User login
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
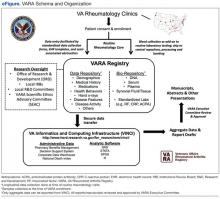
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
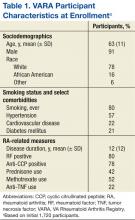
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
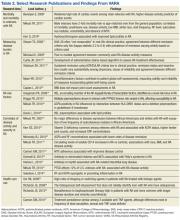
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rheumatoid arthritis (RA) is a systemic autoimmune disease that manifests primarily in the joints, leading to substantial morbidity, reduced survival, and enormous health care costs. As a result, RA exerts a major impact on patients and health care systems. U.S. military veterans and active-duty personnel have traditionally been underrepresented in RA research, likely due in part to the challenges posed by conducting investigations across federal facilities or the common refrain that such populations are not generalizable to the demographic groups (eg, younger women) most prone to develop RA.
Although RA is 3 to 4 times more common in women than in men (the latter comprising about 90% of the U.S. veteran population), its relevance to the VA health system has grown with the increase in women veterans. Well-defined risk factors for RA, such as cigarette smoking, are highly prevalent in these populations, as are comorbid conditions that frequently complicate its disease course, most notably cardiovascular disease.1 Men with RA, a disease demographic common in the VA, seem to experience a more severe disease arthritis course than do women with RA and more commonly have extra-articular manifestations, which are known to contribute to worse outcomes.2 Yet, data from predominantly male RA cohorts are sparse.
To address this gap in RA research, the VA Rheumatoid Arthritis Registry (VARA) was established in 2002 with its first patient enrolled in early 2003. Since its early inception, the registry has served as a research resource not only for VA investigators, but also for their collaborators, the VA health system, and U.S. veteran patients. This report reviews the resources available in VARA, the important insights gained in these efforts, and implications for both patients and health systems providing care. Future directions and opportunities for VARA and other disease registries are provided.
Registry Background
The VARA is a prospective, observational, multicenter study that includes VAMCs in 12 cities (Birmingham, Alabama; Brooklyn, New York; Dallas, Texas; Denver, Colorado; Jackson, Mississippi; Iowa City, Iowa; Little Rock, Arkansas; Omaha, Nebraska; Portland, Oregon; Philadelphia, Pennsylvania; Salt Lake City, Utah; and Washington, DC). In addition to support from VA research, this multicenter effort has been supported by the VA Office of Research Development, the National Institutes of Health, industry, and nonprofit foundations. The VARA serves as a repository linking banked serum, plasma, and DNA samples with an array of patient-level information, including sociodemographics, medical history, medications, comorbid conditions, longitudinal disease activity measures, and other variables (eFigure).
Clinical data are entered by investigators during routine rheumatologic care, facilitated by the use of standardized patient note templates in the VA Computerized Patient Record System, semi-automated data abstraction, and a secure intranet-based platform. With regulatory approvals, including approval of the VARA Scientific and Ethics Advisory Committee (SEAC), registry data are accessed using the VA Informatics and Computer Infrastructure (VINCI), allowing for secure linkage with detailed administrative data, including medication dispensing, diagnostic and procedural codes, and vital status.
The VARA includes > 2,200 veteran patients, all having provided informed consent, aged ≥ 18 years at disease onset, and satisfying the American College of Rheumatology (ACR) classification criteria for RA (Table 1). Serum, plasma, and DNA samples are collected at enrollment and banked in a central biorepository housed at the Nebraska Western-Iowa VA Health Care System in Omaha. In addition to providing ethical and scientific review, the VARA SEAC also provides oversight for biospecimen access. Upon receipt of specimens, the central biobank performs standardized laboratory assays on serum, including C-reactive protein (CRP), rheumatoid factor (RF), and anticyclic citrullinated (anti-CCP) antibody. These data are made available for all future investigations.
Vara Research Insights
The VARA has served as a valuable resource for a wide scope of clinical and clinical-translational research, ranging from studies of disease outcomes and their determinants, genetic and environmental risk factors, the validation of biomarkers, and health care resource utilization, among others (Table 2).
Mortality and Morbidity
The VARA researchers observed a more than 2-fold increase in mortality risk among men with RA compared with age-matched men without RA in the general U.S. population (standardized mortality ratio [SMR] 2.1; 95% confidence interval, 1.8-2.5), a risk that seems to be higher than that observed in other RA cohorts.3 Of the variables associated with mortality in this group, several potentially modifiable factors can be identified, including high erythrocyte sedimentation rate (ESR); elevated Disease Activity Score (DAS)-28 (a composite measure of disease activity including assessments of 28 joints); prednisone use; and low body weight. Patients with a body mass index < 20 kg/m2 (considered underweight) had an SMR > 5.0. Based on more recent VARA evaluations, this association seems to be driven primarily by prior weight loss rather than absolute body weight.4
Related: Methotrexate: Finding the Right Starting Dose
In contrast to oral prednisone use, which is associated with increased mortality risk, the use of methotrexate (MTX), the most commonly prescribed disease-modifying drug in RA, was associated with about a 40% reduction in all-cause mortality.3 This finding was consistent with data from other groups demonstrating that MTX use, alone or in combination with other treatments, is associated with substantial reductions in RA-related mortality, a benefit that seems to result from a robust cardioprotective effect in this population.5 Indeed, prior examinations of a VARA subpopulation revealed high rates of major acute coronary events during observation, a risk that was higher with increased disease activity.1 Studies are now underway in non-RA patients to examine the effectiveness of MTX in secondary cardiovascular disease prevention.
Although not associated with a reduced mortality risk in a previous study, hydroxychloroquine (HCQ) seems to be associated with favorable changes in lipid profiles.3 The VARA participants using HCQ were far more likely to achieve target lipid goals than were participants not using HCQ, including total cholesterol to high-density lipoprotein cholesterol (HDL-C) ratio and HDL-C to low-density lipoprotein cholesterol ratio.6 Importantly, these lipid changes appeared soon after HCQ initiation but were lost within 1 year of discontinuation. These results, coupled with data from separate groups suggesting that HCQ may also improve insulin resistance and even prevent the onset of diabetes, suggest that HCQ could play an important adjuvant treatment role by reducing cardiovascular morbidity in RA.7
Measurement Pitfalls
Proposed best practices in RA management increasingly call for the adoption of a “treat-to-target” approach, with the goal of achieving and maintaining patients in a state of low disease activity or remission.8 Although this strategy receives broad endorsement, its routine implementation is limited in the absence of a single universally accepted method for quantifying disease activity or assessing treatment response in the clinical setting. Indeed, several different measures of RA disease activity have been proposed, including at least 1 that was developed by VARA investigators.9
In a prior study, only poor to modest agreement was found among various proposed measures of treatment response and similar differences among the many proposed definitions of clinical remission.9-11 Moreover, important limitations with the validity and reliability of the patient global health assessment in clinical practice was observed. This reflected, at least in part, the contributions of many non-RA factors to its value.12 This is important, because the patient global health assessment is common to several composite disease activity measures, including remission criteria published by both the ACR and European League Against Rheumatism.13
RA Risk Factors
As part of a large collaborative consortium, VARA has been instrumental in studies examining risk factors for developing RA. These efforts have included reports of novel genetic risk factors in addition to others highlighting the importance of both gene-gene and gene-environment interactions in disease susceptibility.14-16 Among existing literature, these reports inform future efforts to further the understanding of RA pathogenesis in addition to those working to identify methods of risk stratification and disease prevention.
Disease Activity and Severity
The VARA has served as an important resource for studies examining biomarkers and other predictive factors in RA. In addition to serving as important diagnostic tools in the clinic, a recent report highlighted the potential synergistic role of RF and anti-CCP antibody in promoting disease inflammation.17 In this study, patients who were positive for both autoantibodies had much higher disease activity compared with sero-negative patients or individuals with just 1 positive autoantibody. Likewise, patients who were positive for both RF and anti-CCP had higher serum concentrations of CRP and several proinflammatory cytokines than did patients who were sero-negative or who had only 1 positive autoantibody.
In vitro studies done in parallel corroborated these observations, demonstrating for the first time that anticitrullinated protein antibody (ACPA)-containing immune complexes stimulated macrophage production of cytokines, which was further enhanced in the presence of RF. Other biomarkers investigated have included 25-hydroxy vitamin D, soluble forms of CD14 and autoantibodies to deiminated histones, neutrophil extracellular traps, and citrullinated heat shock protein.18-21
Related: The Golden Era of Treatment in Rheumatology
Of high relevance to the VA, VARA has demonstrated robust associations of treatment noncompliance, posttraumatic stress disorder (PTSD), and cigarette smoking with worse RA outcomes.22-24 In a longitudinal study of about 1,500 VARA enrollees, PTSD was independently associated with higher pain levels, tender joint counts, and self-reported disability in addition to worse patient global well-being.23 In contrast, PTSD demonstrated no associations with measures more commonly attributed to ongoing inflammation, including swollen joint counts, ESR, or DAS-28 scores. In addition to demonstrating associations of PTSD with a more severe RA course, these findings suggest that the higher disease burden observed in patients with comorbid PTSD may be attributable to noninflammatory factors that may call for management strategies beyond disease-modifying therapies.
Cigarette smoking is a well-known risk factor for RA, and emerging data, including preliminary results from VARA, suggest that smoking may render a detrimental impact on outcomes.25 Current or former smoking (observed in about 4 of 5 VARA enrollees) is associated with higher ACPA and RF levels, relevant because these autoantibodies are predictive of worse long-term outcomes, including the accrual of joint damage.24-26 Disease activity of VARA participants, measured with multiple clinical measures and an array of proinflammatory cytokines, was higher among current smokers and significantly lower in former smokers, with the former smoking group demonstrating disease activity levels approaching that of never smokers.24 In addition to its benefit in other chronic health conditions, these results suggest that smoking cessation may be a viable approach in ameliorating the systemic inflammatory effects of RA.
Health Care Use
The economic and societal burden posed by RA is enormous and growing. A large proportion of this growth relates to the near exponential increase in direct treatment costs accompanying the emergence of highly effective biologic therapies. Capitalizing on direct links between the VARA and administrative databases maintained in VINCI (eFigure), a recent investigation focused on the use of agents targeting tumor necrosis factor (TNF).27 These efforts have shown that among the 3 most commonly prescribed TNF inhibitors, persistence on initial treatment is similar over time, although important differences exist across agents in the frequency with which patients with RA undergo dose escalation. Recognizing that several reports have demonstrated their cost-effectiveness in RA, annual VA costs for a course of anti-TNF therapy approximated $13,000 to $17,000 per patient treated, and higher costs did not seem to translate into improved patient outcomes.27
Future Directions
Several recent initiatives have been undertaken within the VARA with the goal of expanding the breadth and depth of research that it supports. Ongoing efforts will link VARA with data from the National Death Index, allowing for examinations of cause-specific mortality. Given the high frequency of VA beneficiaries receiving dual care outside the VA system, future links with datasets, such as those from Medicare, will be essential to assure a more optimal capture of relevant health outcomes. Indeed, in recent surveys, almost 1 in 2 VARA participants reported the receipt of dual care, which was most common in those aged > 65 years or receiving prior joint replacement surgery (Pascale Schwab, MD, written communication, April 1, 2015).
Efforts are underway to add other well-annotated specimens to the biorepository, such as synovial fluid and tissues obtained during routine care. The VARA investigators, under regulatory approvals, have begun to collect serum samples longitudinally to complement the prospective disease activity assessments already in place. Other efforts will include the full adoption of standardized patient note templates and transitioning data entry from a decentralized and semi-automated process to one that is centralized and fully automated. This change will reduce the resources required for site investigators and study personnel.
Other Rheumatic Disease Registries
The VA health care system is the largest integrated health system in the U.S. and as such, represents an ideal setting for the investigation of chronic health conditions and patient outcomes. The assets and potential of this system have been at least partially borne out in VARA over the past decade and now extend to other rheumatic disease registries in the VA, including those focused on spondyloarthritis (PULSAR) and gout (Crystal registry). Together, these registries are poised to provide valuable information about these rheumatic conditions and will continue to serve as models for patient registries from other medical disciplines in the VA and elsewhere.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.
1. Banerjee S, Compton AP, Hooker RS, et al. Cardiovascular outcomes in male veterans with rheumatoid arthritis. Am J Cardiol. 2008;101(8):1201-1205.
2. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41(5):817-822.
3. Mikuls TR, Fay BT, Michaud K, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatol (Oxford). 2011;50(1):101-109.
4. Baker JF, Billig E, Cannon GW, Caplan L, Majithia V, Mikuls TR. Weight loss and risk of death in rheumatoid arthritis [abstract 1391]. Arthritis Rheumatol. 2014;66(suppl 10):S613-S614.
5. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173-1177.
6. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken). 2014;66(11):1619-1626.
7. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187-193.
8. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
9. Michaud K, Mikuls TR, Call SE, et al. Poor to modest agreement between rheumatoid arthritis response measures in clinical practice. Clin Exp Rheumatol. 2009;27(4):633-640.
10. Shahouri SH, Michaud K, Mikuls TR, et al. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204-3215.
11. Shaver TS, Anderson JD, Weidensaul DN, et al. The problem of rheumatoid arthritis disease activity and remission in clinical practice. J Rheumatol. 2008;35(6):1015-1022.
12. Masri KR, Shaver TS, Shahouri SH, et al. Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol. 2012;39(6):1139-1145.
13. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis. 2008;67(10):1360-1364.
14. Gregersen PK, Amos CI, Lee AT, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820-823.
15. Briggs FB, Ramsay PP, Madden E, et al. Supervised machine learning and logistic regression identifies novel epistatic risk factors with PTPN22 for rheumatoid arthritis. Genes Immun. 2010;11(3):199-208.
16. Mikuls TR, Gould KA, Bynoté KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213.
17. Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813-821.
18. Kerr GS, Sabahi I, Richards JS, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53-59.
19. Mikuls TR, LeVan TD, Sayles H, et al. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38(12):2509-2516.
20. Dwivedi N, Upadhyay J, Neeli I, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982-992.
21. Harlow L, Rosas IO, Gochuico BR, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869-879.
22. Cannon GW, Mikuls TR, Hayden CL, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken). 2011;63(12):1680-1690.
23. Mikuls TR, Padala PR, Sayles HR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227-234.
24. Sokolove J, Sayles H, Wagner CA, et al. Smoking status is associated with inflammatory cytokine profile and disease activity: decreased inflammation and disease improvement with smoking cessation? [abstract 348]. Arthritis Rheumatol. 2014;66(suppl 10):S146.
25. Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465-471.
26. Hecht C, Englbrecht M, Rech J, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA [published online ahead of print August 12, 2014]. Ann Rheum Dis. doi: 10.1136/annrheumdis -2014-205428.
27. Cannon GW, DuVall SL, Haroldsen CL, et al. Persistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritis. J Rheumatol. 2014;41(10):1935-1943.
28. Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155.
29. Caplan L, Davis LA, Bright CM, et al. Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken). 2013;65(1):101-106.
30. Davis LA, Whitfield E, Cannon GW, et al. Association of rheumatoid arthritis susceptibility gene with lipid profiles in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(6):1014-1021.
31. Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480-1484.
32. Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292-1297.
33. Oei HB, Hooker RS, Cipher DJ, Reimold A. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):926-934.
34. Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12(4):434-440.
35. Richards JS, Cannon GW, Hayden CL, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(12):1864-1870.