patients before treatment; after treatment no patient had severe or moderate regurgitation, and after 1 year 63% had no regurgitation, 24% had trace, and 12% had mild regurgitation (figures total 99% because of rounding). The 1-year rate of 97% of patients free from dysfunction of their implanted valve appeared to surpass the 94% rate seen in the pivotal trial (Circulation 2010;122:507-16). Dr. Armstrong suggested that if this represented truly improved performance, it may have resulted from lessons during the prior trial on how to best deploy the transcatheter pulmonary valve.
"We learned that the need for reinterventions in the prior trial was primarily due to stent fracture," she said. To reduce the fracture risk, she and her associates routinely began by placing stainless steel stents in the pulmonary artery, an approach known as "pre-stenting," which makes "the risk of fracture go down significantly," she said. Investigators in the new study used pre-stents in 76% of the cases, compared with 35% in the first study. "Our practice now is to pre-stent almost all patients," Dr. Armstrong said.
The results also showed that high right-ventricular pressure prior to valve placement was the only variable independently associated with subsequent valve dysfunction. "Patients who go into the procedure with a very stenotic conduit are probably at higher risk for transcatheter pulmonary valve dysfunction down the road," she said.
The study was sponsored by Medtronic, which markets the Melody transcatheter pulmonary valve. Dr. Armstrong said that she has received consultant fees and honoraria from Siemens Healthcare and St. Jude Medical, and has received research funding from Medtronic and Edwards Lifesciences.
On Twitter @mitchelzoler
The importance of this study cannot be overestimated. Congenital heart disease is the No. 1 birth defect worldwide, affecting nearly 1% of all live births. Our success in treating infants, children, and teenagers with congenital heart disease has produced an epidemic of young adults with congenital heart disease, an estimated 1.5-2 million Americans today
These young adults need to undergo multiple major heart surgeries during their life. The transcatheter pulmonary valve provides, for the first time, a technology to treat a common problem for patients with congenital heart disease in a way that produces excellent results without the need for surgery.
The most impressive part of the findings is that freedom from valve dysfunction after 1 year was even greater than in the initial trial cohort. These results show that this technology is both transferable and can improve. This was very important work for a population that desperately needs improved treatments.
Dr. Evan M. Zahn is director of the congenital heart program of the Heart Institute of Cedar’s Sinai Medical Center in Los Angeles. He said that he has received consulting fees and honoraria from Medtronic and research grants from Edwards Lifesciences. Dr. Zahn was a coinvestigator in the pivotal trial of the Melody transcatheter pulmonary valve system when he was on the staff at Miami Children’s Hospital He made these comments during a press conference.
The importance of this study cannot be overestimated. Congenital heart disease is the No. 1 birth defect worldwide, affecting nearly 1% of all live births. Our success in treating infants, children, and teenagers with congenital heart disease has produced an epidemic of young adults with congenital heart disease, an estimated 1.5-2 million Americans today
These young adults need to undergo multiple major heart surgeries during their life. The transcatheter pulmonary valve provides, for the first time, a technology to treat a common problem for patients with congenital heart disease in a way that produces excellent results without the need for surgery.
The most impressive part of the findings is that freedom from valve dysfunction after 1 year was even greater than in the initial trial cohort. These results show that this technology is both transferable and can improve. This was very important work for a population that desperately needs improved treatments.
Dr. Evan M. Zahn is director of the congenital heart program of the Heart Institute of Cedar’s Sinai Medical Center in Los Angeles. He said that he has received consulting fees and honoraria from Medtronic and research grants from Edwards Lifesciences. Dr. Zahn was a coinvestigator in the pivotal trial of the Melody transcatheter pulmonary valve system when he was on the staff at Miami Children’s Hospital He made these comments during a press conference.
The importance of this study cannot be overestimated. Congenital heart disease is the No. 1 birth defect worldwide, affecting nearly 1% of all live births. Our success in treating infants, children, and teenagers with congenital heart disease has produced an epidemic of young adults with congenital heart disease, an estimated 1.5-2 million Americans today
These young adults need to undergo multiple major heart surgeries during their life. The transcatheter pulmonary valve provides, for the first time, a technology to treat a common problem for patients with congenital heart disease in a way that produces excellent results without the need for surgery.
The most impressive part of the findings is that freedom from valve dysfunction after 1 year was even greater than in the initial trial cohort. These results show that this technology is both transferable and can improve. This was very important work for a population that desperately needs improved treatments.
Dr. Evan M. Zahn is director of the congenital heart program of the Heart Institute of Cedar’s Sinai Medical Center in Los Angeles. He said that he has received consulting fees and honoraria from Medtronic and research grants from Edwards Lifesciences. Dr. Zahn was a coinvestigator in the pivotal trial of the Melody transcatheter pulmonary valve system when he was on the staff at Miami Children’s Hospital He made these comments during a press conference.
WASHINGTON – A transcatheter pulmonary valve system that provides a new right ventricle to pulmonary artery conduit to congenital heart disease patients without the need for open heart surgery performed a little better in a real world registry at 10 U.S. centers than it had in the pivotal trial that led to the system’s 2010 FDA approval.
The new results "confirm the strong performance of the Melody transcatheter pulmonary valve achieved by real world providers with results comparable to the U.S. investigational device exemption [IDE] trial," Dr. Aimee K. Armstrong said at the annual meeting of the American College of Cardiology. The "high level" of 97% freedom from transcatheter pulmonary valve (TPV) dysfunction at 1 year "was better than in the IDE trial," where the level reached 94%, noted Dr. Armstrong, an interventional cardiologist in the pediatric cardiac catheterization laboratory at the University of Michigan in Ann Arbor.
The registry study, which the Food and Drug Administration mandated when it approved the Melody valve in 2010, ran during July 2010 to July 2012 at 10 U.S. centers that had not participated in the pivotal trial. The 99 patients who received an implant that stayed in place for at least 1 day ranged from 5 to 45 years old, with an average age of 20 years.
Although patient follow-up averaged 22 months, the study’s primary endpoint was acceptable hemodynamic function within the conduit at 6 months, with a prespecified performance goal of 75% of patients achieving this outcome. The outcome actually occurred in 97% of the 90 evaluable patients at 6 months, and in 88% of all 99 patients who received a conduit. The difference between each of these rates and the performance goal was statistically significant, Dr. Armstrong said.
The transcatheter valve showed excellent performance by other criteria as well. Acceptable hemodynamic function continued through 1 year in 94% of the 87 implanted patients with evaluable data at 12 months, which translated to 83% of the entire 99 patients in the implanted group. Severe or moderate pulmonary valve regurgitation existed in 85% of the patients before treatment; after treatment no patient had severe or moderate regurgitation, and after 1 year 63% had no regurgitation, 24% had trace, and 12% had mild regurgitation (figures total 99% because of rounding). The 1-year rate of 97% of patients free from dysfunction of their implanted valve appeared to surpass the 94% rate seen in the pivotal trial (Circulation 2010;122:507-16). Dr. Armstrong suggested that if this represented truly improved performance, it may have resulted from lessons during the prior trial on how to best deploy the transcatheter pulmonary valve.
"We learned that the need for reinterventions in the prior trial was primarily due to stent fracture," she said. To reduce the fracture risk, she and her associates routinely began by placing stainless steel stents in the pulmonary artery, an approach known as "pre-stenting," which makes "the risk of fracture go down significantly," she said. Investigators in the new study used pre-stents in 76% of the cases, compared with 35% in the first study. "Our practice now is to pre-stent almost all patients," Dr. Armstrong said.
The results also showed that high right-ventricular pressure prior to valve placement was the only variable independently associated with subsequent valve dysfunction. "Patients who go into the procedure with a very stenotic conduit are probably at higher risk for transcatheter pulmonary valve dysfunction down the road," she said.
The study was sponsored by Medtronic, which markets the Melody transcatheter pulmonary valve. Dr. Armstrong said that she has received consultant fees and honoraria from Siemens Healthcare and St. Jude Medical, and has received research funding from Medtronic and Edwards Lifesciences.
On Twitter @mitchelzoler
WASHINGTON – A transcatheter pulmonary valve system that provides a new right ventricle to pulmonary artery conduit to congenital heart disease patients without the need for open heart surgery performed a little better in a real world registry at 10 U.S. centers than it had in the pivotal trial that led to the system’s 2010 FDA approval.
The new results "confirm the strong performance of the Melody transcatheter pulmonary valve achieved by real world providers with results comparable to the U.S. investigational device exemption [IDE] trial," Dr. Aimee K. Armstrong said at the annual meeting of the American College of Cardiology. The "high level" of 97% freedom from transcatheter pulmonary valve (TPV) dysfunction at 1 year "was better than in the IDE trial," where the level reached 94%, noted Dr. Armstrong, an interventional cardiologist in the pediatric cardiac catheterization laboratory at the University of Michigan in Ann Arbor.
The registry study, which the Food and Drug Administration mandated when it approved the Melody valve in 2010, ran during July 2010 to July 2012 at 10 U.S. centers that had not participated in the pivotal trial. The 99 patients who received an implant that stayed in place for at least 1 day ranged from 5 to 45 years old, with an average age of 20 years.
Although patient follow-up averaged 22 months, the study’s primary endpoint was acceptable hemodynamic function within the conduit at 6 months, with a prespecified performance goal of 75% of patients achieving this outcome. The outcome actually occurred in 97% of the 90 evaluable patients at 6 months, and in 88% of all 99 patients who received a conduit. The difference between each of these rates and the performance goal was statistically significant, Dr. Armstrong said.
The transcatheter valve showed excellent performance by other criteria as well. Acceptable hemodynamic function continued through 1 year in 94% of the 87 implanted patients with evaluable data at 12 months, which translated to 83% of the entire 99 patients in the implanted group. Severe or moderate pulmonary valve regurgitation existed in 85% of the patients before treatment; after treatment no patient had severe or moderate regurgitation, and after 1 year 63% had no regurgitation, 24% had trace, and 12% had mild regurgitation (figures total 99% because of rounding). The 1-year rate of 97% of patients free from dysfunction of their implanted valve appeared to surpass the 94% rate seen in the pivotal trial (Circulation 2010;122:507-16). Dr. Armstrong suggested that if this represented truly improved performance, it may have resulted from lessons during the prior trial on how to best deploy the transcatheter pulmonary valve.
"We learned that the need for reinterventions in the prior trial was primarily due to stent fracture," she said. To reduce the fracture risk, she and her associates routinely began by placing stainless steel stents in the pulmonary artery, an approach known as "pre-stenting," which makes "the risk of fracture go down significantly," she said. Investigators in the new study used pre-stents in 76% of the cases, compared with 35% in the first study. "Our practice now is to pre-stent almost all patients," Dr. Armstrong said.
The results also showed that high right-ventricular pressure prior to valve placement was the only variable independently associated with subsequent valve dysfunction. "Patients who go into the procedure with a very stenotic conduit are probably at higher risk for transcatheter pulmonary valve dysfunction down the road," she said.
The study was sponsored by Medtronic, which markets the Melody transcatheter pulmonary valve. Dr. Armstrong said that she has received consultant fees and honoraria from Siemens Healthcare and St. Jude Medical, and has received research funding from Medtronic and Edwards Lifesciences.
On Twitter @mitchelzoler
AT ACC 2014
Key clinical point: The Melody transcatheter pulmonary valve system worked as well in a real world registry as it did in its pivotal trial as a conduit between the right ventricle and pulmonary artery.
Major finding: Acceptable hemodynamic function at 6 months occurred in 88% of implanted patients, significantly surpassing the 75% performance goal.
Data source: A series of 99 patients who received a transcatheter pulmonary valve at any of 10 participating U.S. centers.
Disclosures: The study was sponsored by Medtronic, which markets the Melody transcatheter pulmonary valve. Dr. Armstrong said that she has received consultant fees and honoraria from Siemens Healthcare and St. Jude Medical, and has received research funding from Medtronic and Edwards Lifesciences.