User login
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
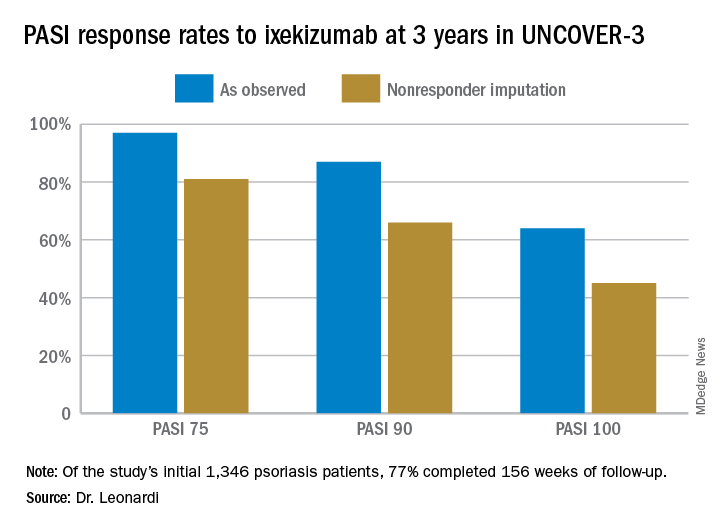
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.

However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.
“Patients fall out because the drug isn’t working well or they’re having a side effect, so over time, you tend to enrich for patients who are doing very well with the as-observed analysis,” the dermatologist explained.
Historically, many industry-sponsored clinical trials reported efficacy outcomes using the as-observed analysis; however, the FDA is increasingly unwilling to accept that approach as the sole analytic method.
At the other extreme is the nonresponder imputation method.
“This is the most stringent statistical package that exists. In fact, when a patient isn’t observed at one of the observation points – for example, at week 8 say the patient has a flat tire and can’t make it to the clinic – they’re counted as a treatment failure. So it’s a very tough statistical package,” according to Dr. Leonardi.
Seventy-seven percent of the initial 1,346 randomized patients in UNCOVER-3 completed 156 weeks of follow-up. To illustrate the importance of paying attention to the details of statistical methodology utilized in reporting efficacy outcomes, he noted that the PASI 75 rate at 156 weeks in study completers on the approved dosing regimen was 97% by the as-observed method, dropping to a still robust 81% by nonresponder imputation. The PASI 90 and -100 rates and static Physician’s Global Assessment (sPGA) results followed suit (see graphic).
Real-world performance
Dr. Leonard’s analysis of ixekizumab’s performance in his own practice included 106 patients placed on the drug following its FDA approval in March 2016, 74% of whom were still on the drug 12 months later. The cohort had a mean disease duration of 15 years. Three-quarters of them had previously received biologic therapy for their psoriasis, most often a tumor necrosis factor inhibitor. The study efficacy endpoints were the sPGA and Dermatology Life Quality Index (DLQI).
Already at 1 month, 30% of ixekizumab-treated patients had an sPGA score of 0, meaning their skin was totally clear. Another 29% had an sPGA of 1, meaning almost clear. At 3 months, 53% of patients had an sPGA of 0 and 21% had an sPGA of 1. Among patients on treatment at 12 months, the rates were 39% and 24% for sPGAs of 0 and 1, respectively. And in patients with an sPGA of 0/1 at 3 months, 73% maintained that score at 12 months, including 47% with an sPGA of 0.
A DLQI score of 0/1, indicative of little or no disease effect upon a patient’s life, was present in 63% of ixekizumab-treated patients at 1 month, 84% at 3 months, and 73% at 12 months.
The value in pushing for PASI 100
The ixekizumab experience in the phase-3 UNCOVER clinical trial program provided the first-ever evidence that incrementally improving psoriasis also provides stepwise improvement in DLQI, a key patient-reported outcome. At week 12 under double-blind conditions, only 4% of ixekizumab-treated patients with less than a PASI 50 response had a DLQI of 0/1. The rate rose to 18.8% in those with a PASI 50 to less than PASI 75 response. In patients with a week-12 PASI 75 to less than PASI 90 response, the DLQI 0/1 rate climbed to 52.3%. At a PASI 90 to less than PASI 100 response, the rate was 66.9%. And 82.9% of patients with a PASI 100 had a DLQI of 0/1. Every step of the way, those DLQI rates were significantly different from each other.
These data are “fascinating,” Dr. Leonardi commented. “If you ever get any inquiries from the friendly insurance carrier and they want to know if you’re improving your patient’s life, this is the kind of data that supports that they’re being improved dramatically.”
Dr. Leonardi noted that ixekizumab isn’t unique in its high rate of clinical effectiveness. That distinction is shared by the other approved IL-17 inhibitors, secukinumab (Cosentyx) and brodalumab (Siliq), as well as the IL-23 inhibitor guselkumab (Tremfya). He refers to these biologics collectively as “high-performance skin-clearance drugs.” He has calculated the number needed to treat (NNT) to achieve a PASI 100 response – complete clearance of the disease – based upon clinical trial data filed with the FDA and/or in the package inserts. The numbers are eye-opening: an NTT of 2.6 for ixekizumab based upon data from the UNCOVER-2 trial, 2.4 for brodalumab, 2.7 for guselkumab, and 3.6 for secukinumab. To help put that into perspective, the NNTs for methotrexate and etanercept (Enbrel) – not so long ago considered state of the art medications for moderate to severe psoriasis – are 25 and 23.3, respectively.
The UNCOVER trial portfolio and Dr. Leonardi’s single-center retrospective study were funded by Eli Lilly, which markets ixekizumab. He reported serving as a consultant to and receiving research funding from that company and more than a dozen others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
