User login
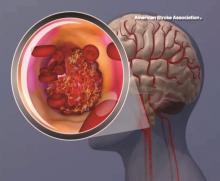
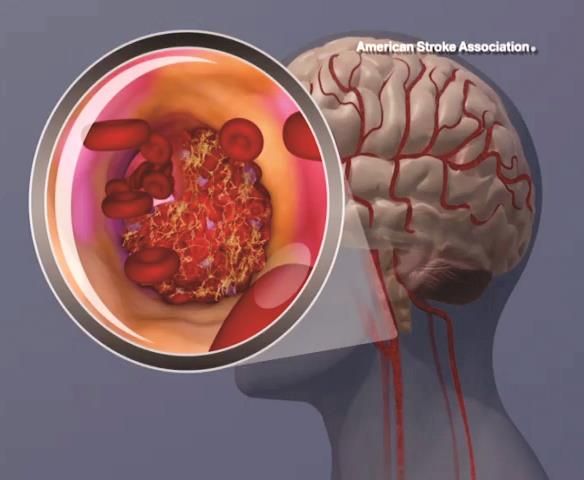
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
BOSTON – Body weight over 100 kg in acute ischemic stroke patients does not reduce the clinical benefit derived from a 90-mg fixed dose of intravenous recombinant tissue plasminogen activator, according to a pooled analysis of data from three randomized clinical trials.
Prior small studies have suggested that the magnitude of benefit with intravenous recombinant tissue plasminogen activator (IV rtPA) is reduced in patients with body weight over 100 kg who receive less than 0.9 mg/kg of IV rtPA under current guidelines. However, in the current study, the rate of favorable outcomes at 90 days – defined as modified Rankin scale score of 0-2 – did not differ significantly between 872 patients with weight at or below 100 kg and 105 with body weight over 100 kg (and up to 190 kg) after adjustment for patient demographics, stroke severity, and 90-day modified Rankin scale score (adjusted odds ratio, 0.99), Shahram Majidi, MD, said at the annual meeting of the American Academy of Neurology.
The results were similar when patients with NIHSS score less than 8 were excluded, and when those with weight over 150 kg were compared with those with weight at 100 kg or less, Dr. Majidi said.
There were only eight patients who weighed more than 150 kg, but those patients did very well at 90 days, and had favorable outcomes that were comparable to those in the lower weight group, he noted.
“Body weight more than 100 kg, and receiving less than 0.9 mg/kg of IV rtPA, did not reduce the benefit of IV rtPA in acute ischemic stroke patients, and our results support the current recommendations from the American Stroke Association,” Dr. Majidi concluded.
Dr. Majidi reported having no disclosures.
Key clinical point:
Major finding: Patients with weight at or below 100 kg and those with weight over 100 kg had a similar rate of favorable outcome at 90 days (adjusted OR, 0.99).
Data source: A pooled analysis of data from 977 patients in three randomized trials.
Disclosures: Dr. Majidi reported having no disclosures.