User login
Gastrointestinal bleeding occurring in hospitalized patients admitted for nongastrointestinal disorders has been extensively studied in intensive care unit patients. However, a systematic study in noncritically ill medical patients has not yet been done. In critically ill patients the incidence of hospital‐acquired gastrointestinal bleeding (GIB) varies from 0.17% to 5%, depending on its definition.16 These bleeding events significantly increase the morbidity and duration of hospitalization.1, 5, 79
Risk factors for bleeding in the intensive care unit include mechanical ventilation, coagulopathy, burns, chronic renal failure, and neurological insults.15 Several studies have found that stress ulcer prophylaxis with histamine‐2 (H2) receptor antagonists, sucralfate, or proton pump inhibitors (PPIs) decreases bleeding in this group of patients, with a relative risk reduction of 29%61%.10, 11 However, use of these drugs outside this high‐risk group has been questioned because of the low overall risk of bleeding.1, 11, 12 Despite their being an unproven benefit in the noncritically ill population, prophylactic H2 antagonists or PPIs are prescribed in an indiscriminant fashion to up to 30%50% of patients admitted to the hospital,13, 14 suggesting that physician preference dictates this practice. To shed light on this issue in noncritically ill patients, we conducted a retrospective casecontrol study in order to identify risk factors that predict hospital‐acquired gastrointestinal bleeding in this group of patients and to assess whether treatment with prophylactic acid suppression was associated with fewer bleeding events. We also sought to characterize the endoscopic lesions in these patients.
MATERIALS AND METHODS
Study Patients
The institutional review board of the Cleveland Clinic Foundation (Cleveland, OH) approved this study. All patients admitted to the General Internal Medicine service between January 1, 1999, and December 31, 2002, were eligible for inclusion. Two types of cases were included: 1) patients admitted for nongastrointestinal illnesses who developed bleeding at least 24 hours after admission and required esophagogastroduodenoscopy (EGD) during hospitalization (designated in‐hospital bleeding), and 2) patients admitted with gastrointestinal bleeding (requiring EGD) who had been hospitalized on the General Medical service during the preceding 4 weeks for a nongastrointestinal illness (designated out‐of‐hospital bleeding). This second group was included to identify risk factors for delayed bleeding that might not be obvious during hospitalization.
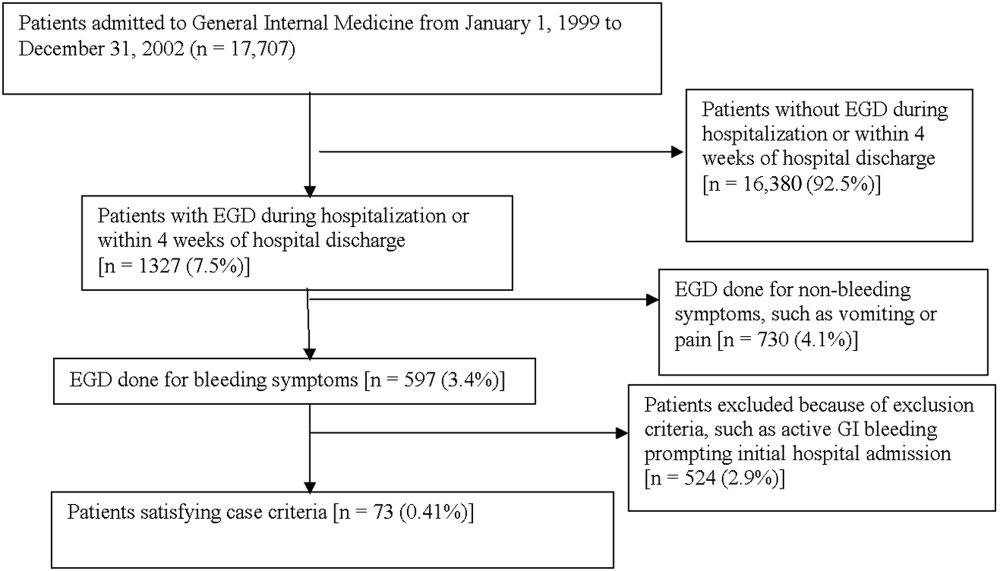
Medical records of all General Medicine patients who underwent EGD were reviewed in a standardized fashion (Fig. 1). We excluded patients with documented gastrointestinal complaints (including bleeding) at the time of the index admission or within 24 hours of admission, bleeding in the intensive care unit (ICU) or in another hospital prior to transfer to the General Medicine service, or a history of gastrointestinal bleeding during the month prior to admission. ICU stay prior to General Medicine admission, if not associated with GI bleeding, was not an exclusion criterion for our study.
Controls, also without any acute gastrointestinal symptoms at admission, were randomly matched to cases in a 1:1 ratio by date of admission. We used this liberal matching strategy because any factors matched for would no longer be eligible to be risk factors for bleeding. If more than one control was admitted on the same day as a case, then a random number was used to select the control.
Definition of Prophylactic Acid Suppression
We defined prophylactic acid suppression as in‐hospital de novo treatment with histamine‐2 receptor antagonists and/or proton pump inhibitors received prior to the onset of any symptoms that would suggest GI bleeding (for cases) or any time during hospitalization (for controls). Patients taking these drugs prior to admission were deemed ineligible for in‐hospital prophylactic acid blockade and were excluded from the related analyses.
Data Collection
We extracted demographic information, medical history, medication usage, and laboratory data by chart review. For those patients readmitted for gastrointestinal bleeding following discharge, data from the initial (nongastrointestinal illnessassociated) hospitalization were recorded. Bleeding symptoms triggering endoscopy were grouped into four categories: 1) melena or hematochezia; 2) hematemesis (frank blood in vomitus or coffee‐grounds emesis); 3) melena or hematochezia plus hematemesis (both 1 and 2); 4) stool positivity for occult blood or unexplained drop in hemoglobin in the absence of overt bleeding. Endoscopic findings were categorized by the nature of the visualized lesions, and if multiple lesions were noted, the endoscopist's impression of the most likely bleeding site was used to define the source of bleeding. We recorded colonoscopy findings for patients undergoing this evaluation.
Statistical Analysis
We analyzed data utilizing JMP 5.1 (SAS Institute, Cary, NC). Random controls were chosen using computer‐generated random numbers. The proportions of patients with various categorical characteristics were compared using the chi‐square test or Fisher's exact test as appropriate. We used the Student t test or Wilcoxon's test to compare continuous variables. Odds ratios and adjusted odds ratios were calculated by logistic regression. Two‐tailed P values less than .05 were considered statistically significant.
RESULTS: Identification of Cases and Controls
Of 17,707 patients admitted to the General Medicine service, 1327 (7.5%) underwent EGD during hospitalization or within 1 month of discharge. Only 73 (0.41%) of the total number of patients met the case definition (Fig. 1). Of these cases, 62 (84.9%) had developed gastrointestinal bleeding during the index hospitalization, whereas 11 (15.1%) were readmitted for bleeding within 4 weeks of hospital discharge. The remaining 1254 patients who underwent EGD were excluded based on exclusion criteria, including an absence of documented bleeding prompting the EGD.
Clinical Risk Factors for Bleeding
In univariate analysis, as shown in Table 1, predictors of GIB included: 1) age (P = .02); 2) admission diagnosis (P = .01); 3) preexisting coronary artery disease (P = .004); 4) treatment with blood‐thinning medications, including warfarin (P = .0004), intravenous heparin (P = .0003), and clopidogrel (P = .02); and 5) treatment with PPIs (P = .02). After adjusting for the use of full‐dose anticoagulation and/or clopidogrel, the only of these risk factors that remained significantly associated with GIB was treatment with PPIs prior to hospitalization (adjusted OR = 2.1; 95% CI 1.17.0; P = .04), suggesting that PPI treatment in the outpatient setting may be a marker for GI vulnerability.
| Characteristic | Cases n = 73 | Controls n = 73 | Unadjusted | Adjusted for treatment with full‐dose anticoagulants or clopidogrel | ||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value (2‐tailed) | Odds ratio (95% CI) | P value (2‐tailed) | |||
| ||||||
| Demographics | ||||||
| Women | 36 (49.3%) | 29 (39.7%) | 1.5 (0.82.9) | .24 | 1.6 (0.83.3) | .19 |
| Age (years), mean (SD) | 71.6 (13.7) | 65.7 (17.2) | 1.5 (1.12.1)c | .02 | 1.3 (0.91.8) | .19 |
| Caucasian | 42 (58.3%) | 32 (44.4%) | 1.7 (0.93.4) | .09 | 1.3 (0.62.6) | .50 |
| Nursing home residents | 5 (6.9%) | 5 (6.9%) | 1.0 (0.33.7) | >.99 | 0.5 (0.12.2) | .35 |
| Admission diagnosisa | .01d | .30d | ||||
| Cardiovascular (non‐thrombotic) | 15 (20.5%) | 6 (8.2%) | 2.9 (1.18.5) | .04 | 2.1 (0.76.5) | .19 |
| Arterial or venous thrombosis | 13 (17.8%) | 2 (2.7%) | 7.9 (2.050.4) | .009 | 3.3 (0.822.1) | .15 |
| Infection | 21 (28.8%) | 24 (32.9%) | 0.8 (0.41.7) | .59 | 1.1 (0.52.3) | .86 |
| Pulmonary (noninfectious) | 4 (5.5%) | 10 (13.7%) | 0.4 (0.11.2) | .10 | 0.5 (0.11.7) | .31 |
| Altered level of consciousness | 7 (9.6%) | 10 (13.7%) | 0.7 (0.21.8) | .44 | 0.7 (0.22.2) | .59 |
| Other | 13 (17.8%) | 21 (28.8%) | 0.5 (0.21.2) | .12 | 0.6 (0.31.5) | .29 |
| Baseline medical conditions | ||||||
| Diabetes mellitus | 28 (38.4%) | 25 (34.3%) | 1.2 (0.62.4) | .61 | 1.3 (0.62.7) | .48 |
| Hypertension | 50 (68.5%) | 48 (65.8%) | 1.1 (0.62.3) | .72 | 1.2 (0.52.5) | .71 |
| Coronary artery disease | 36 (49.3%) | 19 (26.0%) | 2.8 (1.45.6) | .004 | 2.0 (1.04.3) | .06 |
| Atrial fibrillation | 18 (24.7%) | 10 (13.7%) | 2.1 (0.95.0) | .09 | 1.4 (0.53.6) | .49 |
| Congestive heart failure | 25 (34.3%) | 16 (21.9%) | 1.9 (0.93.9) | .10 | 1.5 (0.73.3) | .35 |
| Renal insufficiency (creatinine > 2) | 18 (24.7%) | 11 (15.1%) | 1.8 (0.84.4) | .14 | 1.9 (0.84.7) | .33 |
| Chronic obstructive pulmonary disease | 21 (28.8%) | 20 (27.4%) | 1.1 (0.52.2) | .85 | 1.5 (0.73.4) | .29 |
| Stroke | 13 (17.8%) | 16 (21.9%) | 0.8 (0.31.7) | .53 | 0.7 (0.31.6) | .39 |
| Active malignancy | 6 (8.2%) | 8 (11.0%) | 0.7 (0.32.2) | .57 | 1.0 (0.33.5) | .80 |
| Gastroesophageal reflux (GERD) | 10 (13.7%) | 10 (13.7%) | 1.0 (0.42.6) | >.99 | 1.0 (0.32.7) | .92 |
| Liver disease | 7 (9.6%) | 6 (8.2%) | 1.2 (0.43.9) | .77 | 1.4 (0.44.9) | .59 |
| Peptic ulcer disease | 13 (17.8%) | 5 (6.9%) | 2.9 (1.09.6) | .04 | 2.7 (0.99.4) | .09 |
| Colonic disease (diverticulosis, polyp, or AVM) | 7 (9.6%) | 4 (5.5%) | 1.8 (0.57.3) | .34 | 1.2 (0.35.2) | .79 |
| Prior gastrointestinal hemorrhage | 15 (20.1%) | 7 (9.6%) | 2.4 (1.06.8) | .06 | 2.0 (0.75.8) | .20 |
| Tobacco abuse (current smoking) | 9 (12.3%) | 18 (24.7%) | 0.4 (0.21.0) | .05 | 0.6 (0.21.5) | .26 |
| Heavy drinking (>8 drinks/day) | 2 (2.7%) | 2 (2.7%) | 1.0 (0.18.5) | >.99 | 1.3 (0.111.7) | .83 |
| Medication exposure prior to bleeding (excluding acid blockade)b | ||||||
| Aspirin (with or without NSAID) | 34 (46.6%) | 32 (43.8%) | 1.1 (0.62.1) | .74 | 0.7 (0.31.5) | .42 |
| Nonselective NSAID (without aspirin) | 3 (4.1%) | 5 (6.9%) | 0.6 (0.12.5) | .72 | 0.6 (0.12.6) | .44 |
| COX‐2 inhibitors | 3 (4.1%) | 7 (9.6%) | 0.4 (0.11.5) | .18 | 0.3 (0.11.4) | .15 |
| Glucocorticoids | 17 (23.3%) | 20 (27.4%) | 0.8 (0.41.7) | .57 | 0.9 (0.42.1) | .89 |
| Warfarin | 24 (32.9%) | 7 (9.6%) | 4.6 (1.912.4) | .004 | N/A | N/A |
| Unfractionated heparin, UFH (full‐dose intravenous | 23 (31.5%) | 6 (20.7%) | 5.1 (2.114.8) | .0003 | N/A | N/A |
| Full‐dose low‐molecular‐weight heparin (LMWH) | 2 (2.7%) | 0 (0%) | infinity | .50 | N/A | N/A |
| Clopidogrel | 9 (12.3%) | 2 (2.7%) | 5.0 (1.233.5) | .02 | N/A | N/A |
| Prophylactic LMWH or UFH (among 103 patients not on full‐dose anticoagulants) | 19 (47.5%) | 32 (50.8%) | 0.9 (0.41.9) | .74 | N/A | N/A |
| Any treatment with warfarin, full‐dose UFH, full‐ dose LMWH, and/or clopidogrel | 41 (56.2%) | 14 (19.2%) | 5.4 (2.611.7) | <.0001 | N/A | N/A |
| Gastric acid suppression (prior to any gastrointestinal hemorrhage) | ||||||
| H2‐receptor antagonists (H2RA) (total) | 11 (15.1%) | 19 (26.0%) | 0.5 (0.21.1) | .10 | 0.6 (0.31.5) | .31 |
| Taken prior to admission | 6 (8.2%) | 9 (12.3%) | 0.6 (0.21.9) | .41 | 0.6 (0.22.1) | .47 |
| Started de novo at admission | 5 (6.9%) | 10 (13.7%) | 0.5 (0.11.4) | .17 | 0.7 (0.22.2) | .53 |
| Proton‐pump inhibitor (PPI) (total) | 28 (38.6%) | 16 (21.9%) | 2.2 (1.14.7) | .03 | 2.1 (1.04.6) | .07 |
| Taken prior to admission | 20 (27.4%) | 9 (12.3%) | 2.2 (1.14.7) | .02 | 2.7 (1.17.0) | .04 |
| Started de novo at admission | 8 (11.0%) | 7 (9.6%) | 1.2 (0.43.5) | .79 | 1.0 (0.33.2) | .99 |
| Any treatment with PPI or H2RA prior to hemorrhage (total) | 39 (53.4%) | 33 (45.2%) | 1.4 (0.72.7) | .32 | 1.5 (0.73.0) | .28 |
| Taken prior to admission | 26 (35.6%) | 18 (24.7%) | 1.7 (0.83.5) | .15 | 1.7 (0.83.7) | .18 |
| Started de novo at admission (among the 102 patients not taking prior to admission) | 13 (27.7%) | 15 (27.3%) | 1.0 (0.42.4) | .97 | 1.1 (0.42.9) | .80 |
Among patients on warfarin, the peak international normalized ratio (median [IQR]) was 3.0 (1.25.0) for cases and 1.9 (1.64.8) for controls (P = .52). For those on heparin (23 cases and 6 controls), the median peak activated partial thromboplastin time (aPTT) was 67 (5082) and 128 (67180) seconds for cases and controls, respectively (P = .03), a surprising finding that was likely a result of type III error and small sample size.
Outcomes
We found no evidence of major complications from bleeding, as shown in Table 2. As expected, cases were more likely to receive blood transfusions than were controls, but clinically serious outcomes were uncommon in both groups.
| Characteristic | Cases n = 73 | Controls n = 73 | P value |
|---|---|---|---|
| |||
| Pulmonary complicationsa | 4 ( 5.5%) | 2 (2.7%) | .68 |
| Cardiac complicationsb | 4 ( 5.5%) | 3 (4.1%) | >.99 |
| Acute renal failure requiring dialysis | 0 ( 0.0%) | 1 (1.4%) | >.99 |
| Stroke or transient cerebral ischemia | 1 ( 1.4%) | 1 (1.4%) | >.99 |
| Transfer to intensive care unit | 9 (12.3%) | 4 (5.5%) | .14 |
| Blood transfusion required | 46 (63.0%) | 3 (4.1%) | <.0001 |
| All‐cause mortality | 3 ( 4.1%) | 2 (2.7%) | >.99 |
Gastrointestinal Symptoms and Endoscopic Findings
Bleeding symptoms prompting EGD and associated endoscopic findings are shown in Table 3. Findings on colonoscopy (performed in 34 patients) are included. Overall, 54 (74%) patients had a detected abnormality on EGD and/or colonoscopy that was believed to be a likely source of bleeding by the endoscopist, and 19 (26%) had no apparent culprit lesions. Melena and stool positivity for occult blood were the most common manifestations of gastrointestinal bleeding (77%) and also accounted for all the normal endoscopic evaluations. Of the 21 ulcers, 18 (85.7%) had a clean base, 1 (4.8%) had a red spot, and 2 (9.5%) had an adherent clot. None had a bleeding vessel. Endoscopic treatment was performed in one patient and angiography in one patient. A possible gastric stromal tumor (not the source of bleeding) was seen in one patient, but no mucosal malignant lesions were identified. Of the 73 cases, 41 (56.2% of cases and 0.2% of the total cohort of 17,707 patients) had culprit lesions that might have been preventable with gastric acid suppression (including peptic ulcers, esophagitis, and duodenitis).
| Most likely primary source of bleeding based on EGD with or without colonoscopya | Hematemesis only n = 10 (13.7% of cases) | Melena or hematochezia n = 33 (45.2% of cases) | Hematemesis plus either melena or hematochezia n = 4 (5.5% of cases) | Occult blood (+) and/or drop in hemoglobin (without overt bleeding) n = 26 (35.6% of cases) |
|---|---|---|---|---|
| ||||
| Normal (no lesions identified n = 19 (26.0% of cases) | 0 | 12 | 0 | 7 |
| Peptic ulcer n = 21 (28.8% of cases) | 4 | 10 | 0 | 7 |
| Esophagitis n = 8 (11.0% of cases) | 2 | 2 | 2 | 2 |
| Gastritis or duodenitis n = 12 (16.4% of cases) | 1 | 6 | 1 | 4 |
| Lower GI source only n = 1 (1.4% of cases) | 0 | 0 | 0 | 1 |
| Miscellaneous upper GI sourceb n = 12 (16.4% of cases) | 3 | 3 | 1 | 5 |
Prophylactic Gastric Acid Suppression
One hundred and two patients were not taking any acid‐suppressive prophylaxis on admission to the hospital. Of these patients, on admission 28 (27.5%) were prescribed either histamine‐2 receptor antagonists or proton pump inhibitors. We identified no clinical features associated with the prescriptions for these medications (Table 4), suggesting that physician preference, rather than perceived risk factors for bleeding, determined which patients received prophylactic acid blockade. There was no association between this prophylaxis and GI bleeding, but because of the small size of our sample, the confidence interval was wide (OR = 1.0; 95% CI 0.42.4; P = .97). In the analysis of the subgroup of patients receiving anticoagulation or clopidogrel, prophylaxis showed a nonsignificant trend toward benefit (OR = 0.71; 95% CI 0.23.9; P = .67). There was no significant interaction between the presence of anticoagulation or clopidogrel and prophylaxis (P = .61). Similarly, when we excluded those without prior GI bleeding from analysis, there was still no apparent protective effect of acid‐suppressive prophylaxis (OR = 1.0; 95% CI 0.42.5; P = .97). Finally, there was no significant association between the use of prophylaxis and lesions (theoretically) preventable by acid blockade (OR = 0.9; 95% CI 0.32.3; P = .84).
| Characteristic | Prophylaxis | |||
|---|---|---|---|---|
| Initiated n = 28 (27.5%) | Withheld n = 74 (72.5%) | Odds ratio (95% CI) | P value | |
| ||||
| Cases | ||||
| All lesions | 13 (46.4%) | 34 (46.0%) | 1.0 (0.42.4) | .97 |
| Lesions preventable with acid blockadeb | 8 (28.6%) | 20 (27.0%) | 1.1 (0.42.8) | .88 |
| Demographics | ||||
| Age, in years (SD) | 70.3 (18.6) | 66.9 (15.7) | 1.2 (0.81.9) | .40 |
| Female | 10 (35.7%) | 36 (48.7%) | 0.6 (0.21.4) | .24 |
| Medical history | ||||
| Prior gastrointestinal bleeding | 4 (14.3%) | 7 ( 9.5%) | 1.6 (0.45.8) | .49 |
| History of GERD | 1 ( 3.6%) | 4 ( 5.4%) | 0.6 (0.04.6) | >.99 |
| History of peptic ulcer disease | 3 (10.7%) | 5 ( 6.8%) | 1.7 (0.37.3) | .68 |
| Hospitalization variables | ||||
| Transferred from ICU | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Cardiovascular admission diagnosis | 7 (25.0%) | 21 (28.4%) | 0.8 (0.32.2) | .73 |
| Medication exposure | ||||
| Aspirin (with or without NSAID) | 13 (46.4%) | 30 (40.5%) | 1.3 (0.53.1) | .59 |
| NSAID alone (nonselective) | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Glucocorticoids | 6 (21.4%) | 19 (25.7%) | 0.8 (0.32.2) | .65 |
| Warfarin, clopidogrel, or IV heparin | 9 (32.1%) | 28 (37.8%) | 0.8 (0.31.9) | .59 |
DISCUSSION
Our data suggest that the incidence of hospital‐acquired gastrointestinal bleeding in noncritically ill medical patients is low (approximately 0.4%) and that treatment with anticoagulants or clopidogrel predisposes to this complication. Anticoagulation is a well‐known risk factor for gastrointestinal bleeding, with an estimated odds ratio of 2416; our study confirmed this risk.
Although some studies have questioned the utility of prophylactic acid blockade in the intensive care unit,15 the weight of current evidence supports prophylaxis in selected critically ill patients. In a randomized double‐blind study of 1200 mechanically ventilated patients, the relative risk of gastrointestinal bleeding in patients treated with ranitidine was 0.44 (95% CI 0.210.92 P = .02).11 Many experts discourage indiscriminant use of prophylaxes, even by patients in intensive care units, recommending that it be used only in patients with established risk factors for bleeding.1, 12
Despite the absence of evidence of any benefit of the use of prophylactic acid blockade outside the intensive care unit, this practice is common. In our study, 27.5% of patients who were not on outpatient acid suppression medications (PPIs or H2 antagonists) were started on them on admission to the hospital, presumably as prophylaxes, as we excluded patients admitted for acute gastrointestinal complaints. Other studies have reported prophylaxis rates of 30%50%.13, 14 Many patients started on this prophylaxis during hospitalization go on to take these drugs following discharge, creating an unnecessary economic burden.13, 14 In our study, GI prophylaxis did not appear to prevent hospital‐acquired gastrointestinal bleeding. However, the odds ratio associating the use of prophylactic acid suppression with gastrointestinal bleeding (1.0) was associated with a wide 95% confidence interval (0.42.4), so we cannot exclude the possibility that these medications might provide a relative risk reduction that we were unable to detect. Finally, although gastrointestinal bleeding in the intensive care unit is associated with significant morbidity and mortality,8, 9 we found no evidence to suggest that gastrointestinal bleeding in our patients was associated with poor outcomes.
In interpreting the data from this study, it is important to note that the definition of hospital‐acquired gastrointestinal bleeding in the literature has been inconsistent. Some studies have required that bleeding be hemodynamically significant1, 2, 5, 11a stringent criterion that may be present in only 10%15% of patients with bleeding16whereas other studies defined gastrointestinal bleeding on the basis of occult‐blood‐positive nasogastric aspirates or positive endoscopic findings.7, 15 Because the definition used in the present study required a hard clinical event (EGD), it excluded bleeding events that were considered clinically insignificant by treating physicians. We justified this definition on our belief that any bleeding that warrants invasive evaluation is clinically relevant because it is expensive and puts the patient at some physical risk. Even though some of our patients were diagnosed with GIB without obvious melena or hematemesis (ie, based on stool positivity for occult blood), many of these patients had significant drops in hemoglobin during hospitalization, which, accompanied by occult blood positivity, justified inpatient EGD. We do not believe our definition of GI bleeding was too restrictive, at least for our institution, as physicians at the Cleveland Clinic generally pursue inpatient EGD with clinically apparent gastrointestinal bleeding; we maintain that bleeding that is minor enough not to change management is of limited clinical relevance. Nevertheless, the threshold for EGD at a given institution could affect the rate of EGD for soft indications and the overall prevalence of nosocomial GI bleeding based on our definition.
It also is worth noting that our definition of nosocomial bleeding encompassed some patients with recent hospitalization on the medical service who bled following discharge (15% of cases in this study). This inclusion criterion was chosen because of our concern that the stress of hospitalization might lead to complications even after discharge. We chose an arbitrary postdischarge cutoff of 4 weeks. When we excluded these patients from analysis, the results were similar (data not shown). Although it is possible that we missed some patient who presented to other institutions with GI bleeding following discharge from the Cleveland Clinic, we suspect that the number of such patients was very small based on current referral patterns.
We do not have complete information to determine exactly why patients were on acid‐suppressive therapy prior to admission, but the available data suggest that many had gastroesophageal reflux disease (GERD), PUD, or prior GI bleeding. For this reason, we focused the investigation of the potential efficacy of prophylactic initiation of acid blockade among patients who at presentation were not taking these medications, as prior GERD (or undocumented GIB) leading to chronic use of acid blockade may predispose to subsequent GIB. Although we analyzed only those patients who had newly started taking acid‐suppressive medications, we acknowledge that a few of them may have been started on these medications for other reasons, like chest pain or GERD. However, the evidence suggests that an overwhelming number are started on these medications for the sole purpose of GI prophylaxis.13, 14
Our study was limited by its retrospective casecontrol design. However, because of the low prevalence of hospital‐acquired gastrointestinal bleeding outside the critical care unit, a prospective study would have to enroll thousands of patients in order to generate statistically meaningful results.
In summary, hospital‐acquired gastrointestinal bleeding outside the intensive care unit is uncommon, with an incidence of about 0.4% according to our definition of bleeding. We found no evidence that these bleeding episodes are associated with increased mortality or with occult malignancy. Furthermore, we found no evidence that prophylactic gastric acid suppression prevents these events, and only 41 patients (0.2% of the total cohort) had lesions that might be preventable with gastric acid blockade. We discourage the indiscriminant use of prophylactic acid suppressants in general medical patients.
Acknowledgements
The authors thank Donna M. Richey and Betty Lou Harrison for clerical support.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,, et al.Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.Crit Care Med.1999;27:2812–2817.
- ,,,,.Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit.Am J Med.1984;76:623–630.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators.J Am Geriatr Soc.2001;49:126–133.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case–control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,.Characterization of gastrointestinal bleeding in severely ill hospitalized patients.Crit Care Med.2000;28:46–50.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,,, et al.The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients.Crit Care.2001;5:368–375.
- ,,,.Risks for developing critical illness with GI hemorrhage.Chest.2000;118:473–478.
- .Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis.Scand J GastroenterolSuppl.1995;210:48–52.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–122.
- ,,, et al.Prophylaxis for stress‐related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single‐blind study.Ann Intern Med.1994;121:568–575.
- .Low incidence of hemodynamic instability in patients with gastrointestinal hemorrhage.South Med J.1996;89:386–390.
Gastrointestinal bleeding occurring in hospitalized patients admitted for nongastrointestinal disorders has been extensively studied in intensive care unit patients. However, a systematic study in noncritically ill medical patients has not yet been done. In critically ill patients the incidence of hospital‐acquired gastrointestinal bleeding (GIB) varies from 0.17% to 5%, depending on its definition.16 These bleeding events significantly increase the morbidity and duration of hospitalization.1, 5, 79
Risk factors for bleeding in the intensive care unit include mechanical ventilation, coagulopathy, burns, chronic renal failure, and neurological insults.15 Several studies have found that stress ulcer prophylaxis with histamine‐2 (H2) receptor antagonists, sucralfate, or proton pump inhibitors (PPIs) decreases bleeding in this group of patients, with a relative risk reduction of 29%61%.10, 11 However, use of these drugs outside this high‐risk group has been questioned because of the low overall risk of bleeding.1, 11, 12 Despite their being an unproven benefit in the noncritically ill population, prophylactic H2 antagonists or PPIs are prescribed in an indiscriminant fashion to up to 30%50% of patients admitted to the hospital,13, 14 suggesting that physician preference dictates this practice. To shed light on this issue in noncritically ill patients, we conducted a retrospective casecontrol study in order to identify risk factors that predict hospital‐acquired gastrointestinal bleeding in this group of patients and to assess whether treatment with prophylactic acid suppression was associated with fewer bleeding events. We also sought to characterize the endoscopic lesions in these patients.
MATERIALS AND METHODS
Study Patients
The institutional review board of the Cleveland Clinic Foundation (Cleveland, OH) approved this study. All patients admitted to the General Internal Medicine service between January 1, 1999, and December 31, 2002, were eligible for inclusion. Two types of cases were included: 1) patients admitted for nongastrointestinal illnesses who developed bleeding at least 24 hours after admission and required esophagogastroduodenoscopy (EGD) during hospitalization (designated in‐hospital bleeding), and 2) patients admitted with gastrointestinal bleeding (requiring EGD) who had been hospitalized on the General Medical service during the preceding 4 weeks for a nongastrointestinal illness (designated out‐of‐hospital bleeding). This second group was included to identify risk factors for delayed bleeding that might not be obvious during hospitalization.
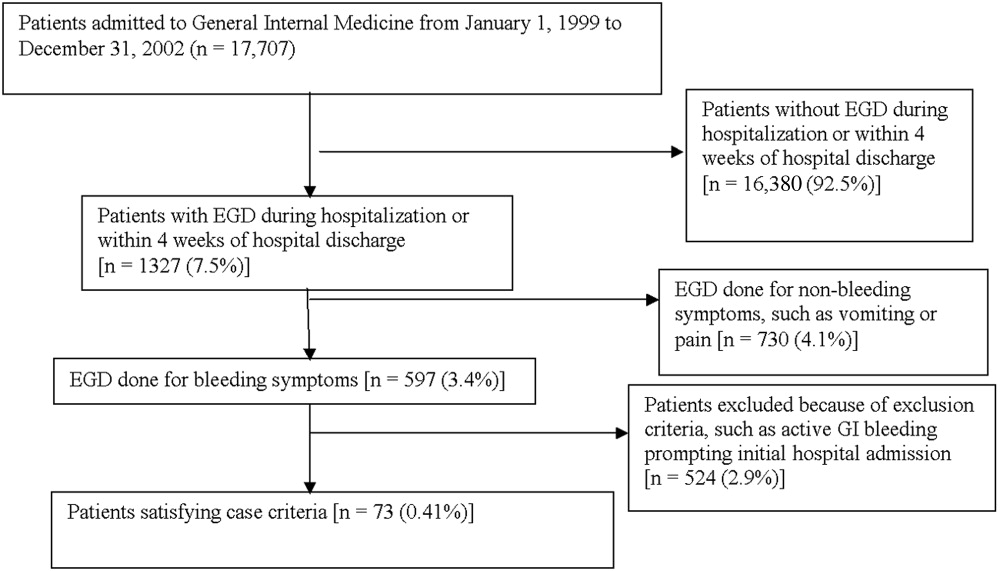
Medical records of all General Medicine patients who underwent EGD were reviewed in a standardized fashion (Fig. 1). We excluded patients with documented gastrointestinal complaints (including bleeding) at the time of the index admission or within 24 hours of admission, bleeding in the intensive care unit (ICU) or in another hospital prior to transfer to the General Medicine service, or a history of gastrointestinal bleeding during the month prior to admission. ICU stay prior to General Medicine admission, if not associated with GI bleeding, was not an exclusion criterion for our study.

Controls, also without any acute gastrointestinal symptoms at admission, were randomly matched to cases in a 1:1 ratio by date of admission. We used this liberal matching strategy because any factors matched for would no longer be eligible to be risk factors for bleeding. If more than one control was admitted on the same day as a case, then a random number was used to select the control.
Definition of Prophylactic Acid Suppression
We defined prophylactic acid suppression as in‐hospital de novo treatment with histamine‐2 receptor antagonists and/or proton pump inhibitors received prior to the onset of any symptoms that would suggest GI bleeding (for cases) or any time during hospitalization (for controls). Patients taking these drugs prior to admission were deemed ineligible for in‐hospital prophylactic acid blockade and were excluded from the related analyses.
Data Collection
We extracted demographic information, medical history, medication usage, and laboratory data by chart review. For those patients readmitted for gastrointestinal bleeding following discharge, data from the initial (nongastrointestinal illnessassociated) hospitalization were recorded. Bleeding symptoms triggering endoscopy were grouped into four categories: 1) melena or hematochezia; 2) hematemesis (frank blood in vomitus or coffee‐grounds emesis); 3) melena or hematochezia plus hematemesis (both 1 and 2); 4) stool positivity for occult blood or unexplained drop in hemoglobin in the absence of overt bleeding. Endoscopic findings were categorized by the nature of the visualized lesions, and if multiple lesions were noted, the endoscopist's impression of the most likely bleeding site was used to define the source of bleeding. We recorded colonoscopy findings for patients undergoing this evaluation.
Statistical Analysis
We analyzed data utilizing JMP 5.1 (SAS Institute, Cary, NC). Random controls were chosen using computer‐generated random numbers. The proportions of patients with various categorical characteristics were compared using the chi‐square test or Fisher's exact test as appropriate. We used the Student t test or Wilcoxon's test to compare continuous variables. Odds ratios and adjusted odds ratios were calculated by logistic regression. Two‐tailed P values less than .05 were considered statistically significant.
RESULTS: Identification of Cases and Controls
Of 17,707 patients admitted to the General Medicine service, 1327 (7.5%) underwent EGD during hospitalization or within 1 month of discharge. Only 73 (0.41%) of the total number of patients met the case definition (Fig. 1). Of these cases, 62 (84.9%) had developed gastrointestinal bleeding during the index hospitalization, whereas 11 (15.1%) were readmitted for bleeding within 4 weeks of hospital discharge. The remaining 1254 patients who underwent EGD were excluded based on exclusion criteria, including an absence of documented bleeding prompting the EGD.
Clinical Risk Factors for Bleeding
In univariate analysis, as shown in Table 1, predictors of GIB included: 1) age (P = .02); 2) admission diagnosis (P = .01); 3) preexisting coronary artery disease (P = .004); 4) treatment with blood‐thinning medications, including warfarin (P = .0004), intravenous heparin (P = .0003), and clopidogrel (P = .02); and 5) treatment with PPIs (P = .02). After adjusting for the use of full‐dose anticoagulation and/or clopidogrel, the only of these risk factors that remained significantly associated with GIB was treatment with PPIs prior to hospitalization (adjusted OR = 2.1; 95% CI 1.17.0; P = .04), suggesting that PPI treatment in the outpatient setting may be a marker for GI vulnerability.
| Characteristic | Cases n = 73 | Controls n = 73 | Unadjusted | Adjusted for treatment with full‐dose anticoagulants or clopidogrel | ||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value (2‐tailed) | Odds ratio (95% CI) | P value (2‐tailed) | |||
| ||||||
| Demographics | ||||||
| Women | 36 (49.3%) | 29 (39.7%) | 1.5 (0.82.9) | .24 | 1.6 (0.83.3) | .19 |
| Age (years), mean (SD) | 71.6 (13.7) | 65.7 (17.2) | 1.5 (1.12.1)c | .02 | 1.3 (0.91.8) | .19 |
| Caucasian | 42 (58.3%) | 32 (44.4%) | 1.7 (0.93.4) | .09 | 1.3 (0.62.6) | .50 |
| Nursing home residents | 5 (6.9%) | 5 (6.9%) | 1.0 (0.33.7) | >.99 | 0.5 (0.12.2) | .35 |
| Admission diagnosisa | .01d | .30d | ||||
| Cardiovascular (non‐thrombotic) | 15 (20.5%) | 6 (8.2%) | 2.9 (1.18.5) | .04 | 2.1 (0.76.5) | .19 |
| Arterial or venous thrombosis | 13 (17.8%) | 2 (2.7%) | 7.9 (2.050.4) | .009 | 3.3 (0.822.1) | .15 |
| Infection | 21 (28.8%) | 24 (32.9%) | 0.8 (0.41.7) | .59 | 1.1 (0.52.3) | .86 |
| Pulmonary (noninfectious) | 4 (5.5%) | 10 (13.7%) | 0.4 (0.11.2) | .10 | 0.5 (0.11.7) | .31 |
| Altered level of consciousness | 7 (9.6%) | 10 (13.7%) | 0.7 (0.21.8) | .44 | 0.7 (0.22.2) | .59 |
| Other | 13 (17.8%) | 21 (28.8%) | 0.5 (0.21.2) | .12 | 0.6 (0.31.5) | .29 |
| Baseline medical conditions | ||||||
| Diabetes mellitus | 28 (38.4%) | 25 (34.3%) | 1.2 (0.62.4) | .61 | 1.3 (0.62.7) | .48 |
| Hypertension | 50 (68.5%) | 48 (65.8%) | 1.1 (0.62.3) | .72 | 1.2 (0.52.5) | .71 |
| Coronary artery disease | 36 (49.3%) | 19 (26.0%) | 2.8 (1.45.6) | .004 | 2.0 (1.04.3) | .06 |
| Atrial fibrillation | 18 (24.7%) | 10 (13.7%) | 2.1 (0.95.0) | .09 | 1.4 (0.53.6) | .49 |
| Congestive heart failure | 25 (34.3%) | 16 (21.9%) | 1.9 (0.93.9) | .10 | 1.5 (0.73.3) | .35 |
| Renal insufficiency (creatinine > 2) | 18 (24.7%) | 11 (15.1%) | 1.8 (0.84.4) | .14 | 1.9 (0.84.7) | .33 |
| Chronic obstructive pulmonary disease | 21 (28.8%) | 20 (27.4%) | 1.1 (0.52.2) | .85 | 1.5 (0.73.4) | .29 |
| Stroke | 13 (17.8%) | 16 (21.9%) | 0.8 (0.31.7) | .53 | 0.7 (0.31.6) | .39 |
| Active malignancy | 6 (8.2%) | 8 (11.0%) | 0.7 (0.32.2) | .57 | 1.0 (0.33.5) | .80 |
| Gastroesophageal reflux (GERD) | 10 (13.7%) | 10 (13.7%) | 1.0 (0.42.6) | >.99 | 1.0 (0.32.7) | .92 |
| Liver disease | 7 (9.6%) | 6 (8.2%) | 1.2 (0.43.9) | .77 | 1.4 (0.44.9) | .59 |
| Peptic ulcer disease | 13 (17.8%) | 5 (6.9%) | 2.9 (1.09.6) | .04 | 2.7 (0.99.4) | .09 |
| Colonic disease (diverticulosis, polyp, or AVM) | 7 (9.6%) | 4 (5.5%) | 1.8 (0.57.3) | .34 | 1.2 (0.35.2) | .79 |
| Prior gastrointestinal hemorrhage | 15 (20.1%) | 7 (9.6%) | 2.4 (1.06.8) | .06 | 2.0 (0.75.8) | .20 |
| Tobacco abuse (current smoking) | 9 (12.3%) | 18 (24.7%) | 0.4 (0.21.0) | .05 | 0.6 (0.21.5) | .26 |
| Heavy drinking (>8 drinks/day) | 2 (2.7%) | 2 (2.7%) | 1.0 (0.18.5) | >.99 | 1.3 (0.111.7) | .83 |
| Medication exposure prior to bleeding (excluding acid blockade)b | ||||||
| Aspirin (with or without NSAID) | 34 (46.6%) | 32 (43.8%) | 1.1 (0.62.1) | .74 | 0.7 (0.31.5) | .42 |
| Nonselective NSAID (without aspirin) | 3 (4.1%) | 5 (6.9%) | 0.6 (0.12.5) | .72 | 0.6 (0.12.6) | .44 |
| COX‐2 inhibitors | 3 (4.1%) | 7 (9.6%) | 0.4 (0.11.5) | .18 | 0.3 (0.11.4) | .15 |
| Glucocorticoids | 17 (23.3%) | 20 (27.4%) | 0.8 (0.41.7) | .57 | 0.9 (0.42.1) | .89 |
| Warfarin | 24 (32.9%) | 7 (9.6%) | 4.6 (1.912.4) | .004 | N/A | N/A |
| Unfractionated heparin, UFH (full‐dose intravenous | 23 (31.5%) | 6 (20.7%) | 5.1 (2.114.8) | .0003 | N/A | N/A |
| Full‐dose low‐molecular‐weight heparin (LMWH) | 2 (2.7%) | 0 (0%) | infinity | .50 | N/A | N/A |
| Clopidogrel | 9 (12.3%) | 2 (2.7%) | 5.0 (1.233.5) | .02 | N/A | N/A |
| Prophylactic LMWH or UFH (among 103 patients not on full‐dose anticoagulants) | 19 (47.5%) | 32 (50.8%) | 0.9 (0.41.9) | .74 | N/A | N/A |
| Any treatment with warfarin, full‐dose UFH, full‐ dose LMWH, and/or clopidogrel | 41 (56.2%) | 14 (19.2%) | 5.4 (2.611.7) | <.0001 | N/A | N/A |
| Gastric acid suppression (prior to any gastrointestinal hemorrhage) | ||||||
| H2‐receptor antagonists (H2RA) (total) | 11 (15.1%) | 19 (26.0%) | 0.5 (0.21.1) | .10 | 0.6 (0.31.5) | .31 |
| Taken prior to admission | 6 (8.2%) | 9 (12.3%) | 0.6 (0.21.9) | .41 | 0.6 (0.22.1) | .47 |
| Started de novo at admission | 5 (6.9%) | 10 (13.7%) | 0.5 (0.11.4) | .17 | 0.7 (0.22.2) | .53 |
| Proton‐pump inhibitor (PPI) (total) | 28 (38.6%) | 16 (21.9%) | 2.2 (1.14.7) | .03 | 2.1 (1.04.6) | .07 |
| Taken prior to admission | 20 (27.4%) | 9 (12.3%) | 2.2 (1.14.7) | .02 | 2.7 (1.17.0) | .04 |
| Started de novo at admission | 8 (11.0%) | 7 (9.6%) | 1.2 (0.43.5) | .79 | 1.0 (0.33.2) | .99 |
| Any treatment with PPI or H2RA prior to hemorrhage (total) | 39 (53.4%) | 33 (45.2%) | 1.4 (0.72.7) | .32 | 1.5 (0.73.0) | .28 |
| Taken prior to admission | 26 (35.6%) | 18 (24.7%) | 1.7 (0.83.5) | .15 | 1.7 (0.83.7) | .18 |
| Started de novo at admission (among the 102 patients not taking prior to admission) | 13 (27.7%) | 15 (27.3%) | 1.0 (0.42.4) | .97 | 1.1 (0.42.9) | .80 |
Among patients on warfarin, the peak international normalized ratio (median [IQR]) was 3.0 (1.25.0) for cases and 1.9 (1.64.8) for controls (P = .52). For those on heparin (23 cases and 6 controls), the median peak activated partial thromboplastin time (aPTT) was 67 (5082) and 128 (67180) seconds for cases and controls, respectively (P = .03), a surprising finding that was likely a result of type III error and small sample size.
Outcomes
We found no evidence of major complications from bleeding, as shown in Table 2. As expected, cases were more likely to receive blood transfusions than were controls, but clinically serious outcomes were uncommon in both groups.
| Characteristic | Cases n = 73 | Controls n = 73 | P value |
|---|---|---|---|
| |||
| Pulmonary complicationsa | 4 ( 5.5%) | 2 (2.7%) | .68 |
| Cardiac complicationsb | 4 ( 5.5%) | 3 (4.1%) | >.99 |
| Acute renal failure requiring dialysis | 0 ( 0.0%) | 1 (1.4%) | >.99 |
| Stroke or transient cerebral ischemia | 1 ( 1.4%) | 1 (1.4%) | >.99 |
| Transfer to intensive care unit | 9 (12.3%) | 4 (5.5%) | .14 |
| Blood transfusion required | 46 (63.0%) | 3 (4.1%) | <.0001 |
| All‐cause mortality | 3 ( 4.1%) | 2 (2.7%) | >.99 |
Gastrointestinal Symptoms and Endoscopic Findings
Bleeding symptoms prompting EGD and associated endoscopic findings are shown in Table 3. Findings on colonoscopy (performed in 34 patients) are included. Overall, 54 (74%) patients had a detected abnormality on EGD and/or colonoscopy that was believed to be a likely source of bleeding by the endoscopist, and 19 (26%) had no apparent culprit lesions. Melena and stool positivity for occult blood were the most common manifestations of gastrointestinal bleeding (77%) and also accounted for all the normal endoscopic evaluations. Of the 21 ulcers, 18 (85.7%) had a clean base, 1 (4.8%) had a red spot, and 2 (9.5%) had an adherent clot. None had a bleeding vessel. Endoscopic treatment was performed in one patient and angiography in one patient. A possible gastric stromal tumor (not the source of bleeding) was seen in one patient, but no mucosal malignant lesions were identified. Of the 73 cases, 41 (56.2% of cases and 0.2% of the total cohort of 17,707 patients) had culprit lesions that might have been preventable with gastric acid suppression (including peptic ulcers, esophagitis, and duodenitis).
| Most likely primary source of bleeding based on EGD with or without colonoscopya | Hematemesis only n = 10 (13.7% of cases) | Melena or hematochezia n = 33 (45.2% of cases) | Hematemesis plus either melena or hematochezia n = 4 (5.5% of cases) | Occult blood (+) and/or drop in hemoglobin (without overt bleeding) n = 26 (35.6% of cases) |
|---|---|---|---|---|
| ||||
| Normal (no lesions identified n = 19 (26.0% of cases) | 0 | 12 | 0 | 7 |
| Peptic ulcer n = 21 (28.8% of cases) | 4 | 10 | 0 | 7 |
| Esophagitis n = 8 (11.0% of cases) | 2 | 2 | 2 | 2 |
| Gastritis or duodenitis n = 12 (16.4% of cases) | 1 | 6 | 1 | 4 |
| Lower GI source only n = 1 (1.4% of cases) | 0 | 0 | 0 | 1 |
| Miscellaneous upper GI sourceb n = 12 (16.4% of cases) | 3 | 3 | 1 | 5 |
Prophylactic Gastric Acid Suppression
One hundred and two patients were not taking any acid‐suppressive prophylaxis on admission to the hospital. Of these patients, on admission 28 (27.5%) were prescribed either histamine‐2 receptor antagonists or proton pump inhibitors. We identified no clinical features associated with the prescriptions for these medications (Table 4), suggesting that physician preference, rather than perceived risk factors for bleeding, determined which patients received prophylactic acid blockade. There was no association between this prophylaxis and GI bleeding, but because of the small size of our sample, the confidence interval was wide (OR = 1.0; 95% CI 0.42.4; P = .97). In the analysis of the subgroup of patients receiving anticoagulation or clopidogrel, prophylaxis showed a nonsignificant trend toward benefit (OR = 0.71; 95% CI 0.23.9; P = .67). There was no significant interaction between the presence of anticoagulation or clopidogrel and prophylaxis (P = .61). Similarly, when we excluded those without prior GI bleeding from analysis, there was still no apparent protective effect of acid‐suppressive prophylaxis (OR = 1.0; 95% CI 0.42.5; P = .97). Finally, there was no significant association between the use of prophylaxis and lesions (theoretically) preventable by acid blockade (OR = 0.9; 95% CI 0.32.3; P = .84).
| Characteristic | Prophylaxis | |||
|---|---|---|---|---|
| Initiated n = 28 (27.5%) | Withheld n = 74 (72.5%) | Odds ratio (95% CI) | P value | |
| ||||
| Cases | ||||
| All lesions | 13 (46.4%) | 34 (46.0%) | 1.0 (0.42.4) | .97 |
| Lesions preventable with acid blockadeb | 8 (28.6%) | 20 (27.0%) | 1.1 (0.42.8) | .88 |
| Demographics | ||||
| Age, in years (SD) | 70.3 (18.6) | 66.9 (15.7) | 1.2 (0.81.9) | .40 |
| Female | 10 (35.7%) | 36 (48.7%) | 0.6 (0.21.4) | .24 |
| Medical history | ||||
| Prior gastrointestinal bleeding | 4 (14.3%) | 7 ( 9.5%) | 1.6 (0.45.8) | .49 |
| History of GERD | 1 ( 3.6%) | 4 ( 5.4%) | 0.6 (0.04.6) | >.99 |
| History of peptic ulcer disease | 3 (10.7%) | 5 ( 6.8%) | 1.7 (0.37.3) | .68 |
| Hospitalization variables | ||||
| Transferred from ICU | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Cardiovascular admission diagnosis | 7 (25.0%) | 21 (28.4%) | 0.8 (0.32.2) | .73 |
| Medication exposure | ||||
| Aspirin (with or without NSAID) | 13 (46.4%) | 30 (40.5%) | 1.3 (0.53.1) | .59 |
| NSAID alone (nonselective) | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Glucocorticoids | 6 (21.4%) | 19 (25.7%) | 0.8 (0.32.2) | .65 |
| Warfarin, clopidogrel, or IV heparin | 9 (32.1%) | 28 (37.8%) | 0.8 (0.31.9) | .59 |
DISCUSSION
Our data suggest that the incidence of hospital‐acquired gastrointestinal bleeding in noncritically ill medical patients is low (approximately 0.4%) and that treatment with anticoagulants or clopidogrel predisposes to this complication. Anticoagulation is a well‐known risk factor for gastrointestinal bleeding, with an estimated odds ratio of 2416; our study confirmed this risk.
Although some studies have questioned the utility of prophylactic acid blockade in the intensive care unit,15 the weight of current evidence supports prophylaxis in selected critically ill patients. In a randomized double‐blind study of 1200 mechanically ventilated patients, the relative risk of gastrointestinal bleeding in patients treated with ranitidine was 0.44 (95% CI 0.210.92 P = .02).11 Many experts discourage indiscriminant use of prophylaxes, even by patients in intensive care units, recommending that it be used only in patients with established risk factors for bleeding.1, 12
Despite the absence of evidence of any benefit of the use of prophylactic acid blockade outside the intensive care unit, this practice is common. In our study, 27.5% of patients who were not on outpatient acid suppression medications (PPIs or H2 antagonists) were started on them on admission to the hospital, presumably as prophylaxes, as we excluded patients admitted for acute gastrointestinal complaints. Other studies have reported prophylaxis rates of 30%50%.13, 14 Many patients started on this prophylaxis during hospitalization go on to take these drugs following discharge, creating an unnecessary economic burden.13, 14 In our study, GI prophylaxis did not appear to prevent hospital‐acquired gastrointestinal bleeding. However, the odds ratio associating the use of prophylactic acid suppression with gastrointestinal bleeding (1.0) was associated with a wide 95% confidence interval (0.42.4), so we cannot exclude the possibility that these medications might provide a relative risk reduction that we were unable to detect. Finally, although gastrointestinal bleeding in the intensive care unit is associated with significant morbidity and mortality,8, 9 we found no evidence to suggest that gastrointestinal bleeding in our patients was associated with poor outcomes.
In interpreting the data from this study, it is important to note that the definition of hospital‐acquired gastrointestinal bleeding in the literature has been inconsistent. Some studies have required that bleeding be hemodynamically significant1, 2, 5, 11a stringent criterion that may be present in only 10%15% of patients with bleeding16whereas other studies defined gastrointestinal bleeding on the basis of occult‐blood‐positive nasogastric aspirates or positive endoscopic findings.7, 15 Because the definition used in the present study required a hard clinical event (EGD), it excluded bleeding events that were considered clinically insignificant by treating physicians. We justified this definition on our belief that any bleeding that warrants invasive evaluation is clinically relevant because it is expensive and puts the patient at some physical risk. Even though some of our patients were diagnosed with GIB without obvious melena or hematemesis (ie, based on stool positivity for occult blood), many of these patients had significant drops in hemoglobin during hospitalization, which, accompanied by occult blood positivity, justified inpatient EGD. We do not believe our definition of GI bleeding was too restrictive, at least for our institution, as physicians at the Cleveland Clinic generally pursue inpatient EGD with clinically apparent gastrointestinal bleeding; we maintain that bleeding that is minor enough not to change management is of limited clinical relevance. Nevertheless, the threshold for EGD at a given institution could affect the rate of EGD for soft indications and the overall prevalence of nosocomial GI bleeding based on our definition.
It also is worth noting that our definition of nosocomial bleeding encompassed some patients with recent hospitalization on the medical service who bled following discharge (15% of cases in this study). This inclusion criterion was chosen because of our concern that the stress of hospitalization might lead to complications even after discharge. We chose an arbitrary postdischarge cutoff of 4 weeks. When we excluded these patients from analysis, the results were similar (data not shown). Although it is possible that we missed some patient who presented to other institutions with GI bleeding following discharge from the Cleveland Clinic, we suspect that the number of such patients was very small based on current referral patterns.
We do not have complete information to determine exactly why patients were on acid‐suppressive therapy prior to admission, but the available data suggest that many had gastroesophageal reflux disease (GERD), PUD, or prior GI bleeding. For this reason, we focused the investigation of the potential efficacy of prophylactic initiation of acid blockade among patients who at presentation were not taking these medications, as prior GERD (or undocumented GIB) leading to chronic use of acid blockade may predispose to subsequent GIB. Although we analyzed only those patients who had newly started taking acid‐suppressive medications, we acknowledge that a few of them may have been started on these medications for other reasons, like chest pain or GERD. However, the evidence suggests that an overwhelming number are started on these medications for the sole purpose of GI prophylaxis.13, 14
Our study was limited by its retrospective casecontrol design. However, because of the low prevalence of hospital‐acquired gastrointestinal bleeding outside the critical care unit, a prospective study would have to enroll thousands of patients in order to generate statistically meaningful results.
In summary, hospital‐acquired gastrointestinal bleeding outside the intensive care unit is uncommon, with an incidence of about 0.4% according to our definition of bleeding. We found no evidence that these bleeding episodes are associated with increased mortality or with occult malignancy. Furthermore, we found no evidence that prophylactic gastric acid suppression prevents these events, and only 41 patients (0.2% of the total cohort) had lesions that might be preventable with gastric acid blockade. We discourage the indiscriminant use of prophylactic acid suppressants in general medical patients.
Acknowledgements
The authors thank Donna M. Richey and Betty Lou Harrison for clerical support.
Gastrointestinal bleeding occurring in hospitalized patients admitted for nongastrointestinal disorders has been extensively studied in intensive care unit patients. However, a systematic study in noncritically ill medical patients has not yet been done. In critically ill patients the incidence of hospital‐acquired gastrointestinal bleeding (GIB) varies from 0.17% to 5%, depending on its definition.16 These bleeding events significantly increase the morbidity and duration of hospitalization.1, 5, 79
Risk factors for bleeding in the intensive care unit include mechanical ventilation, coagulopathy, burns, chronic renal failure, and neurological insults.15 Several studies have found that stress ulcer prophylaxis with histamine‐2 (H2) receptor antagonists, sucralfate, or proton pump inhibitors (PPIs) decreases bleeding in this group of patients, with a relative risk reduction of 29%61%.10, 11 However, use of these drugs outside this high‐risk group has been questioned because of the low overall risk of bleeding.1, 11, 12 Despite their being an unproven benefit in the noncritically ill population, prophylactic H2 antagonists or PPIs are prescribed in an indiscriminant fashion to up to 30%50% of patients admitted to the hospital,13, 14 suggesting that physician preference dictates this practice. To shed light on this issue in noncritically ill patients, we conducted a retrospective casecontrol study in order to identify risk factors that predict hospital‐acquired gastrointestinal bleeding in this group of patients and to assess whether treatment with prophylactic acid suppression was associated with fewer bleeding events. We also sought to characterize the endoscopic lesions in these patients.
MATERIALS AND METHODS
Study Patients
The institutional review board of the Cleveland Clinic Foundation (Cleveland, OH) approved this study. All patients admitted to the General Internal Medicine service between January 1, 1999, and December 31, 2002, were eligible for inclusion. Two types of cases were included: 1) patients admitted for nongastrointestinal illnesses who developed bleeding at least 24 hours after admission and required esophagogastroduodenoscopy (EGD) during hospitalization (designated in‐hospital bleeding), and 2) patients admitted with gastrointestinal bleeding (requiring EGD) who had been hospitalized on the General Medical service during the preceding 4 weeks for a nongastrointestinal illness (designated out‐of‐hospital bleeding). This second group was included to identify risk factors for delayed bleeding that might not be obvious during hospitalization.
Medical records of all General Medicine patients who underwent EGD were reviewed in a standardized fashion (Fig. 1). We excluded patients with documented gastrointestinal complaints (including bleeding) at the time of the index admission or within 24 hours of admission, bleeding in the intensive care unit (ICU) or in another hospital prior to transfer to the General Medicine service, or a history of gastrointestinal bleeding during the month prior to admission. ICU stay prior to General Medicine admission, if not associated with GI bleeding, was not an exclusion criterion for our study.

Controls, also without any acute gastrointestinal symptoms at admission, were randomly matched to cases in a 1:1 ratio by date of admission. We used this liberal matching strategy because any factors matched for would no longer be eligible to be risk factors for bleeding. If more than one control was admitted on the same day as a case, then a random number was used to select the control.
Definition of Prophylactic Acid Suppression
We defined prophylactic acid suppression as in‐hospital de novo treatment with histamine‐2 receptor antagonists and/or proton pump inhibitors received prior to the onset of any symptoms that would suggest GI bleeding (for cases) or any time during hospitalization (for controls). Patients taking these drugs prior to admission were deemed ineligible for in‐hospital prophylactic acid blockade and were excluded from the related analyses.
Data Collection
We extracted demographic information, medical history, medication usage, and laboratory data by chart review. For those patients readmitted for gastrointestinal bleeding following discharge, data from the initial (nongastrointestinal illnessassociated) hospitalization were recorded. Bleeding symptoms triggering endoscopy were grouped into four categories: 1) melena or hematochezia; 2) hematemesis (frank blood in vomitus or coffee‐grounds emesis); 3) melena or hematochezia plus hematemesis (both 1 and 2); 4) stool positivity for occult blood or unexplained drop in hemoglobin in the absence of overt bleeding. Endoscopic findings were categorized by the nature of the visualized lesions, and if multiple lesions were noted, the endoscopist's impression of the most likely bleeding site was used to define the source of bleeding. We recorded colonoscopy findings for patients undergoing this evaluation.
Statistical Analysis
We analyzed data utilizing JMP 5.1 (SAS Institute, Cary, NC). Random controls were chosen using computer‐generated random numbers. The proportions of patients with various categorical characteristics were compared using the chi‐square test or Fisher's exact test as appropriate. We used the Student t test or Wilcoxon's test to compare continuous variables. Odds ratios and adjusted odds ratios were calculated by logistic regression. Two‐tailed P values less than .05 were considered statistically significant.
RESULTS: Identification of Cases and Controls
Of 17,707 patients admitted to the General Medicine service, 1327 (7.5%) underwent EGD during hospitalization or within 1 month of discharge. Only 73 (0.41%) of the total number of patients met the case definition (Fig. 1). Of these cases, 62 (84.9%) had developed gastrointestinal bleeding during the index hospitalization, whereas 11 (15.1%) were readmitted for bleeding within 4 weeks of hospital discharge. The remaining 1254 patients who underwent EGD were excluded based on exclusion criteria, including an absence of documented bleeding prompting the EGD.
Clinical Risk Factors for Bleeding
In univariate analysis, as shown in Table 1, predictors of GIB included: 1) age (P = .02); 2) admission diagnosis (P = .01); 3) preexisting coronary artery disease (P = .004); 4) treatment with blood‐thinning medications, including warfarin (P = .0004), intravenous heparin (P = .0003), and clopidogrel (P = .02); and 5) treatment with PPIs (P = .02). After adjusting for the use of full‐dose anticoagulation and/or clopidogrel, the only of these risk factors that remained significantly associated with GIB was treatment with PPIs prior to hospitalization (adjusted OR = 2.1; 95% CI 1.17.0; P = .04), suggesting that PPI treatment in the outpatient setting may be a marker for GI vulnerability.
| Characteristic | Cases n = 73 | Controls n = 73 | Unadjusted | Adjusted for treatment with full‐dose anticoagulants or clopidogrel | ||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value (2‐tailed) | Odds ratio (95% CI) | P value (2‐tailed) | |||
| ||||||
| Demographics | ||||||
| Women | 36 (49.3%) | 29 (39.7%) | 1.5 (0.82.9) | .24 | 1.6 (0.83.3) | .19 |
| Age (years), mean (SD) | 71.6 (13.7) | 65.7 (17.2) | 1.5 (1.12.1)c | .02 | 1.3 (0.91.8) | .19 |
| Caucasian | 42 (58.3%) | 32 (44.4%) | 1.7 (0.93.4) | .09 | 1.3 (0.62.6) | .50 |
| Nursing home residents | 5 (6.9%) | 5 (6.9%) | 1.0 (0.33.7) | >.99 | 0.5 (0.12.2) | .35 |
| Admission diagnosisa | .01d | .30d | ||||
| Cardiovascular (non‐thrombotic) | 15 (20.5%) | 6 (8.2%) | 2.9 (1.18.5) | .04 | 2.1 (0.76.5) | .19 |
| Arterial or venous thrombosis | 13 (17.8%) | 2 (2.7%) | 7.9 (2.050.4) | .009 | 3.3 (0.822.1) | .15 |
| Infection | 21 (28.8%) | 24 (32.9%) | 0.8 (0.41.7) | .59 | 1.1 (0.52.3) | .86 |
| Pulmonary (noninfectious) | 4 (5.5%) | 10 (13.7%) | 0.4 (0.11.2) | .10 | 0.5 (0.11.7) | .31 |
| Altered level of consciousness | 7 (9.6%) | 10 (13.7%) | 0.7 (0.21.8) | .44 | 0.7 (0.22.2) | .59 |
| Other | 13 (17.8%) | 21 (28.8%) | 0.5 (0.21.2) | .12 | 0.6 (0.31.5) | .29 |
| Baseline medical conditions | ||||||
| Diabetes mellitus | 28 (38.4%) | 25 (34.3%) | 1.2 (0.62.4) | .61 | 1.3 (0.62.7) | .48 |
| Hypertension | 50 (68.5%) | 48 (65.8%) | 1.1 (0.62.3) | .72 | 1.2 (0.52.5) | .71 |
| Coronary artery disease | 36 (49.3%) | 19 (26.0%) | 2.8 (1.45.6) | .004 | 2.0 (1.04.3) | .06 |
| Atrial fibrillation | 18 (24.7%) | 10 (13.7%) | 2.1 (0.95.0) | .09 | 1.4 (0.53.6) | .49 |
| Congestive heart failure | 25 (34.3%) | 16 (21.9%) | 1.9 (0.93.9) | .10 | 1.5 (0.73.3) | .35 |
| Renal insufficiency (creatinine > 2) | 18 (24.7%) | 11 (15.1%) | 1.8 (0.84.4) | .14 | 1.9 (0.84.7) | .33 |
| Chronic obstructive pulmonary disease | 21 (28.8%) | 20 (27.4%) | 1.1 (0.52.2) | .85 | 1.5 (0.73.4) | .29 |
| Stroke | 13 (17.8%) | 16 (21.9%) | 0.8 (0.31.7) | .53 | 0.7 (0.31.6) | .39 |
| Active malignancy | 6 (8.2%) | 8 (11.0%) | 0.7 (0.32.2) | .57 | 1.0 (0.33.5) | .80 |
| Gastroesophageal reflux (GERD) | 10 (13.7%) | 10 (13.7%) | 1.0 (0.42.6) | >.99 | 1.0 (0.32.7) | .92 |
| Liver disease | 7 (9.6%) | 6 (8.2%) | 1.2 (0.43.9) | .77 | 1.4 (0.44.9) | .59 |
| Peptic ulcer disease | 13 (17.8%) | 5 (6.9%) | 2.9 (1.09.6) | .04 | 2.7 (0.99.4) | .09 |
| Colonic disease (diverticulosis, polyp, or AVM) | 7 (9.6%) | 4 (5.5%) | 1.8 (0.57.3) | .34 | 1.2 (0.35.2) | .79 |
| Prior gastrointestinal hemorrhage | 15 (20.1%) | 7 (9.6%) | 2.4 (1.06.8) | .06 | 2.0 (0.75.8) | .20 |
| Tobacco abuse (current smoking) | 9 (12.3%) | 18 (24.7%) | 0.4 (0.21.0) | .05 | 0.6 (0.21.5) | .26 |
| Heavy drinking (>8 drinks/day) | 2 (2.7%) | 2 (2.7%) | 1.0 (0.18.5) | >.99 | 1.3 (0.111.7) | .83 |
| Medication exposure prior to bleeding (excluding acid blockade)b | ||||||
| Aspirin (with or without NSAID) | 34 (46.6%) | 32 (43.8%) | 1.1 (0.62.1) | .74 | 0.7 (0.31.5) | .42 |
| Nonselective NSAID (without aspirin) | 3 (4.1%) | 5 (6.9%) | 0.6 (0.12.5) | .72 | 0.6 (0.12.6) | .44 |
| COX‐2 inhibitors | 3 (4.1%) | 7 (9.6%) | 0.4 (0.11.5) | .18 | 0.3 (0.11.4) | .15 |
| Glucocorticoids | 17 (23.3%) | 20 (27.4%) | 0.8 (0.41.7) | .57 | 0.9 (0.42.1) | .89 |
| Warfarin | 24 (32.9%) | 7 (9.6%) | 4.6 (1.912.4) | .004 | N/A | N/A |
| Unfractionated heparin, UFH (full‐dose intravenous | 23 (31.5%) | 6 (20.7%) | 5.1 (2.114.8) | .0003 | N/A | N/A |
| Full‐dose low‐molecular‐weight heparin (LMWH) | 2 (2.7%) | 0 (0%) | infinity | .50 | N/A | N/A |
| Clopidogrel | 9 (12.3%) | 2 (2.7%) | 5.0 (1.233.5) | .02 | N/A | N/A |
| Prophylactic LMWH or UFH (among 103 patients not on full‐dose anticoagulants) | 19 (47.5%) | 32 (50.8%) | 0.9 (0.41.9) | .74 | N/A | N/A |
| Any treatment with warfarin, full‐dose UFH, full‐ dose LMWH, and/or clopidogrel | 41 (56.2%) | 14 (19.2%) | 5.4 (2.611.7) | <.0001 | N/A | N/A |
| Gastric acid suppression (prior to any gastrointestinal hemorrhage) | ||||||
| H2‐receptor antagonists (H2RA) (total) | 11 (15.1%) | 19 (26.0%) | 0.5 (0.21.1) | .10 | 0.6 (0.31.5) | .31 |
| Taken prior to admission | 6 (8.2%) | 9 (12.3%) | 0.6 (0.21.9) | .41 | 0.6 (0.22.1) | .47 |
| Started de novo at admission | 5 (6.9%) | 10 (13.7%) | 0.5 (0.11.4) | .17 | 0.7 (0.22.2) | .53 |
| Proton‐pump inhibitor (PPI) (total) | 28 (38.6%) | 16 (21.9%) | 2.2 (1.14.7) | .03 | 2.1 (1.04.6) | .07 |
| Taken prior to admission | 20 (27.4%) | 9 (12.3%) | 2.2 (1.14.7) | .02 | 2.7 (1.17.0) | .04 |
| Started de novo at admission | 8 (11.0%) | 7 (9.6%) | 1.2 (0.43.5) | .79 | 1.0 (0.33.2) | .99 |
| Any treatment with PPI or H2RA prior to hemorrhage (total) | 39 (53.4%) | 33 (45.2%) | 1.4 (0.72.7) | .32 | 1.5 (0.73.0) | .28 |
| Taken prior to admission | 26 (35.6%) | 18 (24.7%) | 1.7 (0.83.5) | .15 | 1.7 (0.83.7) | .18 |
| Started de novo at admission (among the 102 patients not taking prior to admission) | 13 (27.7%) | 15 (27.3%) | 1.0 (0.42.4) | .97 | 1.1 (0.42.9) | .80 |
Among patients on warfarin, the peak international normalized ratio (median [IQR]) was 3.0 (1.25.0) for cases and 1.9 (1.64.8) for controls (P = .52). For those on heparin (23 cases and 6 controls), the median peak activated partial thromboplastin time (aPTT) was 67 (5082) and 128 (67180) seconds for cases and controls, respectively (P = .03), a surprising finding that was likely a result of type III error and small sample size.
Outcomes
We found no evidence of major complications from bleeding, as shown in Table 2. As expected, cases were more likely to receive blood transfusions than were controls, but clinically serious outcomes were uncommon in both groups.
| Characteristic | Cases n = 73 | Controls n = 73 | P value |
|---|---|---|---|
| |||
| Pulmonary complicationsa | 4 ( 5.5%) | 2 (2.7%) | .68 |
| Cardiac complicationsb | 4 ( 5.5%) | 3 (4.1%) | >.99 |
| Acute renal failure requiring dialysis | 0 ( 0.0%) | 1 (1.4%) | >.99 |
| Stroke or transient cerebral ischemia | 1 ( 1.4%) | 1 (1.4%) | >.99 |
| Transfer to intensive care unit | 9 (12.3%) | 4 (5.5%) | .14 |
| Blood transfusion required | 46 (63.0%) | 3 (4.1%) | <.0001 |
| All‐cause mortality | 3 ( 4.1%) | 2 (2.7%) | >.99 |
Gastrointestinal Symptoms and Endoscopic Findings
Bleeding symptoms prompting EGD and associated endoscopic findings are shown in Table 3. Findings on colonoscopy (performed in 34 patients) are included. Overall, 54 (74%) patients had a detected abnormality on EGD and/or colonoscopy that was believed to be a likely source of bleeding by the endoscopist, and 19 (26%) had no apparent culprit lesions. Melena and stool positivity for occult blood were the most common manifestations of gastrointestinal bleeding (77%) and also accounted for all the normal endoscopic evaluations. Of the 21 ulcers, 18 (85.7%) had a clean base, 1 (4.8%) had a red spot, and 2 (9.5%) had an adherent clot. None had a bleeding vessel. Endoscopic treatment was performed in one patient and angiography in one patient. A possible gastric stromal tumor (not the source of bleeding) was seen in one patient, but no mucosal malignant lesions were identified. Of the 73 cases, 41 (56.2% of cases and 0.2% of the total cohort of 17,707 patients) had culprit lesions that might have been preventable with gastric acid suppression (including peptic ulcers, esophagitis, and duodenitis).
| Most likely primary source of bleeding based on EGD with or without colonoscopya | Hematemesis only n = 10 (13.7% of cases) | Melena or hematochezia n = 33 (45.2% of cases) | Hematemesis plus either melena or hematochezia n = 4 (5.5% of cases) | Occult blood (+) and/or drop in hemoglobin (without overt bleeding) n = 26 (35.6% of cases) |
|---|---|---|---|---|
| ||||
| Normal (no lesions identified n = 19 (26.0% of cases) | 0 | 12 | 0 | 7 |
| Peptic ulcer n = 21 (28.8% of cases) | 4 | 10 | 0 | 7 |
| Esophagitis n = 8 (11.0% of cases) | 2 | 2 | 2 | 2 |
| Gastritis or duodenitis n = 12 (16.4% of cases) | 1 | 6 | 1 | 4 |
| Lower GI source only n = 1 (1.4% of cases) | 0 | 0 | 0 | 1 |
| Miscellaneous upper GI sourceb n = 12 (16.4% of cases) | 3 | 3 | 1 | 5 |
Prophylactic Gastric Acid Suppression
One hundred and two patients were not taking any acid‐suppressive prophylaxis on admission to the hospital. Of these patients, on admission 28 (27.5%) were prescribed either histamine‐2 receptor antagonists or proton pump inhibitors. We identified no clinical features associated with the prescriptions for these medications (Table 4), suggesting that physician preference, rather than perceived risk factors for bleeding, determined which patients received prophylactic acid blockade. There was no association between this prophylaxis and GI bleeding, but because of the small size of our sample, the confidence interval was wide (OR = 1.0; 95% CI 0.42.4; P = .97). In the analysis of the subgroup of patients receiving anticoagulation or clopidogrel, prophylaxis showed a nonsignificant trend toward benefit (OR = 0.71; 95% CI 0.23.9; P = .67). There was no significant interaction between the presence of anticoagulation or clopidogrel and prophylaxis (P = .61). Similarly, when we excluded those without prior GI bleeding from analysis, there was still no apparent protective effect of acid‐suppressive prophylaxis (OR = 1.0; 95% CI 0.42.5; P = .97). Finally, there was no significant association between the use of prophylaxis and lesions (theoretically) preventable by acid blockade (OR = 0.9; 95% CI 0.32.3; P = .84).
| Characteristic | Prophylaxis | |||
|---|---|---|---|---|
| Initiated n = 28 (27.5%) | Withheld n = 74 (72.5%) | Odds ratio (95% CI) | P value | |
| ||||
| Cases | ||||
| All lesions | 13 (46.4%) | 34 (46.0%) | 1.0 (0.42.4) | .97 |
| Lesions preventable with acid blockadeb | 8 (28.6%) | 20 (27.0%) | 1.1 (0.42.8) | .88 |
| Demographics | ||||
| Age, in years (SD) | 70.3 (18.6) | 66.9 (15.7) | 1.2 (0.81.9) | .40 |
| Female | 10 (35.7%) | 36 (48.7%) | 0.6 (0.21.4) | .24 |
| Medical history | ||||
| Prior gastrointestinal bleeding | 4 (14.3%) | 7 ( 9.5%) | 1.6 (0.45.8) | .49 |
| History of GERD | 1 ( 3.6%) | 4 ( 5.4%) | 0.6 (0.04.6) | >.99 |
| History of peptic ulcer disease | 3 (10.7%) | 5 ( 6.8%) | 1.7 (0.37.3) | .68 |
| Hospitalization variables | ||||
| Transferred from ICU | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Cardiovascular admission diagnosis | 7 (25.0%) | 21 (28.4%) | 0.8 (0.32.2) | .73 |
| Medication exposure | ||||
| Aspirin (with or without NSAID) | 13 (46.4%) | 30 (40.5%) | 1.3 (0.53.1) | .59 |
| NSAID alone (nonselective) | 2 ( 7.1%) | 4 ( 5.4%) | 1.3 (0.27.3) | .67 |
| Glucocorticoids | 6 (21.4%) | 19 (25.7%) | 0.8 (0.32.2) | .65 |
| Warfarin, clopidogrel, or IV heparin | 9 (32.1%) | 28 (37.8%) | 0.8 (0.31.9) | .59 |
DISCUSSION
Our data suggest that the incidence of hospital‐acquired gastrointestinal bleeding in noncritically ill medical patients is low (approximately 0.4%) and that treatment with anticoagulants or clopidogrel predisposes to this complication. Anticoagulation is a well‐known risk factor for gastrointestinal bleeding, with an estimated odds ratio of 2416; our study confirmed this risk.
Although some studies have questioned the utility of prophylactic acid blockade in the intensive care unit,15 the weight of current evidence supports prophylaxis in selected critically ill patients. In a randomized double‐blind study of 1200 mechanically ventilated patients, the relative risk of gastrointestinal bleeding in patients treated with ranitidine was 0.44 (95% CI 0.210.92 P = .02).11 Many experts discourage indiscriminant use of prophylaxes, even by patients in intensive care units, recommending that it be used only in patients with established risk factors for bleeding.1, 12
Despite the absence of evidence of any benefit of the use of prophylactic acid blockade outside the intensive care unit, this practice is common. In our study, 27.5% of patients who were not on outpatient acid suppression medications (PPIs or H2 antagonists) were started on them on admission to the hospital, presumably as prophylaxes, as we excluded patients admitted for acute gastrointestinal complaints. Other studies have reported prophylaxis rates of 30%50%.13, 14 Many patients started on this prophylaxis during hospitalization go on to take these drugs following discharge, creating an unnecessary economic burden.13, 14 In our study, GI prophylaxis did not appear to prevent hospital‐acquired gastrointestinal bleeding. However, the odds ratio associating the use of prophylactic acid suppression with gastrointestinal bleeding (1.0) was associated with a wide 95% confidence interval (0.42.4), so we cannot exclude the possibility that these medications might provide a relative risk reduction that we were unable to detect. Finally, although gastrointestinal bleeding in the intensive care unit is associated with significant morbidity and mortality,8, 9 we found no evidence to suggest that gastrointestinal bleeding in our patients was associated with poor outcomes.
In interpreting the data from this study, it is important to note that the definition of hospital‐acquired gastrointestinal bleeding in the literature has been inconsistent. Some studies have required that bleeding be hemodynamically significant1, 2, 5, 11a stringent criterion that may be present in only 10%15% of patients with bleeding16whereas other studies defined gastrointestinal bleeding on the basis of occult‐blood‐positive nasogastric aspirates or positive endoscopic findings.7, 15 Because the definition used in the present study required a hard clinical event (EGD), it excluded bleeding events that were considered clinically insignificant by treating physicians. We justified this definition on our belief that any bleeding that warrants invasive evaluation is clinically relevant because it is expensive and puts the patient at some physical risk. Even though some of our patients were diagnosed with GIB without obvious melena or hematemesis (ie, based on stool positivity for occult blood), many of these patients had significant drops in hemoglobin during hospitalization, which, accompanied by occult blood positivity, justified inpatient EGD. We do not believe our definition of GI bleeding was too restrictive, at least for our institution, as physicians at the Cleveland Clinic generally pursue inpatient EGD with clinically apparent gastrointestinal bleeding; we maintain that bleeding that is minor enough not to change management is of limited clinical relevance. Nevertheless, the threshold for EGD at a given institution could affect the rate of EGD for soft indications and the overall prevalence of nosocomial GI bleeding based on our definition.
It also is worth noting that our definition of nosocomial bleeding encompassed some patients with recent hospitalization on the medical service who bled following discharge (15% of cases in this study). This inclusion criterion was chosen because of our concern that the stress of hospitalization might lead to complications even after discharge. We chose an arbitrary postdischarge cutoff of 4 weeks. When we excluded these patients from analysis, the results were similar (data not shown). Although it is possible that we missed some patient who presented to other institutions with GI bleeding following discharge from the Cleveland Clinic, we suspect that the number of such patients was very small based on current referral patterns.
We do not have complete information to determine exactly why patients were on acid‐suppressive therapy prior to admission, but the available data suggest that many had gastroesophageal reflux disease (GERD), PUD, or prior GI bleeding. For this reason, we focused the investigation of the potential efficacy of prophylactic initiation of acid blockade among patients who at presentation were not taking these medications, as prior GERD (or undocumented GIB) leading to chronic use of acid blockade may predispose to subsequent GIB. Although we analyzed only those patients who had newly started taking acid‐suppressive medications, we acknowledge that a few of them may have been started on these medications for other reasons, like chest pain or GERD. However, the evidence suggests that an overwhelming number are started on these medications for the sole purpose of GI prophylaxis.13, 14
Our study was limited by its retrospective casecontrol design. However, because of the low prevalence of hospital‐acquired gastrointestinal bleeding outside the critical care unit, a prospective study would have to enroll thousands of patients in order to generate statistically meaningful results.
In summary, hospital‐acquired gastrointestinal bleeding outside the intensive care unit is uncommon, with an incidence of about 0.4% according to our definition of bleeding. We found no evidence that these bleeding episodes are associated with increased mortality or with occult malignancy. Furthermore, we found no evidence that prophylactic gastric acid suppression prevents these events, and only 41 patients (0.2% of the total cohort) had lesions that might be preventable with gastric acid blockade. We discourage the indiscriminant use of prophylactic acid suppressants in general medical patients.
Acknowledgements
The authors thank Donna M. Richey and Betty Lou Harrison for clerical support.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,, et al.Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.Crit Care Med.1999;27:2812–2817.
- ,,,,.Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit.Am J Med.1984;76:623–630.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators.J Am Geriatr Soc.2001;49:126–133.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case–control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,.Characterization of gastrointestinal bleeding in severely ill hospitalized patients.Crit Care Med.2000;28:46–50.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,,, et al.The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients.Crit Care.2001;5:368–375.
- ,,,.Risks for developing critical illness with GI hemorrhage.Chest.2000;118:473–478.
- .Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis.Scand J GastroenterolSuppl.1995;210:48–52.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–122.
- ,,, et al.Prophylaxis for stress‐related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single‐blind study.Ann Intern Med.1994;121:568–575.
- .Low incidence of hemodynamic instability in patients with gastrointestinal hemorrhage.South Med J.1996;89:386–390.
- ,,, et al.Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group.N Engl J Med.1994;330:377–381.
- ,,, et al.Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.Crit Care Med.1999;27:2812–2817.
- ,,,,.Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit.Am J Med.1984;76:623–630.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons. Cardiovascular Health Study Investigators.J Am Geriatr Soc.2001;49:126–133.
- ,.Gastrointestinal bleeding in the hospitalized patient: a case–control study to assess risk factors, causes, and outcome.Am J Med.1998;104:349–354.
- ,,.Characterization of gastrointestinal bleeding in severely ill hospitalized patients.Crit Care Med.2000;28:46–50.
- ,,,,.Clinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxis.Am J Gastroenterol.2000;95:2801–2806.
- ,,, et al.The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients.Crit Care.2001;5:368–375.
- ,,,.Risks for developing critical illness with GI hemorrhage.Chest.2000;118:473–478.
- .Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis.Scand J GastroenterolSuppl.1995;210:48–52.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- ,.Stress ulcer: is routine prophylaxis necessary?Am J Gastroenterol.1995;90:708–712.
- ,,, et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17:1503–1506.
- ,,.Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95:3118–122.
- ,,, et al.Prophylaxis for stress‐related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single‐blind study.Ann Intern Med.1994;121:568–575.
- .Low incidence of hemodynamic instability in patients with gastrointestinal hemorrhage.South Med J.1996;89:386–390.
Copyright © 2006 Society of Hospital Medicine