User login
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
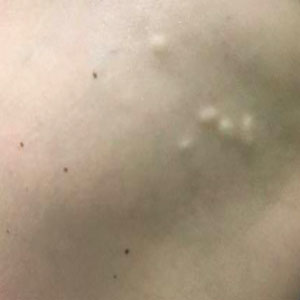
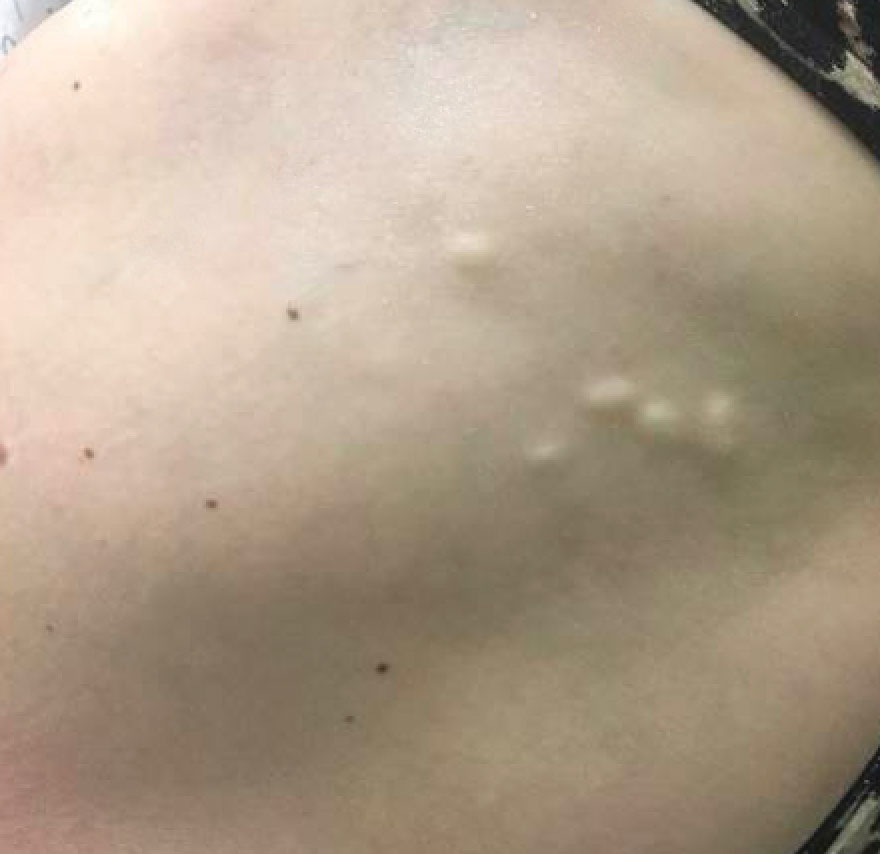
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.
Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
Practice Points
- Glatiramer acetate is a subcutaneous injection utilized for relapsing-remitting multiple sclerosis, and common adverse effects include injection-site reactions such as calcinosis cutis.
- Development of calcinosis cutis in association with glatiramer acetate is not an indication for medication discontinuation.
- Dermatologists should be aware of this potential association, and treatment should be considered in cases of symptomatic calcinosis cutis.