User login
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
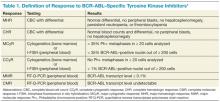
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Chronic myelogenous leukemia (CML) is caused by the constitutively active BCR-ABL fusion protein that results from t(9;22), the Philadelphia (Ph+) chromosome. Chronic myelogenous leukemia typically evolves through 3 clinical phases: an indolent chronic phase, an accelerated phase, and a terminal blast phase analogous to acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Fortunately, today more than 80% of patients are diagnosed in the chronic phase of the disease.1
Before the development of the tyrosine kinase inhibitor (TKI) imatinib, > 20% of the patients with chronic phase CML progressed to the blast phase every year.2 Based on data from 8 years of follow-up with imatinib therapy, the rate of progression to the advanced phases of CML is about 1% per year, with freedom from progression at 92%.3 For the majority of patients with chronic phase CML, due to advances in treatment, the disease does not affect mortality.
For those who progress to the terminal blast phase of CML, survival is typically measured in months unless allogeneic stem cell transplant (allo-SCT) is an option. This article will review one of the major remaining problems in CML: how to manage blast phase CML.
Definition and Diagnosis
Defining blast phase CML can be confusing, because different criteria have been proposed, none of which are biologically based. The most widely used definition is set forth by the European LeukemiaNet, recommending 30% blasts in the blood or bone marrow or the presence of extramedullary disease.1 Clinically, blast phase CML may present with constitutional symptoms, bone pain, or symptoms related to cytopenias (fatigue, dyspnea, bleeding, infections).
Diagnostic workup should include a complete blood cell count (CBC) with differential, bone marrow analysis with conventional cytogenetics, flow cytometry to determine whether the blast phase is of myeloid or lymphoid origin, and molecular mutational analysis of the BCR-ABL tyrosine kinase domain to help guide the choice of TKI. If age and performance status are favorable, a donor search for allo-SCT should be started promptly.
Chronic myelogenous leukemia cells that contain the BCR-ABL kinase protein are genetically unstable.4,5 Additional cytogenetic aberrations (ACAs) are seen in up to 80% of those with blast phase CML and are the most consistent predictor of blast transformation in those with chronic phase CML.6 Chromosomal changes are broken down into the nonrandom, “major route” ACAs (trisomy 8, additional Ph+ chromosome, isochromosome 17q, trisomy 19), considered likely to be involved in the evolution of CML, and the more random “minor route” ACAs, which may denote nothing more than the instability of the genome.5,7 Mutations of the BCR-ABL tyrosine kinase domain are also seen in the majority of those in blast phase CML and, depending on the specific mutation, can negatively predict the response to certain TKI therapies.4
Prognosis
The single most important prognostic indicator for patients with CML remains the length of response to initial BCR-ABL–specific TKI therapy. Only a very small minority of patients are refractory to TKIs from the beginning; these are the patients with the worst prognosis.8 If the response to treatment seems inadequate, then the health care professional should first verify with the patient that he or she is taking the medicine as prescribed.1 Lack of adherence continues to be the most common reason for less-than-ideal outcomes or fluctuations in response and plays a critical role in treatment with TKI therapy, with worse outcomes when < 90% of doses are taken.9
Other features associated with a poor prognosis include cytogenetic clonal evolution, > 50% blasts, and/or extramedullary disease.7,10,11 At the time of imatinib failure, detection of mutations of the BCR-ABL tyrosine kinase domain correlates to worse 4-year event-free survival.12 Showing the instability of the genome in CML, patients who harbor mutations of the BCR-ABL domain have a higher likelihood of relapse associated with further mutations and, therefore, potentially further TKI resistance.13 Once CML has progressed to the blast phase, life expectancy is, on average, less than a year.11
Treatment Strategy
Currently, the most effective treatment strategy in blast phase CML is to prevent the transformation from chronic phase from ever occurring. Management of blast phase CML depends on 2 factors: (1) previous therapies; and (2) type of blast phase—myeloid or lymphoid. The goal of treatment is to knock the disease back to a clinical remission and/or a chronic phase for a long enough period to get the patient to allo-SCT if age, performance status, and suitable donor allow for it.
Using single-agent imatinib for blast phase CML has been tried in patients who have never been on TKI therapy before. Hematologic responses were seen in the majority of patients, but any form of cytogenetic response was seen in fewer than 20% of patients. Median overall survival, although better than with previous conventional chemotherapies, was still measured in months.6 A patient with blast phase CML who has never been on BCR-ABL–specific TKIs is very rare now; at a minimum, the patient has usually tried at least 1 TKI previously.
If blast phase CML develops while a patient is taking imatinib, treatment with a second-generation TKIs—nilotinib or dasatinib— should be attempted if the BCR-ABL tyrosine kinase domain analysis shows no resistant mutations.14 Both nilotinib and dasatinib have been tried as single agents in patients with imatinib-refractory CML or who are unable to tolerate imatinib.15,16 Cytogenetic response rates were 2 to 4 times higher for these agents than for imatinib when used in blast phase CML.
Table 1 reviews the common definitions of response, including cytogenetic response, to TKIs in CML. The pattern of response is usually very predictable: First, a hematologic response is seen, then a cytogenetic response, and finally, a hoped-for molecular response. Interestingly, in these studies, not all patients with blast phase CML who experienced a cytogenetic response had a hematologic response. This makes CBCs less reliable for assessing response and other peripheral blood tests, such as the interphase fluorescence in situ hybridization (I-FISH) test or the quantitative reverse transcriptase polymerase chain reaction (RT-Q-PCR) test, more important. Unfortunately, this improved cytogenetic response in blast phase CML did not translate to long-term survival advantage; median survival with these second- generation TKIs was still less than a year without transplant. If the T315I mutation is present, then clinical trials involving ponatinib or one of the newest non–FDA-approved TKIs should be considered.
Recent data involving ponatinib suggest similar response and survival rates to nilotinib and dasatinib, but this was in more heavily-pretreated CML patients who had resistance to, or unacceptable adverse effects from the second-generation TKIs or who had the BCR-ABL T315I mutation.17
In late 2013, ponatinib was voluntarily suspended from marketing and sales by its manufacturer due to a worrisome rate of serious arterial thromboembolic events reported in clinical trials and in postmarketing experience. However, the FDA reintroduced ponatinib in 2014 once additional safety measures were put in place, such as changes to the black box warning and review of the risk of arterial and venous thrombosis and occlusions.18
Table 2 compares the results between these newer TKIs in blast phase CML. If the patient can tolerate it, a combination of TKI with AML or ALL-type induction chemotherapy, preferably in a clinical trial setting, provides the best opportunity to return the patient to the chronic phase. If this is achieved, then allo-SCT represents the best chance for sustained remission or cure; allo-SCT was standard first-line therapy prior to the advent of BCR-ABL–specific TKIs. Tyrosine kinase inhibitor exposure prior to allo-SCT does not seem to affect transplantation outcomes.19 Allo-SCT while still in blast phase is discouraged because of its high risks with minimal benefit; disease-free survival rates are <10%.19 Although no scientific data support this, maintenance TKI posttransplantation seems logical, with monitoring of BCR-ABL transcript levels every 3 months.
Conclusion
With the advent of TKI therapy, the overall prognosis of CML has changed drastically. Unfortunately, the success seen with these novel agents in the chronic phase of CML has not translated into success in the blast phase of CML. Therefore, the best way to manage blast phase CML is to prevent this transformation from ever happening. The deeper and more rapid the cytogenetic and molecular response after TKI initiation, the better the long-term outcome for the patient.
If the patient progresses though TKI therapy, then combining a different TKI with a conventional induction chemotherapy regimen for acute leukemia should be tried; the goal is to achieve a remission that lasts long enough for the patient to be able to undergo allo-SCT. If the patient is not a candidate for allo-SCT, then the prognosis is extremely poor, and clinical trials with best supportive care should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.
1. Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M; ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):vii72-vii77.
2. Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1(4):493-500.
3. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts). 2009;114(22):abstract 1126.
4. Fabarius A, Leitner A, Hochhaus A, et al, Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) and the German CML Study Group. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760-6768.
5. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76-94.
6. Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747.
7. Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia [published online ahead of print November 12, 2013]. Leuk Lymphoma. doi:10.3109/10428194.2013.845883.
8. Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541-4546.
9. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
10. Cervantes F, Rozman M, Rosell J, Urbano-Ispizua A, Montserrat E, Rozman C. A study of prognostic factors in blast crisis of Philadelphia chromosome-positive chronic myelogenous leukemia. Br J Haematol. 1990;76(1):27-32.
11. Wadhwa J, Szydlo RM, Apperley JF, et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood. 2002;99(7):2304-2309.
12. Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica. 2011;96(6):918-921.
13. Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant BCR-ABL kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171.
14. Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208-1215.
15. Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959-962.
16. Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852-3861.
17. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796.
18. Food and Drug Administration. FDA Drug Safety Communication: FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. U.S. Food and Drug Administration Website. http://www.fda.gov/drugs/drugsafety/ucm379554.htm. Updated December 20, 2013. Accessed June 13, 2014.
19. Khoury HJ, Kukreja M, Goldman JM, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012;47(6):810-816.