User login
Case Report
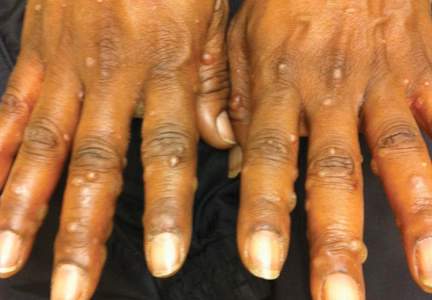
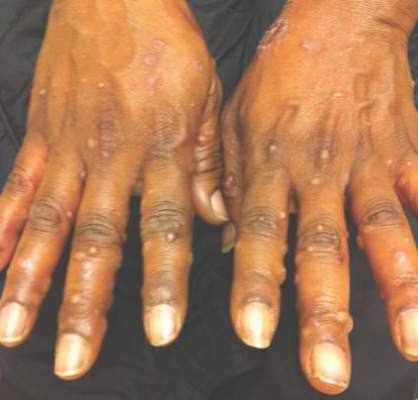
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.
The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
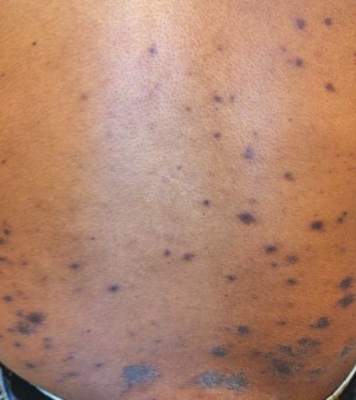
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.
Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
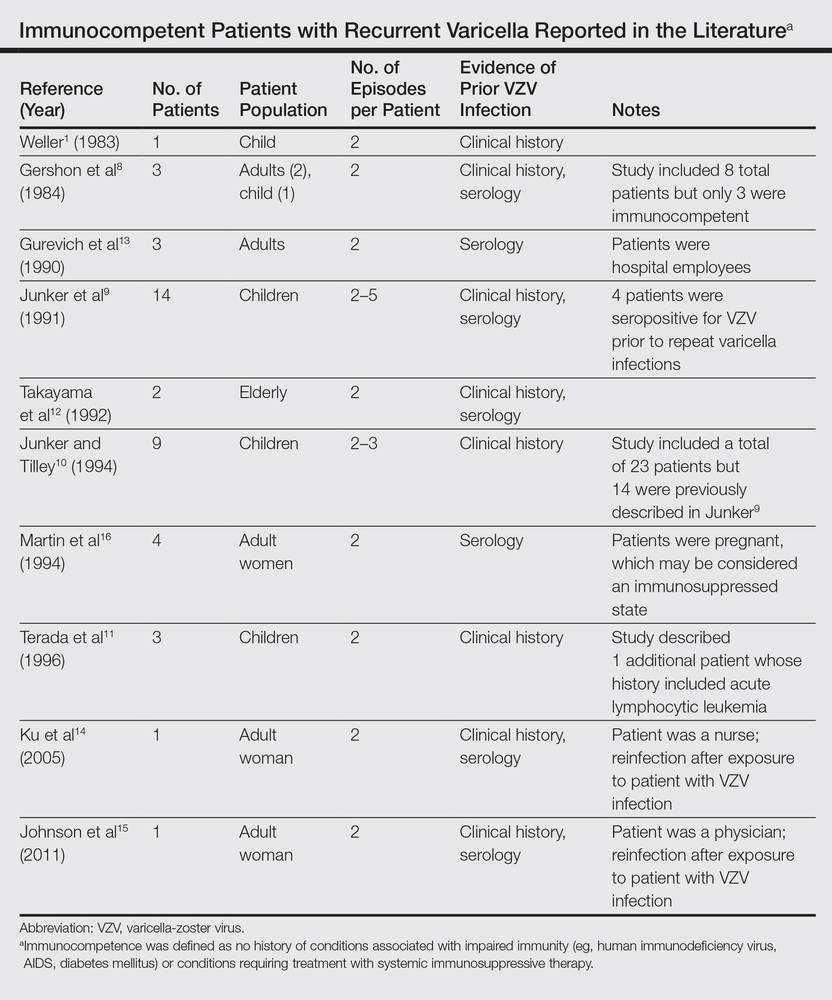
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
Case Report
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.

The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.

Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
Case Report
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.

The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.

Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
Practice Points
- Varicella is a viral exanthem that manifests as generalized and pruritic papules, vesicles, and crusted lesions and is the initial expression of infection with the varicella-zoster virus (VZV).
- Secondary expression of VZV infection is typified by herpes zoster, where painful papules and vesicles are confined within a single dermatome.
- Although contradictory to dogma and public perception, varicella may occur more than once; therefore, patients with a generalized pruritic exanthem should be screened for varicella, even if they report a history of varicella.