User login
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints, leading to joint destruction, with significant long-term morbidity and mortality. Over the past quarter century, multiple new therapies and approaches have been introduced, so patients newly diagnosed with RA can realistically expect to be in remission while taking their medications. However, many of the most commonly used medications are costly, making RA care one of the most expensive per patient.1 Early treatment with disease-modifying antirheumatic drugs (DMARDs) and treating all patients to the target of low-disease activity are critical keys to optimal outcomes.
Methotrexate (MTX) is a highly effective and economical first-line DMARD that is recommended as the initial therapy for most patients.2,3 Unfortunately, one-half to two-thirds of patients will not have complete responses and, therefore, require additional therapy. Fortunately, there are more than a dozen therapies that, when added to MTX, have been shown to be better than MTX alone. However, since some of these options use conventional DMARDs and others require biologics, there exist very different economic as well as potential toxicity implications. Understanding how best to treat patients with RA with active disease while on an appropriate dose of MTX is important for both medical and economic reasons.
Despite this being a seminal question for the past 15 years, no blinded trial had addressed this issue before the VA Cooperative Studies Program (CSP) Rheumatoid Arthritis: Comparison of Active Therapies (RACAT) trial. This was true for several reasons, likely including the considerable cost of conducting such a trial and the low priority of this research question for the pharmaceutical industry. Industry-funded trials in RA often focus on new indications, and these studies often fail to address the questions most relevant to the day-to-day care of patients.
For example, it is often not particularly helpful to the clinician that patients placed on “therapy A” are doing better or worse than those placed on “therapy B” after 1 year of the same treatment. Such rigid protocols do not mimic what is done in the clinic: A patient’s treatment program is often changed much earlier than 1 year when it is not working. Therefore, RACAT was designed to more closely mirror clinical practice and to test the strategy of starting conventional therapy before biologic therapy, with the option of changing therapy for nonresponders—similar to what most clinicians would do in practice. This article explores the lessons learned from this landmark trial and highlights the critical role that the VA CSP played.
Trial Background
The RACAT trial, a comparative effectiveness, randomized, double-blind, noninferiority trial, originated as a joint effort of investigators from the VA and the Rheumatology and Arthritis Investigational Network (RAIN) and subsequently involved Canadian enrollment sites. The RACAT results were published in the New England Journal of Medicine in 2013, and its investigative team was awarded the 2014 Lee C. Howley Sr. Prize by the Arthritis Foundation for conducting the most important arthritis research worldwide from the previous year.4
The RACAT originated with a letter of intent to the VA CSP in 2003. The central question to be addressed was whether biologic therapy should be added first in patients with active RA despite MTX or whether clinicians should first add the much less expensive but very effective combination of conventional therapies, including sulfasalazine (SSZ) and hydroxychloroquine to MTX.5,6 This led to the 48-week, binational, multicenter, randomized, double-blind, noninferiority trial comparing the strategy of initially adding hydroxychloroquine and SSZ to MTX (triple therapy group) in patients with active disease despite MTX compared with the strategy of first adding etanercept to MTX.4 Etanercept is among the most commonly used biologic agents approved for RA. Etanercept works by targeting tumor necrosis factor, a pro-inflammatory cytokine central in disease pathogenesis. Both RACAT treatment groups were switched in a blinded fashion to the other therapy at 24 weeks if they did not have a clinically significant improvement. The primary endpoint was a change in the disease activity score (DAS28) from baseline to 48 weeks. An important secondary endpoint was the comparison of radiographic progression of disease at 48 weeks as measured by the validated modified Sharp scoring method. Additionally, and very importantly, economic and functional outcomes were assessed. To conduct the trial, investigators and patients participated from 16 VA sites in addition to 8 Canadian and 12 RAIN sites. The study was sponsored and primarily funded by the VA CSP, VA Office of Research and Development with additional funding coming from the Canadian Institutes of Health Research (CIHR) and from the National Institutes of Health.
Trial Design
To understand the RACAT trial design, one must appreciate the landscape of RA trials conducted in the early-to-mid 2000s. At that time, there had been an explosion of new biologic therapies for RA. Most of the trials were placebo-controlled studies with nonresponders to MTX being placed on placebo vs active drug.7 For ethical and legal reasons, however, clinicians do not treat patients with placebo, especially when highly effective therapies exist, thus limiting the relevance of the classic placebo-controlled trial in RA.8 One of the main tenets of RA therapy in this century has been to use effective therapies to treat patients with active RA with the goal of achieving (and maintaining) either low-disease activity or remission as measured by a composite scoring system, most commonly the DAS28. In order to do this in the framework of a designed research trial, therapies commonly need to be escalated when patients are not doing well, similar to what is done in clinical practice.
The RACAT trial was a comparative effectiveness trial. Comparative effectiveness is not a new idea; in fact, it is precisely how many clinicians practice medicine. It is simply comparing 2 or more treatments to determine which is more effective. Since the inception of the RACAT trial, the American Recovery and Reinvestment Act of 2009 provided $1.1 billion for major expansion of comparative effectiveness research. This changing landscape of federally funded research has highlighted the growing national interest in this type of trial.
This trial design posed several barriers as it applies to the study medications. Methotrexate, hydroxychloroquine, and SSZ are generic medications most often taken orally (MTX is available for parenteral administration). In contrast, etanercept is most often given as a subcutaneous injection and currently is not available in a biosimilar (generic) form in the U.S.; thus, the medication and its delivery device are proprietary. Because this was a double-blind, noninferiority trial, the study required both etanercept-active medication and placebo in identical delivery devices. The makers of etanercept donated placebo etanercept to make blinding possible. The VA, along with CIHR, purchased active etanercept for all trial participants, including those from Canada and the RAIN network. The VA research pharmacy in Albuquerque, New Mexico, was responsible for blinding all active and placebo drugs used in the trial and made these drugs available to all patients, even those not eligible for VA care.In a precedent-setting effort for rheumatology research, RACAT culminated from the collaborations among the private sector, the Canadian health system, and VA. The VA CSP was responsible for the collection of the clinical data, data analyses (Massachusetts Veterans Epidemiology Research and Information Center, VA Boston Healthcare System [VABHS]); the collection of economic data (VA Palo Alto Health Care System); the provision of and payment for the study medications; and the preparation and distribution of active etanercept and placebos (New Mexico VA Health Care System [NMVAHCS]). Through this organizational structure, the trial was successfully completed. In addition to placebo etanercept provided by Amgen (Thousand Oaks, CA), Pharmascience (Montreal, Quebec) provided blinded SSZ and blinded placebo. Neither company was involved in the study design nor did they have an active role in the trial. Hydroxychoroquine and matched placebo were provided by the central pharmacy of the NMVAHCS.
Safety Monitoring
As with any treatment study, patient safety was of paramount importance. Through the aforementioned organizational structure, each participating site had administrative team members who were responsible to the VABHS CSP to ensure research adherence and compliance with best practices. Additionally, an independent data and safety monitoring committee (DSMC) monitored the trial for safety and scientific integrity. At the time that the trial began in 2007, there were questions about the relative efficacy of triple therapy vs MTX plus biologic therapy. Because of this question, the DSMC raised concerns that patients may be placed at a higher risk of joint damage if not placed sooner on biologic therapy. As a response to this concern, the blinded radiographic reviewers were asked to read the hand and feet X-rays as the study progressed, allowing the DSMC to watch for any emerging safety signals. There were none, and in fact, the therapies were essentially identical radiographically.
The consequence of this request was multifold. First, patient safety was maintained; second, the study team was forced to navigate the logistic and technologic challenges posed by the reading and interpretation of the radiographs at an earlier time point than was originally planned; and last, as a result, results became available and were disseminated in a relatively narrow time frame. This third point was very important. One of the major concerns of foregoing biologic therapy was the potential for joint damage. Without the radiographic information, the manuscript could not have been completed in a timely fashion.
Trial Results
The trial was a 48-week, double-blind, noninferiority trial in which 353 patients with RA who had active disease despite MTX therapy were randomized to a triple therapy regimen of DMARDs (baseline MTX, plus SSZ and hydroxychloroquine) or baseline MTX plus etanercept (Figure 1). Patients who did not have a clinically significant improvement at 24 weeks according to a prespecified threshold were switched in a blinded fashion to the other therapy.
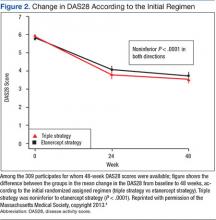
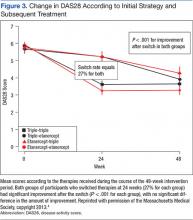
The primary endpoint, the change between baseline and 48 weeks in the DAS28, was similar; thus the strategy of first starting triple therapy was not inferior to first starting etanercept (the change in DAS28 was -2.12 and -2.29 respectively, P < .0001, supporting noninferiority, Figure 2). Both groups had significant improvement over the course of the first 24 weeks (P = .001). A total of 27% of participants in each group switched at 24 weeks (Figure 3). Patients in both groups who switched therapies had improvement after switching (P < .001), and the response after switching did not differ significantly between the 2 groups. Importantly, there were no significant between-group differences in radiographic progression (P = .43), pain and health-related quality of life (QOL), or in medication-associated major adverse events (AEs), although there were numerically more serious infections with etanercept-MTX therapy (12 vs 4). Gastrointestinal AEs were numerically more frequent with triple therapy; whereas infections and skin and subcutaneous AEs were more frequent with etanercept-MTX therapy.
The cost-effectiveness of adding SSZ and hydroxychloroquine to MTX vs adding etanercept to MTX, using a predetermined measurement of QOL, was assessed in this trial.9 These data were initially presented in abstract form at the 2014 American College of Rheumatology national meeting. Considered was the ratio of all the incremental costs between the 2 treatment strategies to the benefits, as measured in quality-adjusted life-years (QALYs), where QOL is measured as an index with 1 being equivalent to full health and 0 being equivalent to death. This incremental cost-effectiveness ratio produces a monetary cost for each QALY, which is an indication of cost-effectiveness and value. Most health care systems currently consider anything that costs < $50,000/QALY to be cost-effective. To be conservative, the trial researchers considered anything up to $100,000 for an additional QALY acceptable.
In the 48-week trial analysis, the use of etanercept first, instead of triple therapy, would incur about $1 million of cost per QALY, far more than the $50,000 to $100,000 deemed to be reasonable value. Biologic therapy use first, had a near-zero chance of being cost-effective and would be cost-effective only after failure of triple therapy; results that were robust to all plausible scenarios.
Economic Implications
As noted in the trial design, economic data were prospectively collected for later analysis. The availability and cost of biologic treatments have become a critical issue. In 2005, it was reported that the U.S. biologics market reached $52 billion and was noted to have an annual growth of 17%.10 In 2011, 8 of the top 20 drugs sold in the U.S. were biologics, and year-to-year biologics spending has grown by 6.6%. In 2013, the top 100 biologics in the U.S. had combined sales of $66.3 billion.11 The researchers analysis demonstrated that using a strategy of triple therapy first could result in health care cost savings in the tens of billions of dollars. Importantly, these savings would occur at the same time as patients were receiving optimal care.
Summary
The major conclusions from the RACAT trial in RA patients with active disease despite MTX are the following: 1. The strategy of first starting the conventional DMARD triple therapy combination is noninferior to first starting etanercept, based on both clinical and radiographic outcomes. 2. The triple therapy group had more minor gastrointestinal events, whereas the etanercept group had more infections. Patients in either group who did not respond well to the initial treatment and switched (27% in both groups) improved significantly after the switch. 3. The economic implications of these findings are significant. The incremental cost differences approached $1 million per QALY.
The VA CSP should be congratulated for supporting and funding this trial, which will inform therapeutic decisions in RA for years to come. These results allow clinicians to provide not only optimal health care for their RA patients, but also maximize the value of their health care.
1. Gavan S, Harrison M, Iglesias C, Barton A, Manca A, Payne K. Economics of stratified medicine in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(12):468.
2. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying anti-rheumatic drugs and biologics in the treatment of rheumatoid arthritis (RA). Arthritis Care Res (Hoboken). 2012;64(5):625-639.
3. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492-509.
4. O'Dell JR, Mikuls TR, Taylor TH, et al; CSP 551 RACAT Investigators. Therapies for active rheumatoid arthritis after methotrexate failure. N Eng J Med. 2013;369(4):307-318.
5. Moreland LW, O'Dell JR, Paulus HE, et al; TEAR Investigators. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824-2835.
6. O'Dell JR, Leff R, Paulsen G, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(5):1164-1170.
7. Singh JA, Cameron DR. Summary of AHRQ's comparative effectiveness review of drug therapy for rheumatoid arthritis in adults-an update. J Manag Care Pharm. 2012;18(4)(suppl C):S1-S18.
8. Chan TE. Regulating the placebo effect in clinical practice. Med Law Rev. 2014;23(1):1-26.
9. Bansback N, Phibb S, Sun H, et al. Cost effectiveness of adding etanercept to methotrexate therapy versus first adding sulfasalazine and hydroxychloroquine: a randomized noninferiority trial [American College of Rheumatology abstract 2781]. Arthritis Rheum. 2014;66(suppl 11):S1214.
10. Ernst and Young. Beyond borders: global biotechnology report 2005. Ernst and Young Website. https://www2.eycom.ch/publications/items/biotech-report/2005/2005_EY_Global_Biotech_Report.pdf. Accessed December 3, 2015.
11. Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. Rand Corporation Website. http://www.rand.org/pubs/perspectives/PE127.html. Accessed December 3, 2015.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints, leading to joint destruction, with significant long-term morbidity and mortality. Over the past quarter century, multiple new therapies and approaches have been introduced, so patients newly diagnosed with RA can realistically expect to be in remission while taking their medications. However, many of the most commonly used medications are costly, making RA care one of the most expensive per patient.1 Early treatment with disease-modifying antirheumatic drugs (DMARDs) and treating all patients to the target of low-disease activity are critical keys to optimal outcomes.
Methotrexate (MTX) is a highly effective and economical first-line DMARD that is recommended as the initial therapy for most patients.2,3 Unfortunately, one-half to two-thirds of patients will not have complete responses and, therefore, require additional therapy. Fortunately, there are more than a dozen therapies that, when added to MTX, have been shown to be better than MTX alone. However, since some of these options use conventional DMARDs and others require biologics, there exist very different economic as well as potential toxicity implications. Understanding how best to treat patients with RA with active disease while on an appropriate dose of MTX is important for both medical and economic reasons.
Despite this being a seminal question for the past 15 years, no blinded trial had addressed this issue before the VA Cooperative Studies Program (CSP) Rheumatoid Arthritis: Comparison of Active Therapies (RACAT) trial. This was true for several reasons, likely including the considerable cost of conducting such a trial and the low priority of this research question for the pharmaceutical industry. Industry-funded trials in RA often focus on new indications, and these studies often fail to address the questions most relevant to the day-to-day care of patients.
For example, it is often not particularly helpful to the clinician that patients placed on “therapy A” are doing better or worse than those placed on “therapy B” after 1 year of the same treatment. Such rigid protocols do not mimic what is done in the clinic: A patient’s treatment program is often changed much earlier than 1 year when it is not working. Therefore, RACAT was designed to more closely mirror clinical practice and to test the strategy of starting conventional therapy before biologic therapy, with the option of changing therapy for nonresponders—similar to what most clinicians would do in practice. This article explores the lessons learned from this landmark trial and highlights the critical role that the VA CSP played.
Trial Background
The RACAT trial, a comparative effectiveness, randomized, double-blind, noninferiority trial, originated as a joint effort of investigators from the VA and the Rheumatology and Arthritis Investigational Network (RAIN) and subsequently involved Canadian enrollment sites. The RACAT results were published in the New England Journal of Medicine in 2013, and its investigative team was awarded the 2014 Lee C. Howley Sr. Prize by the Arthritis Foundation for conducting the most important arthritis research worldwide from the previous year.4
The RACAT originated with a letter of intent to the VA CSP in 2003. The central question to be addressed was whether biologic therapy should be added first in patients with active RA despite MTX or whether clinicians should first add the much less expensive but very effective combination of conventional therapies, including sulfasalazine (SSZ) and hydroxychloroquine to MTX.5,6 This led to the 48-week, binational, multicenter, randomized, double-blind, noninferiority trial comparing the strategy of initially adding hydroxychloroquine and SSZ to MTX (triple therapy group) in patients with active disease despite MTX compared with the strategy of first adding etanercept to MTX.4 Etanercept is among the most commonly used biologic agents approved for RA. Etanercept works by targeting tumor necrosis factor, a pro-inflammatory cytokine central in disease pathogenesis. Both RACAT treatment groups were switched in a blinded fashion to the other therapy at 24 weeks if they did not have a clinically significant improvement. The primary endpoint was a change in the disease activity score (DAS28) from baseline to 48 weeks. An important secondary endpoint was the comparison of radiographic progression of disease at 48 weeks as measured by the validated modified Sharp scoring method. Additionally, and very importantly, economic and functional outcomes were assessed. To conduct the trial, investigators and patients participated from 16 VA sites in addition to 8 Canadian and 12 RAIN sites. The study was sponsored and primarily funded by the VA CSP, VA Office of Research and Development with additional funding coming from the Canadian Institutes of Health Research (CIHR) and from the National Institutes of Health.
Trial Design
To understand the RACAT trial design, one must appreciate the landscape of RA trials conducted in the early-to-mid 2000s. At that time, there had been an explosion of new biologic therapies for RA. Most of the trials were placebo-controlled studies with nonresponders to MTX being placed on placebo vs active drug.7 For ethical and legal reasons, however, clinicians do not treat patients with placebo, especially when highly effective therapies exist, thus limiting the relevance of the classic placebo-controlled trial in RA.8 One of the main tenets of RA therapy in this century has been to use effective therapies to treat patients with active RA with the goal of achieving (and maintaining) either low-disease activity or remission as measured by a composite scoring system, most commonly the DAS28. In order to do this in the framework of a designed research trial, therapies commonly need to be escalated when patients are not doing well, similar to what is done in clinical practice.
The RACAT trial was a comparative effectiveness trial. Comparative effectiveness is not a new idea; in fact, it is precisely how many clinicians practice medicine. It is simply comparing 2 or more treatments to determine which is more effective. Since the inception of the RACAT trial, the American Recovery and Reinvestment Act of 2009 provided $1.1 billion for major expansion of comparative effectiveness research. This changing landscape of federally funded research has highlighted the growing national interest in this type of trial.
This trial design posed several barriers as it applies to the study medications. Methotrexate, hydroxychloroquine, and SSZ are generic medications most often taken orally (MTX is available for parenteral administration). In contrast, etanercept is most often given as a subcutaneous injection and currently is not available in a biosimilar (generic) form in the U.S.; thus, the medication and its delivery device are proprietary. Because this was a double-blind, noninferiority trial, the study required both etanercept-active medication and placebo in identical delivery devices. The makers of etanercept donated placebo etanercept to make blinding possible. The VA, along with CIHR, purchased active etanercept for all trial participants, including those from Canada and the RAIN network. The VA research pharmacy in Albuquerque, New Mexico, was responsible for blinding all active and placebo drugs used in the trial and made these drugs available to all patients, even those not eligible for VA care.In a precedent-setting effort for rheumatology research, RACAT culminated from the collaborations among the private sector, the Canadian health system, and VA. The VA CSP was responsible for the collection of the clinical data, data analyses (Massachusetts Veterans Epidemiology Research and Information Center, VA Boston Healthcare System [VABHS]); the collection of economic data (VA Palo Alto Health Care System); the provision of and payment for the study medications; and the preparation and distribution of active etanercept and placebos (New Mexico VA Health Care System [NMVAHCS]). Through this organizational structure, the trial was successfully completed. In addition to placebo etanercept provided by Amgen (Thousand Oaks, CA), Pharmascience (Montreal, Quebec) provided blinded SSZ and blinded placebo. Neither company was involved in the study design nor did they have an active role in the trial. Hydroxychoroquine and matched placebo were provided by the central pharmacy of the NMVAHCS.
Safety Monitoring
As with any treatment study, patient safety was of paramount importance. Through the aforementioned organizational structure, each participating site had administrative team members who were responsible to the VABHS CSP to ensure research adherence and compliance with best practices. Additionally, an independent data and safety monitoring committee (DSMC) monitored the trial for safety and scientific integrity. At the time that the trial began in 2007, there were questions about the relative efficacy of triple therapy vs MTX plus biologic therapy. Because of this question, the DSMC raised concerns that patients may be placed at a higher risk of joint damage if not placed sooner on biologic therapy. As a response to this concern, the blinded radiographic reviewers were asked to read the hand and feet X-rays as the study progressed, allowing the DSMC to watch for any emerging safety signals. There were none, and in fact, the therapies were essentially identical radiographically.
The consequence of this request was multifold. First, patient safety was maintained; second, the study team was forced to navigate the logistic and technologic challenges posed by the reading and interpretation of the radiographs at an earlier time point than was originally planned; and last, as a result, results became available and were disseminated in a relatively narrow time frame. This third point was very important. One of the major concerns of foregoing biologic therapy was the potential for joint damage. Without the radiographic information, the manuscript could not have been completed in a timely fashion.
Trial Results
The trial was a 48-week, double-blind, noninferiority trial in which 353 patients with RA who had active disease despite MTX therapy were randomized to a triple therapy regimen of DMARDs (baseline MTX, plus SSZ and hydroxychloroquine) or baseline MTX plus etanercept (Figure 1). Patients who did not have a clinically significant improvement at 24 weeks according to a prespecified threshold were switched in a blinded fashion to the other therapy.
The primary endpoint, the change between baseline and 48 weeks in the DAS28, was similar; thus the strategy of first starting triple therapy was not inferior to first starting etanercept (the change in DAS28 was -2.12 and -2.29 respectively, P < .0001, supporting noninferiority, Figure 2). Both groups had significant improvement over the course of the first 24 weeks (P = .001). A total of 27% of participants in each group switched at 24 weeks (Figure 3). Patients in both groups who switched therapies had improvement after switching (P < .001), and the response after switching did not differ significantly between the 2 groups. Importantly, there were no significant between-group differences in radiographic progression (P = .43), pain and health-related quality of life (QOL), or in medication-associated major adverse events (AEs), although there were numerically more serious infections with etanercept-MTX therapy (12 vs 4). Gastrointestinal AEs were numerically more frequent with triple therapy; whereas infections and skin and subcutaneous AEs were more frequent with etanercept-MTX therapy.
The cost-effectiveness of adding SSZ and hydroxychloroquine to MTX vs adding etanercept to MTX, using a predetermined measurement of QOL, was assessed in this trial.9 These data were initially presented in abstract form at the 2014 American College of Rheumatology national meeting. Considered was the ratio of all the incremental costs between the 2 treatment strategies to the benefits, as measured in quality-adjusted life-years (QALYs), where QOL is measured as an index with 1 being equivalent to full health and 0 being equivalent to death. This incremental cost-effectiveness ratio produces a monetary cost for each QALY, which is an indication of cost-effectiveness and value. Most health care systems currently consider anything that costs < $50,000/QALY to be cost-effective. To be conservative, the trial researchers considered anything up to $100,000 for an additional QALY acceptable.
In the 48-week trial analysis, the use of etanercept first, instead of triple therapy, would incur about $1 million of cost per QALY, far more than the $50,000 to $100,000 deemed to be reasonable value. Biologic therapy use first, had a near-zero chance of being cost-effective and would be cost-effective only after failure of triple therapy; results that were robust to all plausible scenarios.
Economic Implications
As noted in the trial design, economic data were prospectively collected for later analysis. The availability and cost of biologic treatments have become a critical issue. In 2005, it was reported that the U.S. biologics market reached $52 billion and was noted to have an annual growth of 17%.10 In 2011, 8 of the top 20 drugs sold in the U.S. were biologics, and year-to-year biologics spending has grown by 6.6%. In 2013, the top 100 biologics in the U.S. had combined sales of $66.3 billion.11 The researchers analysis demonstrated that using a strategy of triple therapy first could result in health care cost savings in the tens of billions of dollars. Importantly, these savings would occur at the same time as patients were receiving optimal care.
Summary
The major conclusions from the RACAT trial in RA patients with active disease despite MTX are the following: 1. The strategy of first starting the conventional DMARD triple therapy combination is noninferior to first starting etanercept, based on both clinical and radiographic outcomes. 2. The triple therapy group had more minor gastrointestinal events, whereas the etanercept group had more infections. Patients in either group who did not respond well to the initial treatment and switched (27% in both groups) improved significantly after the switch. 3. The economic implications of these findings are significant. The incremental cost differences approached $1 million per QALY.
The VA CSP should be congratulated for supporting and funding this trial, which will inform therapeutic decisions in RA for years to come. These results allow clinicians to provide not only optimal health care for their RA patients, but also maximize the value of their health care.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints, leading to joint destruction, with significant long-term morbidity and mortality. Over the past quarter century, multiple new therapies and approaches have been introduced, so patients newly diagnosed with RA can realistically expect to be in remission while taking their medications. However, many of the most commonly used medications are costly, making RA care one of the most expensive per patient.1 Early treatment with disease-modifying antirheumatic drugs (DMARDs) and treating all patients to the target of low-disease activity are critical keys to optimal outcomes.
Methotrexate (MTX) is a highly effective and economical first-line DMARD that is recommended as the initial therapy for most patients.2,3 Unfortunately, one-half to two-thirds of patients will not have complete responses and, therefore, require additional therapy. Fortunately, there are more than a dozen therapies that, when added to MTX, have been shown to be better than MTX alone. However, since some of these options use conventional DMARDs and others require biologics, there exist very different economic as well as potential toxicity implications. Understanding how best to treat patients with RA with active disease while on an appropriate dose of MTX is important for both medical and economic reasons.
Despite this being a seminal question for the past 15 years, no blinded trial had addressed this issue before the VA Cooperative Studies Program (CSP) Rheumatoid Arthritis: Comparison of Active Therapies (RACAT) trial. This was true for several reasons, likely including the considerable cost of conducting such a trial and the low priority of this research question for the pharmaceutical industry. Industry-funded trials in RA often focus on new indications, and these studies often fail to address the questions most relevant to the day-to-day care of patients.
For example, it is often not particularly helpful to the clinician that patients placed on “therapy A” are doing better or worse than those placed on “therapy B” after 1 year of the same treatment. Such rigid protocols do not mimic what is done in the clinic: A patient’s treatment program is often changed much earlier than 1 year when it is not working. Therefore, RACAT was designed to more closely mirror clinical practice and to test the strategy of starting conventional therapy before biologic therapy, with the option of changing therapy for nonresponders—similar to what most clinicians would do in practice. This article explores the lessons learned from this landmark trial and highlights the critical role that the VA CSP played.
Trial Background
The RACAT trial, a comparative effectiveness, randomized, double-blind, noninferiority trial, originated as a joint effort of investigators from the VA and the Rheumatology and Arthritis Investigational Network (RAIN) and subsequently involved Canadian enrollment sites. The RACAT results were published in the New England Journal of Medicine in 2013, and its investigative team was awarded the 2014 Lee C. Howley Sr. Prize by the Arthritis Foundation for conducting the most important arthritis research worldwide from the previous year.4
The RACAT originated with a letter of intent to the VA CSP in 2003. The central question to be addressed was whether biologic therapy should be added first in patients with active RA despite MTX or whether clinicians should first add the much less expensive but very effective combination of conventional therapies, including sulfasalazine (SSZ) and hydroxychloroquine to MTX.5,6 This led to the 48-week, binational, multicenter, randomized, double-blind, noninferiority trial comparing the strategy of initially adding hydroxychloroquine and SSZ to MTX (triple therapy group) in patients with active disease despite MTX compared with the strategy of first adding etanercept to MTX.4 Etanercept is among the most commonly used biologic agents approved for RA. Etanercept works by targeting tumor necrosis factor, a pro-inflammatory cytokine central in disease pathogenesis. Both RACAT treatment groups were switched in a blinded fashion to the other therapy at 24 weeks if they did not have a clinically significant improvement. The primary endpoint was a change in the disease activity score (DAS28) from baseline to 48 weeks. An important secondary endpoint was the comparison of radiographic progression of disease at 48 weeks as measured by the validated modified Sharp scoring method. Additionally, and very importantly, economic and functional outcomes were assessed. To conduct the trial, investigators and patients participated from 16 VA sites in addition to 8 Canadian and 12 RAIN sites. The study was sponsored and primarily funded by the VA CSP, VA Office of Research and Development with additional funding coming from the Canadian Institutes of Health Research (CIHR) and from the National Institutes of Health.
Trial Design
To understand the RACAT trial design, one must appreciate the landscape of RA trials conducted in the early-to-mid 2000s. At that time, there had been an explosion of new biologic therapies for RA. Most of the trials were placebo-controlled studies with nonresponders to MTX being placed on placebo vs active drug.7 For ethical and legal reasons, however, clinicians do not treat patients with placebo, especially when highly effective therapies exist, thus limiting the relevance of the classic placebo-controlled trial in RA.8 One of the main tenets of RA therapy in this century has been to use effective therapies to treat patients with active RA with the goal of achieving (and maintaining) either low-disease activity or remission as measured by a composite scoring system, most commonly the DAS28. In order to do this in the framework of a designed research trial, therapies commonly need to be escalated when patients are not doing well, similar to what is done in clinical practice.
The RACAT trial was a comparative effectiveness trial. Comparative effectiveness is not a new idea; in fact, it is precisely how many clinicians practice medicine. It is simply comparing 2 or more treatments to determine which is more effective. Since the inception of the RACAT trial, the American Recovery and Reinvestment Act of 2009 provided $1.1 billion for major expansion of comparative effectiveness research. This changing landscape of federally funded research has highlighted the growing national interest in this type of trial.
This trial design posed several barriers as it applies to the study medications. Methotrexate, hydroxychloroquine, and SSZ are generic medications most often taken orally (MTX is available for parenteral administration). In contrast, etanercept is most often given as a subcutaneous injection and currently is not available in a biosimilar (generic) form in the U.S.; thus, the medication and its delivery device are proprietary. Because this was a double-blind, noninferiority trial, the study required both etanercept-active medication and placebo in identical delivery devices. The makers of etanercept donated placebo etanercept to make blinding possible. The VA, along with CIHR, purchased active etanercept for all trial participants, including those from Canada and the RAIN network. The VA research pharmacy in Albuquerque, New Mexico, was responsible for blinding all active and placebo drugs used in the trial and made these drugs available to all patients, even those not eligible for VA care.In a precedent-setting effort for rheumatology research, RACAT culminated from the collaborations among the private sector, the Canadian health system, and VA. The VA CSP was responsible for the collection of the clinical data, data analyses (Massachusetts Veterans Epidemiology Research and Information Center, VA Boston Healthcare System [VABHS]); the collection of economic data (VA Palo Alto Health Care System); the provision of and payment for the study medications; and the preparation and distribution of active etanercept and placebos (New Mexico VA Health Care System [NMVAHCS]). Through this organizational structure, the trial was successfully completed. In addition to placebo etanercept provided by Amgen (Thousand Oaks, CA), Pharmascience (Montreal, Quebec) provided blinded SSZ and blinded placebo. Neither company was involved in the study design nor did they have an active role in the trial. Hydroxychoroquine and matched placebo were provided by the central pharmacy of the NMVAHCS.
Safety Monitoring
As with any treatment study, patient safety was of paramount importance. Through the aforementioned organizational structure, each participating site had administrative team members who were responsible to the VABHS CSP to ensure research adherence and compliance with best practices. Additionally, an independent data and safety monitoring committee (DSMC) monitored the trial for safety and scientific integrity. At the time that the trial began in 2007, there were questions about the relative efficacy of triple therapy vs MTX plus biologic therapy. Because of this question, the DSMC raised concerns that patients may be placed at a higher risk of joint damage if not placed sooner on biologic therapy. As a response to this concern, the blinded radiographic reviewers were asked to read the hand and feet X-rays as the study progressed, allowing the DSMC to watch for any emerging safety signals. There were none, and in fact, the therapies were essentially identical radiographically.
The consequence of this request was multifold. First, patient safety was maintained; second, the study team was forced to navigate the logistic and technologic challenges posed by the reading and interpretation of the radiographs at an earlier time point than was originally planned; and last, as a result, results became available and were disseminated in a relatively narrow time frame. This third point was very important. One of the major concerns of foregoing biologic therapy was the potential for joint damage. Without the radiographic information, the manuscript could not have been completed in a timely fashion.
Trial Results
The trial was a 48-week, double-blind, noninferiority trial in which 353 patients with RA who had active disease despite MTX therapy were randomized to a triple therapy regimen of DMARDs (baseline MTX, plus SSZ and hydroxychloroquine) or baseline MTX plus etanercept (Figure 1). Patients who did not have a clinically significant improvement at 24 weeks according to a prespecified threshold were switched in a blinded fashion to the other therapy.
The primary endpoint, the change between baseline and 48 weeks in the DAS28, was similar; thus the strategy of first starting triple therapy was not inferior to first starting etanercept (the change in DAS28 was -2.12 and -2.29 respectively, P < .0001, supporting noninferiority, Figure 2). Both groups had significant improvement over the course of the first 24 weeks (P = .001). A total of 27% of participants in each group switched at 24 weeks (Figure 3). Patients in both groups who switched therapies had improvement after switching (P < .001), and the response after switching did not differ significantly between the 2 groups. Importantly, there were no significant between-group differences in radiographic progression (P = .43), pain and health-related quality of life (QOL), or in medication-associated major adverse events (AEs), although there were numerically more serious infections with etanercept-MTX therapy (12 vs 4). Gastrointestinal AEs were numerically more frequent with triple therapy; whereas infections and skin and subcutaneous AEs were more frequent with etanercept-MTX therapy.
The cost-effectiveness of adding SSZ and hydroxychloroquine to MTX vs adding etanercept to MTX, using a predetermined measurement of QOL, was assessed in this trial.9 These data were initially presented in abstract form at the 2014 American College of Rheumatology national meeting. Considered was the ratio of all the incremental costs between the 2 treatment strategies to the benefits, as measured in quality-adjusted life-years (QALYs), where QOL is measured as an index with 1 being equivalent to full health and 0 being equivalent to death. This incremental cost-effectiveness ratio produces a monetary cost for each QALY, which is an indication of cost-effectiveness and value. Most health care systems currently consider anything that costs < $50,000/QALY to be cost-effective. To be conservative, the trial researchers considered anything up to $100,000 for an additional QALY acceptable.
In the 48-week trial analysis, the use of etanercept first, instead of triple therapy, would incur about $1 million of cost per QALY, far more than the $50,000 to $100,000 deemed to be reasonable value. Biologic therapy use first, had a near-zero chance of being cost-effective and would be cost-effective only after failure of triple therapy; results that were robust to all plausible scenarios.
Economic Implications
As noted in the trial design, economic data were prospectively collected for later analysis. The availability and cost of biologic treatments have become a critical issue. In 2005, it was reported that the U.S. biologics market reached $52 billion and was noted to have an annual growth of 17%.10 In 2011, 8 of the top 20 drugs sold in the U.S. were biologics, and year-to-year biologics spending has grown by 6.6%. In 2013, the top 100 biologics in the U.S. had combined sales of $66.3 billion.11 The researchers analysis demonstrated that using a strategy of triple therapy first could result in health care cost savings in the tens of billions of dollars. Importantly, these savings would occur at the same time as patients were receiving optimal care.
Summary
The major conclusions from the RACAT trial in RA patients with active disease despite MTX are the following: 1. The strategy of first starting the conventional DMARD triple therapy combination is noninferior to first starting etanercept, based on both clinical and radiographic outcomes. 2. The triple therapy group had more minor gastrointestinal events, whereas the etanercept group had more infections. Patients in either group who did not respond well to the initial treatment and switched (27% in both groups) improved significantly after the switch. 3. The economic implications of these findings are significant. The incremental cost differences approached $1 million per QALY.
The VA CSP should be congratulated for supporting and funding this trial, which will inform therapeutic decisions in RA for years to come. These results allow clinicians to provide not only optimal health care for their RA patients, but also maximize the value of their health care.
1. Gavan S, Harrison M, Iglesias C, Barton A, Manca A, Payne K. Economics of stratified medicine in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(12):468.
2. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying anti-rheumatic drugs and biologics in the treatment of rheumatoid arthritis (RA). Arthritis Care Res (Hoboken). 2012;64(5):625-639.
3. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492-509.
4. O'Dell JR, Mikuls TR, Taylor TH, et al; CSP 551 RACAT Investigators. Therapies for active rheumatoid arthritis after methotrexate failure. N Eng J Med. 2013;369(4):307-318.
5. Moreland LW, O'Dell JR, Paulus HE, et al; TEAR Investigators. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824-2835.
6. O'Dell JR, Leff R, Paulsen G, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(5):1164-1170.
7. Singh JA, Cameron DR. Summary of AHRQ's comparative effectiveness review of drug therapy for rheumatoid arthritis in adults-an update. J Manag Care Pharm. 2012;18(4)(suppl C):S1-S18.
8. Chan TE. Regulating the placebo effect in clinical practice. Med Law Rev. 2014;23(1):1-26.
9. Bansback N, Phibb S, Sun H, et al. Cost effectiveness of adding etanercept to methotrexate therapy versus first adding sulfasalazine and hydroxychloroquine: a randomized noninferiority trial [American College of Rheumatology abstract 2781]. Arthritis Rheum. 2014;66(suppl 11):S1214.
10. Ernst and Young. Beyond borders: global biotechnology report 2005. Ernst and Young Website. https://www2.eycom.ch/publications/items/biotech-report/2005/2005_EY_Global_Biotech_Report.pdf. Accessed December 3, 2015.
11. Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. Rand Corporation Website. http://www.rand.org/pubs/perspectives/PE127.html. Accessed December 3, 2015.
1. Gavan S, Harrison M, Iglesias C, Barton A, Manca A, Payne K. Economics of stratified medicine in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(12):468.
2. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying anti-rheumatic drugs and biologics in the treatment of rheumatoid arthritis (RA). Arthritis Care Res (Hoboken). 2012;64(5):625-639.
3. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492-509.
4. O'Dell JR, Mikuls TR, Taylor TH, et al; CSP 551 RACAT Investigators. Therapies for active rheumatoid arthritis after methotrexate failure. N Eng J Med. 2013;369(4):307-318.
5. Moreland LW, O'Dell JR, Paulus HE, et al; TEAR Investigators. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824-2835.
6. O'Dell JR, Leff R, Paulsen G, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(5):1164-1170.
7. Singh JA, Cameron DR. Summary of AHRQ's comparative effectiveness review of drug therapy for rheumatoid arthritis in adults-an update. J Manag Care Pharm. 2012;18(4)(suppl C):S1-S18.
8. Chan TE. Regulating the placebo effect in clinical practice. Med Law Rev. 2014;23(1):1-26.
9. Bansback N, Phibb S, Sun H, et al. Cost effectiveness of adding etanercept to methotrexate therapy versus first adding sulfasalazine and hydroxychloroquine: a randomized noninferiority trial [American College of Rheumatology abstract 2781]. Arthritis Rheum. 2014;66(suppl 11):S1214.
10. Ernst and Young. Beyond borders: global biotechnology report 2005. Ernst and Young Website. https://www2.eycom.ch/publications/items/biotech-report/2005/2005_EY_Global_Biotech_Report.pdf. Accessed December 3, 2015.
11. Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. Rand Corporation Website. http://www.rand.org/pubs/perspectives/PE127.html. Accessed December 3, 2015.