User login
Epidermal Tumors Arising on Donor Sites From Autologous Skin Grafts: A Systematic Review
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
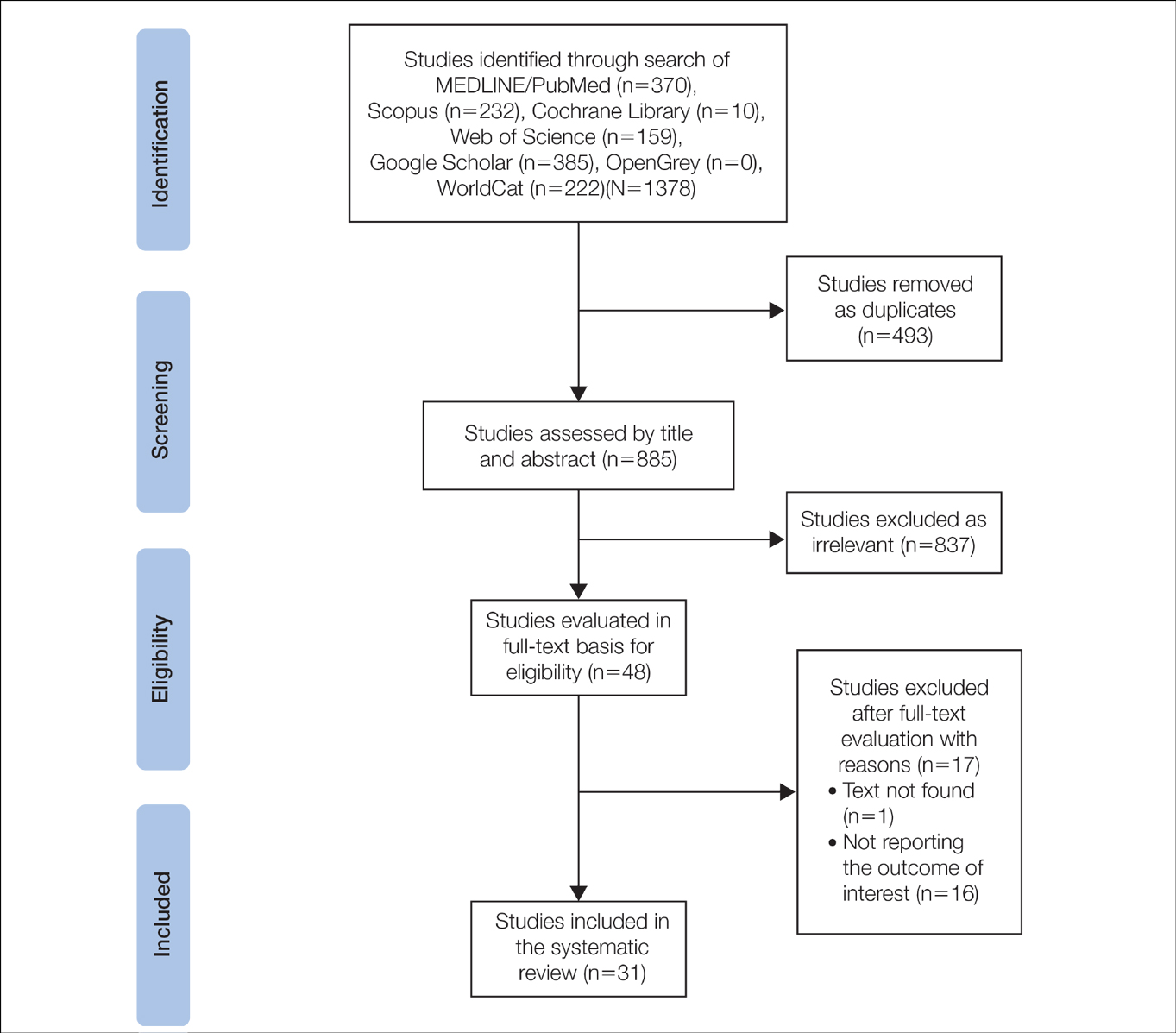
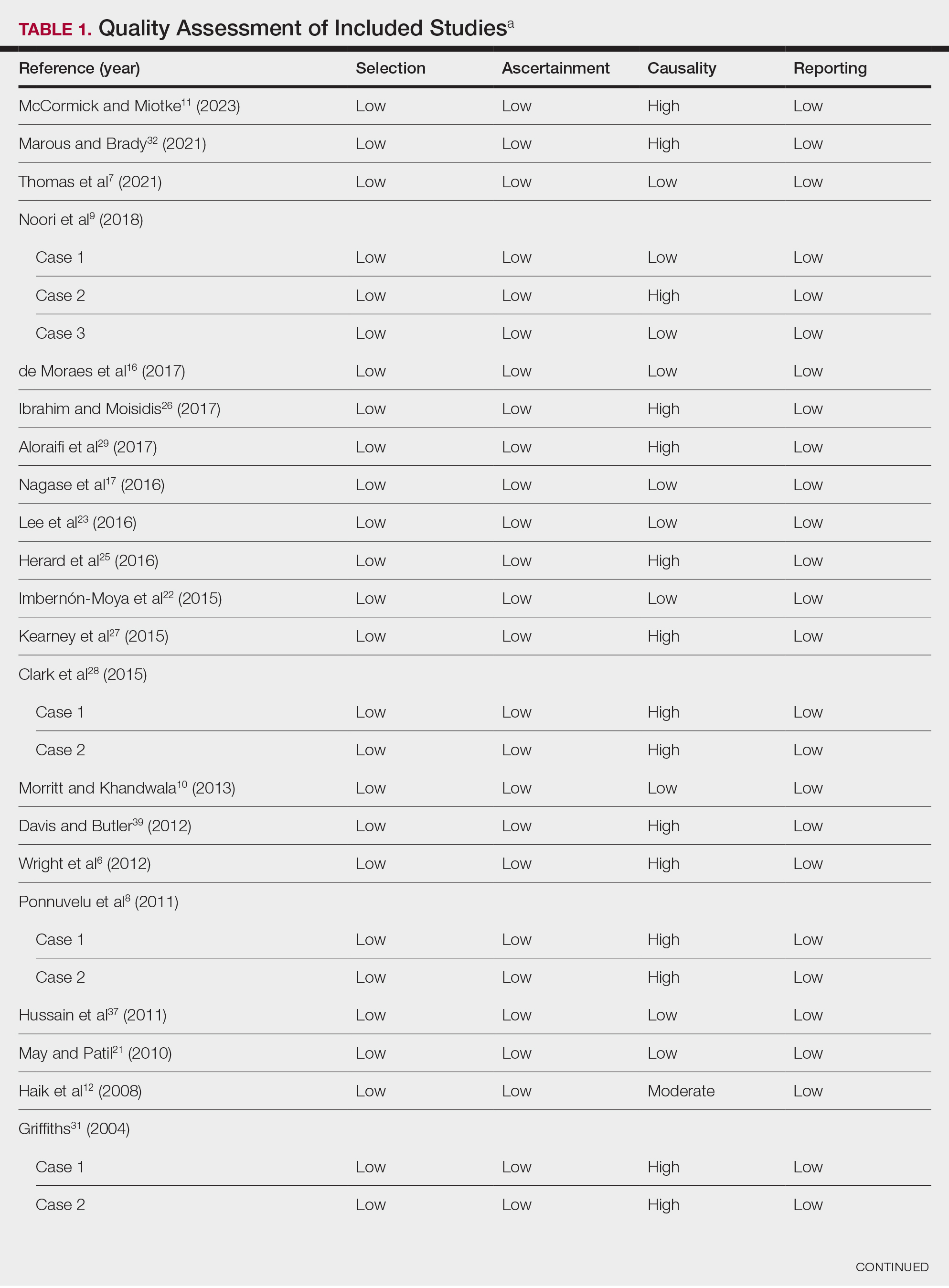
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.
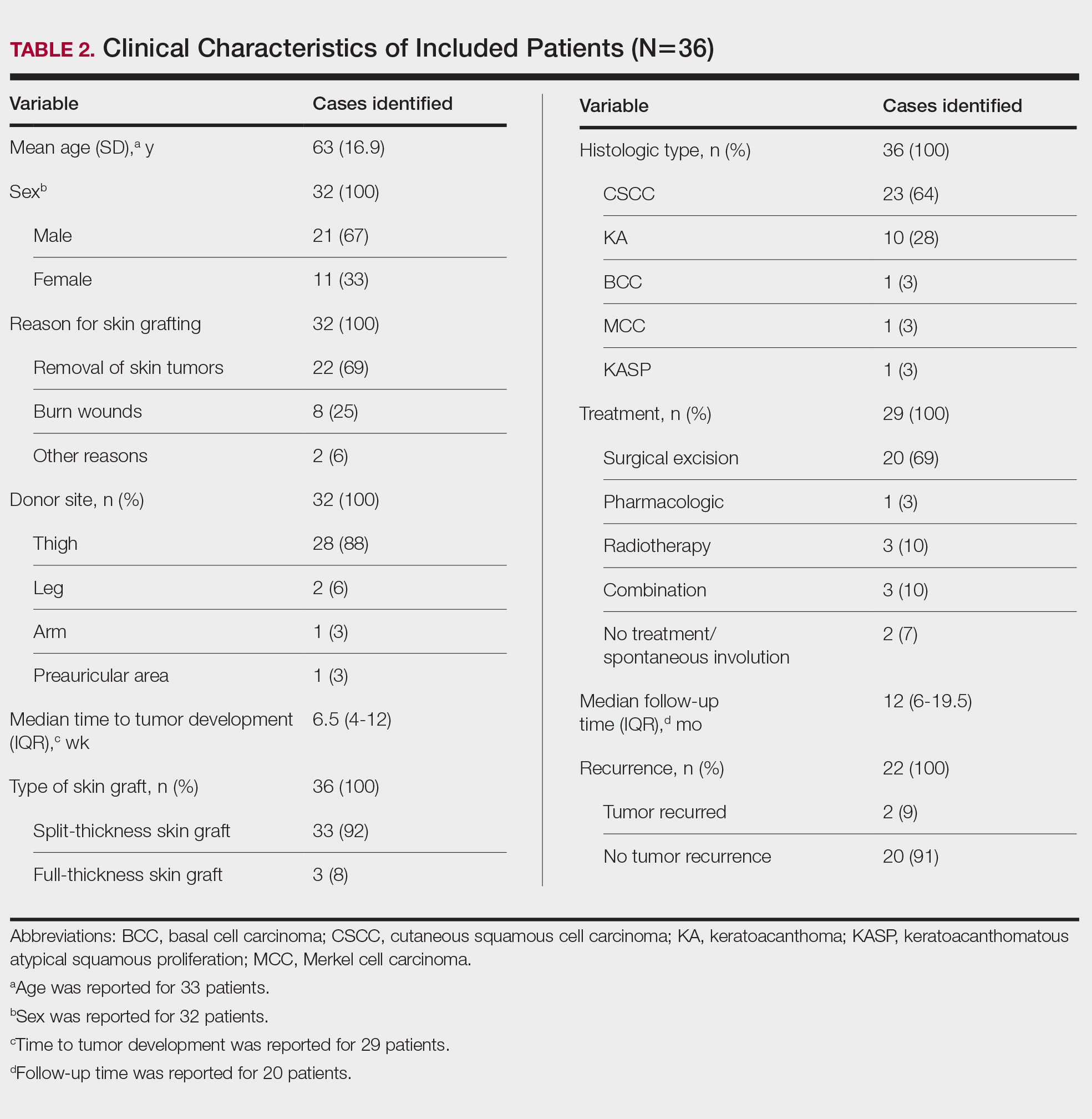
Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
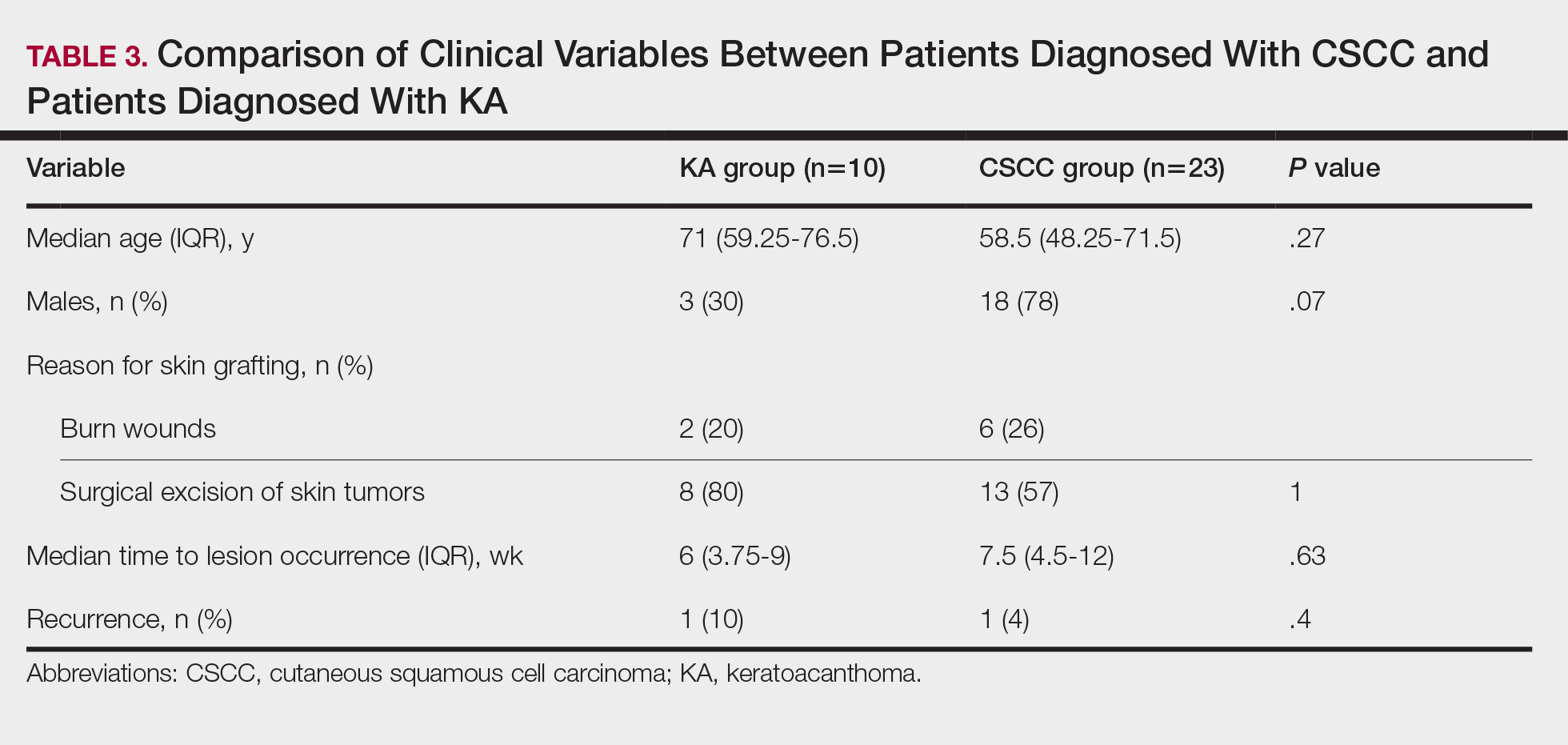
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.
Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.



Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.


Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
Skin grafting is a surgical technique used to cover skin defects resulting from the removal of skin tumors, ulcers, or burn injuries.1-3 Complications can occur at both donor and recipient sites and may include bleeding, hematoma/seroma formation, postoperative pain, infection, scarring, paresthesia, skin pigmentation, graft contracture, and graft failure.1,2,4,5 The development of epidermal tumors is not commonly reported among the complications of skin grafting; however, cases of epidermal tumor development on skin graft donor sites during the postoperative period have been reported.6-12
We performed a systematic review of the literature for cases of epidermal tumor development on skin graft donor sites in patients undergoing autologous skin graft surgery. We present the clinical characteristics of these cases and discuss the nature of these tumors.
Methods
Search Strategy and Study Selection—A literature search was conducted by 2 independent researchers (Z.P. and V.P.) for articles published before December 2022 in the following databases: MEDLINE/PubMed, Web of Science, Scopus, Cochrane Library, OpenGrey, Google Scholar, and WorldCat. Search terms included all possible combinations of the following: keratoacanthoma, molluscum sebaceum, basal cell carcinoma, squamous cell carcinoma, acanthoma, wart, Merkel cell carcinoma, verruca, Bowen disease, keratosis, skin cancer, cutaneous cancer, skin neoplasia, cutaneous neoplasia, and skin tumor. The literature search terms were selected based on the World Health Organization classification of skin tumors.13 Manual bibliography checks were performed on all eligible search results for possible relevant studies. Discrepancies were resolved through discussion and, if needed, mediation by a third researcher (N.C.). To be included, a study had to report a case(s) of epidermal tumor(s) that was confirmed by histopathology and arose on a graft donor site in a patient receiving autologous skin grafts for any reason. No language, geographic, or report date restrictions were set.
Data Extraction, Quality Assessment, and Statistical Analysis—We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Two independent researchers (Z.P. and V.P.) retrieved the data from the included studies. We have used the terms case and patient interchangeably, and 1 month was measured as 4 weeks for simplicity. Disagreements were resolved by discussion and mediation by a third researcher (N.C.). The quality of the included studies was assessed by 2 researchers (M.P. and V.P.) using the tool proposed by Murad et al.15
We used descriptive statistical analysis to analyze clinical characteristics of the included cases. We performed separate descriptive analyses based on the most frequently reported types of epidermal tumors and compared the differences between different groups using the Mann-Whitney U test, χ2 test, and Fisher exact test. The level of significance was set at P<.05. All statistical analyses were conducted using SPSS (version 29).
Results
Literature Search and Characteristics of Included Studies—The initial literature search identified 1378 studies, which were screened based on title and abstract. After removing duplicate and irrelevant studies and evaluating the full text of eligible studies, 31 studies (4 case series and 27 case reports) were included in the systematic review (Figure).6-12,16-39 Quality assessment of the included studies is presented in Table 1.



Clinical Characteristics of Included Patients—Our systematic review included 36 patients with a mean age of 63 years and a male to female ratio of 2:1. The 2 most common causes for skin grafting were burn wounds and surgical excision of skin tumors. Most grafts were harvested from the thighs. The development of a solitary lesion on the donor area was reported in two-thirds of the patients, while more than 1 lesion developed in the remaining one-third of patients. The median time to tumor development was 6.5 weeks. In most cases, a split-thickness skin graft was used.
Cutaneous squamous cell carcinomas (CSCCs) were found in 23 patients, with well-differentiated CSCCs in 19 of these cases. Additionally, keratoacanthomas (KAs) were found in 10 patients. The majority of patients underwent surgical excision of the tumor. The median follow-up time was 12 months, during which recurrences were noted in a small percentage of cases. Clinical characteristics of included patients are presented in Table 2.


Comment
Reasons for Tumor Development on Skin Graft Donor Sites—The etiology behind epidermal tumor development on graft donor sites is unclear. According to one theory, iatrogenic contamination of the donor site during the removal of a primary epidermal tumor could be responsible. However, contemporary surgical procedures dictate the use of different sets of instruments for separate surgical sites. Moreover, this theory cannot explain the occurrence of epidermal tumors on donor sites in patients who have undergone skin grafting for the repair of burn wounds.37
Another theory suggests that hematogenous and/or lymphatic spread can occur from the site of the primary epidermal tumor to the donor site, which has increased vascularization.16,37 However, this theory also fails to provide an explanation for the development of epidermal tumors in patients who receive skin grafts for burn wounds.
A third theory states that the microenvironment of the donor site is key to tumor development. The donor site undergoes acute inflammation due to the trauma from harvesting the skin graft. According to this theory, acute inflammation could promote neoplastic growth and thus explain the development of epidermal tumors on the donor site.8,26 However, the relationship between acute inflammation and carcinogenesis remains unclear. What is known to date is that the development of CSCC has been documented primarily in chronically inflamed tissues, whereas the development of KA—a variant of CSCC with distinctive and more benign clinical characteristics—can be expected in the setting of acute trauma-related inflammation.13,40,41
Based on our systematic review, we propose that well-differentiated CSCC on graft donor sites might actually be misdiagnosed KA, given that the histopathologic differential diagnosis between CSCC and KA is extremely challenging.42 This hypothesis could explain the development of well-differentiated CSCC and KA on graft donor sites.
Conclusion
Development of CSCC and KA on graft donor sites can be listed among the postoperative complications of autologous skin grafting. Patients and physicians should be aware of this potential complication, and donor sites should be monitored for the occurrence of epidermal tumors.
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
- Adams DC, Ramsey ML. Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts. Dermatologic Surg. 2005;31(8, pt 2):1055-1067. doi:10.1111/j.1524-4725.2005.31831
- Shimizu R, Kishi K. Skin graft. Plast Surg Int. 2012;2012:563493. doi:10.1155/2012/563493
- Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. doi:10.1155/2014/582080
- Coughlin MJ, Dockery GD, Crawford ME, et al. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. 2nd ed. Saunders Ltd; 2012.
- Herskovitz I, Hughes OB, Macquhae F, et al. Epidermal skin grafting. Int Wound J. 2016;13(suppl 3):52-56. doi:10.1111/iwj.12631
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266. doi:10.1016/j.bjps.2012.01.022
- Thomas W, Rezzadeh K, Rossi K, et al. Squamous cell carcinoma arising at a skin graft donor site: case report and review of the literature. Plast Surg Case Stud. 2021;7:2513826X211008425. doi:10.1177/2513826X211008425
- Ponnuvelu G, Ng MFY, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169. doi:10.1016/j.surge.2010.08.006
- Noori VJ, Trehan K, Savetamal A, et al. New onset squamous cell carcinoma in previous split-thickness skin graft donor site. Int J Surg. 2018;52:16-19. doi:10.1016/j.ijsu.2018.01.047
- Morritt DG, Khandwala AR. The development of squamous cell carcinomas in split-thickness skin graft donor sites. Eur J Plast Surg. 2013;36:377-380.
- McCormick M, Miotke S. Squamous cell carcinoma at split thickness skin graft donor site: a case report and review of the literature. J Burn Care Res. 2023;44:210-213. doi:10.1093/jbcr/irac137
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893. doi:10.1016/j.burns.2007.06.006
- Elder DE, Massi D, Scolyer RA WR. WHO Classification of Skin Tumours. 4th ed. IARC Press; 2018.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. doi:10.7326/0003-4819-151-4-200908180-00135
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23:60-63. doi:10.1136/bmjebm-2017-110853
- de Moraes LPB, Burchett I, Nicholls S, et al. Large solitary distant metastasis of cutaneous squamous cell carcinoma to skin graft site with complete response following definitive radiotherapy. Int J Bioautomation. 2017;21:103-108.
- Nagase K, Suzuki Y, Misago N, et al. Acute development of keratoacanthoma at a full-thickness skin graft donor site shortly after surgery. J Dermatol. 2016;43:1232-1233. doi:10.1111/1346-8138.13368
- Taylor CD, Snelling CF, Nickerson D, et al. Acute development of invasive squamous cell carcinoma in a split-thickness skin graft donor site. J Burn Care Rehabil. 1998;19:382-385. doi:10.1097/00004630-199809000-00004
- de Delas J, Leache A, Vazquez Doval J, et al. Keratoacanthoma over the donor site of a laminar skin graft. Med Cutan Ibero Lat Am. 1989;17:225-228.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419. doi:10.1016/0007-1226(88)90086-0
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Imbernón-Moya A, Vargas-Laguna E, Lobato-Berezo A, et al. Simultaneous onset of basal cell carcinoma over skin graft and donor site. JAAD Case Rep. 2015;1:244-246. doi:10.1016/j.jdcr.2015.05.004
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:e117-e119. doi:10.1111/ajd.12501
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutelyin a skin graft donor site. J Trauma. 1987;27:681-683. doi:10.1097/00005373-198706000-00017
- Herard C, Arnaud D, Goga D, et al. Rapid onset of squamous cell carcinoma in a thin skin graft donor site. Ann Dermatol Venereol. 2016;143:457-461. doi:10.1016/j.annder.2015.03.027
- Ibrahim A, Moisidis E. Case series: rapidly growing squamous cell carcinoma after cutaneous surgical intervention. JPRAS Open. 2017;14:27-32. doi:10.1016/j.jpra.2017.08.004
- Kearney L, Dolan RT, Parfrey NA, et al. Squamous cell carcinoma arising in a skin graft donor site following melanoma extirpation at a distant site: a case report and review of the literature. JPRAS Open. 2015;3:35-38. doi:10.1016/j.jpra.2015.02.002
- Clark MA, Guitart J, Gerami P, et al. Eruptive keratoacanthomatous atypical squamous proliferations (KASPs) arising in skin graft sites. JAAD Case Rep. 2015;1:274-276. doi:10.1016/j.jdcr.2015.06.009
- Aloraifi F, Mulgrew S, James NK. Secondary Merkel cell carcinoma arising from a graft donor site. J Cutan Med Surg. 2017;21:167-169. doi:10.1177/1203475416676805
- Abadir R, Zurowski S. Case report: squamous cell carcinoma of the skin in both palms, axillary node, donor skin graft site and both soles—associated hyperkeratosis and porokeratosis. Br J Radiol. 1994;67:507-510. doi:10.1259/0007-1285-67-797-507
- Griffiths RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485-501. doi:10.1016/j.bjps.2004.05.007
- Marous M, Brady K. Cutaneous squamous cell carcinoma arising in a split thickness skin graft donor site in a patient with systemic lupus erythematosus. Dermatologic Surg. 2021;47:1106-1107. doi:10.1097/DSS.0000000000002955
- Dibden FA, Fowler M. The multiple growth of molluscum sebaceum in donor and recipient sites of skin graft. Aust N Z J Surg. 1955;25:157-159. doi:10.1111/j.1445-2197.1955.tb05122.x
- Jeremiah BS. Squamous cell carcinoma development on donor area following removal of a split thickness skin graft. Plast Reconstr Surg. 1948;3:718-721.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40(5, pt 2):870-871. doi:10.1053/jd.1999.v40.a94419
- Hamilton SA, Dickson WA, O’Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561. doi:10.1016/s0007-1226(97)91308-4
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692. doi:10.1016/j.bjps.2010.06.004
- Wulsin JH. Keratoacanthoma: a benign cutaneous tumors arising in a skin graft donor site. Am Surg. 1958;24:689-692.
- Davis L, Butler D. Acute development of squamous cell carcinoma in a split-thickness skin graft donor site [abstract]. J Am Acad Dermatol. 2012;66:AB208. doi:10.1016/j.jaad.2011.11.874
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230-232.
- Piotrowski I, Kulcenty K, Suchorska W. Interplay between inflammation and cancer. Reports Pract Oncol Radiother. 2020;25:422-427. doi:10.1016/j.rpor.2020.04.004
- Carr RA, Houghton JP. Histopathologists’ approach to keratoacanthoma: a multisite survey of regional variation in Great Britain and Ireland. J Clin Pathol. 2014;67:637-638. doi:10.1136/jclinpath-2014-202255
Practice Points
- Donor site cutaneous squamous cell carcinoma (CSCC) and keratoacanthoma (KA) can be postoperative complications of autologous skin grafting.
- Surgical excision of donor site CSCC and KA typically is curative.