User login
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
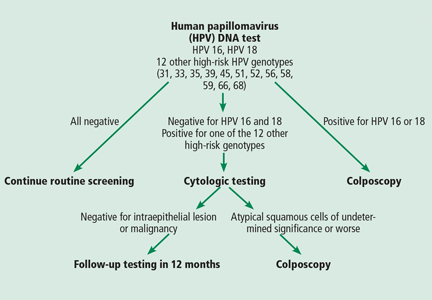
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.