User login
Vertebral Artery Dissection in Active-Duty Soldier Due to Mixed Martial Arts Choke Hold
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
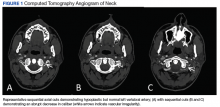
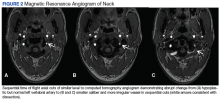
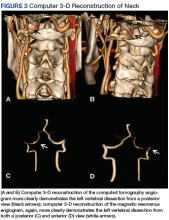
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
Knowledge of the potential dangers of mixed martial arts is valuable for Department of Defense (DoD) health care providers as the military continues to implement combatives training into regular military instruction. This case study presents an active-duty service member who developed a spontaneous vertebral artery dissection (sVAD) during mixed martial arts training, which led to a cerebellar stroke.
To the authors’ knowledge this is the first documented case of a sVAD with associated stroke related to a mixed martial arts choke hold. Understanding the diagnosis, management, and prognosis of this condition will remain important as hand-to-hand combat instruction continues to be a part of regular military training.
Case Presentation
A 39-year-old active-duty male without significant past medical history presented to the emergency department (ED) at the San Antonio Military Medical Center in Texas for evaluation of severe vertigo with associated nausea and vomiting. He had participated in a Jiu-Jitsu match the evening prior to his presentation and reported that he was placed in a choke hold within the last 12 seconds of the match. He denied losing consciousness during this hold.
Once released, he attempted to stand and developed sudden onset vertigo with severe nausea, leading to multiple bouts of emesis. He additionally developed a throbbing, left-sided headache radiating down the left side of his neck. While the vertigo resolved within an hour, he continued to experience bouts of nausea and emesis, prompting him to present to the ED for further evaluation. The patient’s past medical history was remarkable only for multiple prior concussions, and his only medication was occasional ibuprofen. He denied the usage of recreational drugs.
Upon presentation to the ED, the patient’s vital signs were 139/93 mm Hg blood pressure, 73 beats per minute heart rate, 16 breaths per minute respiration, 100% oxygen saturation on room air, and 97.7° F temperature.
The patient demonstrated normal balance and exhibited no nystagmus or limb/truncal ataxia as evaluated with finger-to-nose/heel-to-shin testing and gait exam. Complete blood count, comprehensive metabolic panel, and coagulation panel all demonstrated no abnormalities.
The patient was admitted to the hospital for symptom control and further monitoring. His headache and nausea were managed with medications, and he began antiplatelet therapy with aspirin 325 mg daily. Given the size of his cerebellar infarction, it was decided that he would be monitored in the hospital for 72 hours for the development of significant cerebellar edema. He remained stable throughout his hospitalization and had only a mild headache at the time of discharge.
The patient was last seen 3 months postinjury with no subjective complaints and a completely normal neurologic exam. The treatment plan for the patient is to continue aspirin for 6 months postinjury at which time a repeat CT will be performed to ensure resolution. He has been counseled to avoid heavy lifting and any activity with potential for sudden movement/force of the neck (grappling/wrestling, chiropractic manipulation, roller coasters, or sit-ups) until the repeat CT has been completed.
Discussion
Spontaneous vertebral artery and carotid artery dissections are collectively referred to as sCADs. Spontaneous cervical artery dissections are a rare condition with a higher incidence of internal carotid dissections than are VADs (1.72 vs 0.97 per 100,000 people).1 In contrast to the general stroke population, patients with sCADs are typically younger (mean age 45.3 years); and more than half of the patients are male.1,2
Spontaneous cervical artery dissections are typically characterized by subintimal tears of the vertebral artery leading to the accumulation of an intramural hematoma and creation of a “false lumen” in the arterial wall.3 A sVAD is more often found in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) segments of the vertebral artery than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segments.3-5 The etiology of these injuries is thought to be minor trauma to the neck in the context of a likely underlying connective tissue disease, though no direct association with a particular disease has been shown.
Biopsy evaluation of the superficial temporal arteries of patients with sCADs have revealed pathologic changes of the media and adventitial layers, including vacuolar degeneration and capillary neoangiogenesis, which are not found in the arteries of control patients.5 Although definitive association with a known connective tissue disease is rare, angiographic evidence of fibromuscular dysplasia, a nonspecific marker of connective tissue disease, is noted in as many as 15% to 20% of patients.6 Consequently, routine connective tissue disease screening is not recommended in these patients. One study found that about 40% of sCAD patients can recall minor cervical trauma in the preceding month in comparison to only 10% of other patients with stroke, leading to the moniker of “bottoms-up” or “beauty-parlor strokes” for these injuries. The most common mechanisms of minor neck trauma causing sCADs include tennis and golf swings, yoga, and roller-coaster rides.7,8
Usually symptomatic at presentation, the most frequently encountered sCAD symptoms are head or neck pain (80%), brain ischemia (56%), and Horner syndrome (25%).1 A study of 161 consecutive patients with internal carotid (n = 135) or vertebral artery (n = 26) dissections revealed that headache was reported by 69% of those with sVADs, and when present, was the initial manifestation in 33%. Headaches typically were ipsilateral to the dissection, located posteriorly in 83% of patients, and lasted an average duration of 72 hours. Neck pain, which was noted in 46% of sVAD patients, was predominantly posterior and ipsilateral in location as well.9 Ischemic symptoms of sVAD may include posterior circulation symptoms, such as vertigo, ataxia, diplopia, and leg weakness as well as lateral medullary (Wallenberg) syndrome characterized by dizziness, postural instability, limb hypotonia/ataxia, blurred vision, and nystagmus.
In a study of 169 patients with sCAD, brain ischemia occurred in 77% (131 patients) including 67% (n = 114) with ischemic stroke and 10% (n = 17) with transient ischemic attack. Head and/or neck pain was noted in 88% of those with brain ischemia.4 Etiologies for infarction included thromboembolic (85%), hemodynamic (12%), and mixed (3%).10 Isolated local symptoms are rare with one study of 245 patients with sCAD revealing only 20 (8%) presenting with pain only. Of those with pain only, 6 presented with headache, 2 with neck pain, and 12 with both.11
Diagnosis of sVAD requires a high index of suspicion and is confirmed by diagnostic testing. Previously, invasive angiography was the diagnostic gold standard, but with the improvement in quality of CT and MR angiography, these noninvasive modalities have become the tests of choice. There have been no studies to date revealing a definitive benefit of one modality over the other. A meta-analysis of 25 articles that compared the use of CT and MR angiography for the diagnosis of carotid and VAD revealed similar sensitivity and specificity.12 In contrast, a study involving 10 patients with confirmed sVAD who had both CT and MR angiographies during evaluation showed more total findings consistent with dissection on CT than with MR angiography when graded by 2 neuroradiologists. Additionally, the neuroradiologists subjectively rated CT angiography as preferential to MR in showing the imaging findings of dissection in 8 of 10 cases of vertebral dissection.13
Treatment for sCAD remains heavily debated. The use of IV thrombolysis within the standard time window for acute ischemic stroke is advocated for these patients. A meta-analysis of patients with sCAD vs matched patients with stroke from other causes treated with IV thrombolysis showed no difference in mortality at 3 months (9.0% vs 8.8%) or symptomatic intracranial hemorrhage (3.3% vs 3.0%). Additionally, similar percentages of patients had excellent (30.9% vs 37.4%) and favorable (58.2% vs 52.2%) 3-month functional statuses as expressed by the Modified Rankin Score (mRS).14,15
Debate remains regarding subacute therapy for sCAD with either antiplatelet or anticoagulant therapy. A randomized study of 250 patients with cervical artery dissection (118 carotid, 132 vertebral) in which 126 patients were assigned to antiplatelet therapy and 124 patients were assigned to anticoagulant therapy showed an overall low rate of recurrent stroke (2%). There was no significant difference in efficacy between the therapy groups with stroke or death occurring in 3 antiplatelet patients and 1 anticoagulated patient. Adverse effects were very low in both groups with no deaths and only 1 major bleed in the anticoagulation group. Of note, stroke rates were lower in this study than prior observational studies.16
A nonrandomized study of 88 patients with extracranial sCAD showed overall low rates of recurrent ischemic stroke at 3 months with 1/59 (1.7%) in the antiplatelet group and 1/28 (3.6%) in the anticoagulation group (P 17 Given this low overall rate of recurrent stroke in prior studies, a guideline recommendation for antiplatelet or anticoagulant therapy cannot be made at this time.
The overall prognosis for this condition is fair. Functional status and recurrence risk are favorable, with one study finding a mRS score of 1 Additionally, a historic cohort study of 432 patients with first event of sCAD revealed that after a mean follow-up of 31 months, only 4 (0.9%) patients had a recurrent ischemic stroke either due to incomplete recanalization of the artery (n = 2) or recurrent sCAD (n = 2), and only 4 (0.9%) total recurrences of sCAD were report (2 without associated ischemic strokes).18 Further, a prospective study of 61 patients with confirmed sVAD revealed complete recanalization of 45.9% at 3 months, 62.3% at 6 months, and 63.9% at 12 months, suggesting that recanalization occurs mostly during the initial 6 months. There was no identified association between outcome and complete recanalization with favorable outcomes observed in 55 (90.2%) of patients and no further ischemic symptoms during follow-up.19
Neck maneuvers have been cited as a more common cause of sCAD in several previous studies. One retrospective study found chiropractic neck manipulation to be the etiology in 12 of 141 patients with CT- or MR- confirmed sCAD.20 As noted previously, to the authors’ knowledge this is the first reported case of a sVAD occurring after a mixed martial arts choke hold. While sports-related strokes are rare, one evaluation of 70 published cases found that 80% were due to sCAD. Commonly associated sports in this study included football, yoga, wrestling, tennis, golf, and swimming.21 Grappling-related neck manipulation has been noted as an etiology in a few case reports.
Hyperextension of the neck was deemed to be the etiology in boys aged 11 years and 17 years who developed a sCAD while participating in Judo and backyard wrestling, respectively.22,23 In the martial arts realm, there is a case report of a 26-year-old male who developed a sVAD after rapid head turning during a solo Kung Fu maneuver as well as a report of a 41-year-old male experiencing a right VAD complicated by a posterior infarction several days after straining his neck during a mixed martial arts competition.24,25 The patient denied any choke hold or direct blow to the neck.
The present case is different in that it is the first reported case of a sVAD occurring after a submission maneuver. Prior grappling-related sVADs were associated with hyperextension or rapid acceleration/deceleration forces on the neck. Isometric force to the neck is a rarely described mechanism for development of this injury. Although there are isolated and infrequent forensic case reports of carotid dissection with strangulation injuries, the authors believe this is the first documented case of a sVAD attributed to a combatives submission.
In the context of the military health system, it is important to be aware of this potential complication of combatives as instruction in close-quarters combat continues to be an important part of military training.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
1. Lee VH, Brown RD Jr, Mandrekar J, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809-1812.
2. Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67(6):1050-1052.
3. Schvienk W. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
4. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37(10):2499-2503.
5. Völker W, Dittrich R, Grewe S, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76(17):1463-1471.
6. Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009;40(6):459-466.
7. DeBehnke D, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12(1):27-31.
8. Engelter ST, Grond-Ginsbach C, Metso TM, et al; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology. 2013;80(21):1950-1957.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522.
10. Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;43(5):1354-1361.
11. Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. 2006;77(9):1021-1024.
12. Provenzale J, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;1939(4):1167-1174.
13. Vertinsky AT, Schwartz NE, Fishbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
14. Zinkstok SM, Vergouwen MD, Engelter ST, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011;42(9):2515-2520.
15. Engelter S, Rutgers M, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40(12):3772-3776.
16. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015;14(4):361-367.
17. Kennedy F, Lanfranconi S, Hicks C, et al; CADISS Investigators. Antiplatelets vs. anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012;79(7):686-689.
18. Touze E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347-1351.
19. Arauz A, Marquez J, Artigas C, Balderrama J, Orrego H. Recanalization of vertebral artery dissection. Stroke. 2010;41(4):717-721.
20. Kennell KA, Daghfal MM, Patel SG, et DeSanto JR, Waterman GS, Bertino RE. Cervical artery dissection related to chiropractic manipulation: one institution’s experience. J Fam Pract. 2017;66(9):556-562.
21. McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7(4):298-300.
22. Lannuzel A, Moulin T, Amsallem D, Galmiche J, Rumbach L. Vertebral artery dissection following a judo session: a case report. Neuropediatrics. 1994;25(2):106-108.
23. Gupta V, Dhawan N, Bahl J. Minor trauma causing stroke in a young athlete. Case Rep Neurol Med. 2015;2015: 182875.
24. Pacei F, Valvasorri L, Bet L. Vertebral artery dissection during Kung-Fu training. Neurol Sci. 2014;35(2):331-332.
25. Slowey M, Maw G, Furyk J. Case report on vertebral artery dissection in mixed martial arts. Emerg Med Australas. 2012;24(2):203-206.
Genetic Heart Failure in an Active-Duty Soldier
Left ventricular noncompaction (LVNC) is a rare disorder that is variably classified as a primary genetic cardiomyopathy (CM) by the American Heart Association.1 It is mostly believed to be a congenital abnormality, characterized by the arrest of the typical embryonic myocardial maturation process with the subsequent retention of the trabecular myocardial structure, which defines the early embryonic heart.2
During very early embryonic development, the left ventricular (LV)myocardium is composed of a loose network of fibers separated by deep recesses, which link it with the LV cavity. At 8 weeks of prenatal development, gradual compaction of these fibers occurs, and LVNC is thought to result from the arrest of this normal process.2,3 Significant variability in myocardial involvement exists, ranging from panventricular to isolated apical involvement, likely related to time of arrest of this maturation process.4 The decreased contractile capability and inadequate epicardial coronary system communication of this trabecular endocardium is thought to lead to the clinical manifestations of LVNC.1-7
This report describes the case of a 45-year-old male soldier who presented with a unique case of heart failure, diagnosed via cardiac magnetic resonance imaging (MRI).
Case Study
The patient presented to the San Antonio Military Medical Center emergency department in mid-2011 with increasing dyspnea for several weeks. He also reported significant lower-extremity and scrotal edema. Although the patient had been previously healthy, his recent medical history was remarkable for a severe combat injury suffered while on duty with the U.S. Army in Afghanistan: He was involved in an explosion from an improvised explosive device in August 2009. He was medically evacuated to the U.S., where he required multiple hospitalizations and surgeries. Prior to his current presentation, the patient had been briefly hospitalized for hospital-acquired pneumonia. During this hospitalization, he first noted abnormal swelling of his legs, a finding that was initially attributed to the large sodium load he had received with his IV antibiotics.
DIAGNOSIS
The patient’s vital signs on presentation were notable for 100/83 mm Hg blood pressure, 103 beats per minute (bpm) heart rate, and 18/min respiratory rate with a saturation of 100% on 4 liters of oxygen by nasal cannula. He was conversant but tachypneic and had to pause frequently to catch his breath. His neck veins were notably distended with jugular venous pulsations visible to the angle of the jaw with the patient at 30 degrees. His heart sounds were normal without an S3, but his lungs were notable for bilateral crackles over the lower- to mid-lung fields. He had profound bilateral upper and lower extremity and scrotal pitting edema. He had no lymphadenopathy or skin rashes.
On presentation, the patient’s laboratory results were remarkable for a 444 pg/mL brain natriuretic peptide. A chest X-ray revealed bilateral basilar opacities. An electrocardiogram showed normal sinus rhythm (70 bpm), with normal axis and poor R-wave progression across the precordium. An echocardiogram was performed and notable for a moderately dilated left ventricle with severely depressed systolic function of 10% to 15%, and elevated pulmonary artery pressures. Subsequently, the patient was referred for a coronary angiography, which showed no evidence of coronary atherosclerosis. A cardiac MRI was then performed to evaluate for nonischemic CM, which revealed prominent trabeculations in both ventricles, but most notably in the left ventricle, consistent with a diagnosis of LVNC.
The patient was treated with diuretics, beta-blockers, and an angiotensin-converting enzyme (ACE) inhibitor with improvement in his heart failure symptoms. He was started on systemic anticoagulation with warfarin for his severely depressed LV function. His hospital course was complicated by frequent, nonsustained ventricular tachycardia (VT), and he was referred to the electrophysiology service for implantation of an automated intracardiac cardioverter/defibrillator (AICD) for primary prevention of sudden cardiac death. His clinical course was otherwise unremarkable, and he was discharged after 8 days with complete resolution of his symptoms.
Discussion
The clinical presentation of LVNC is typically due to complications of ventricular dysfunction, including heart failure, arrhythmias, and cardioembolic events. Retrospective studies have shown much variability in the frequency of these complications, likely due to selection bias in earlier studies. These earlier studies had suggested a frequency of heart failure > 50%, but recent studies have shown a more modest frequency of 30% to 35% of affected patients.
Even greater variance has been found in the frequency of arrhythmias, but most studies have shown a frequency of at least 20% for VT. Poor blood flow in the deep intertrabecular recesses in patients with LVNC is additionally thought to lead to a predisposition for mural thrombus formation with an elevated frequency of systemic embolic events, ranging from 5% to 20% among previous studies.1-4,6,8
Much debate remains regarding the genetic association of this condition. The unique character of the resulting myocardium suggests a distinct CM, but the significant genetic heterogeneity with sarcomere protein gene mutations associated with several other CMs, including hypertrophic and dilated CM, suggests that LVNC may simply exist on a phenotypic continuum with these other conditions.4 Inheritance shows additional similarities to these other known CMs with autosomal-
dominant and X-linked modes of transmission shown with familial forms in about 25% of patients.5,7 This has led many to believe that screening of first-degree relatives of clinically affected patients may be appropriate.
The prevalence of LVNC in adults referred for echocardiography is about 0.014% to 1.3%.5 A recent increase in the rate of recognition has raised concerns of possible overdiagnosis, with attempts now made to develop specific imaging diagnostic criteria. Diagnosis of LVNC is most commonly suspected (but can be missed) on echocardiography using 2-D and color Doppler imaging modalities. Echocardiographic findings supporting the diagnosis of LVNC suggested by Oechslin and colleague include:
• Presence of multiple trabeculations, particularly in the LV apex and free wall;
• Multiple deep trabeculation recesses in communication with the LV cavity, usually seen on color Doppler imaging;
• A 2-layered structure of the endomyocardium with ratio of end systolic, noncompacted endocardial layer to compacted epicardial layer > 2 in adults; and
• Absence of other congenital or acquired heart disease, particularly those causing LV outflow obstruction.8
Another proposed standardized method for identifying LVNC via echocardiography by Chin and colleagues focuses on trabeculae at the LV apex on the parasternal short axis and apical views.2,3 LVNC is defined by a ratio of X/Y of ≤ 0.5, where X is the distance from the epicardial surface to the trough of the trabecular recess, and Y is the distance from the epicardial surface to the peak of the trabeculations.
Cardiac MRI is now a more common mode of imaging used for diagnosis of LVNC and often has better imaging characteristics than those of echocardiography. Using a ratio of noncompacted to compacted CM in diastole > 2.3 is suggestive of LVNC with sensitivity and specificity of 86% and 99%.9
The management of LVNC focuses primarily on treatment of complications, including heart failure, rhythm disturbances, and thromboembolic events. Treatment of heart failure is typically the same as for other CMs and includes medical therapy with salt restriction, diuretics, beta-blockers, and ACE inhibitors. In addition, exercise training, as tolerated, is beneficial to improve clinical status.3,10 Electrophysiology studies are often performed in these patients, and implantation of an AICD is typically done in cases of documented, sustained VT, presyncope with inducible VT or severally depressed ejection fraction of < 35%.4,10 Deep intertrabecular recesses and impaired blood flow increase the risk of thrombus formation. Hence, anticoagulation with warfarin (international normalized ratio target 2.3) for those with an impaired LV ejection fraction (< 40%) should be considered for the prevention of cardioembolic events.3,4,6,10
Summary
An active-duty solider with a history of battlefield trauma and multiple hospitalizations was admitted for symptomatic heart failure with cardiac MRI suggestive of LVNC. This condition is a phenotypic result of genetic heterogeneity with significant variability in clinical presentation and a predisposition for heart failure, ventricular arrhythmias, and systemic embolic events. The etiology of this patient’s clinical presentation remains unclear, and additional research is needed to understand whether his recent trauma and multiple hospitalizations played a role in the manifestation of his disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
2. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507-513.
3. Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187-192.
4. Oechslin E, Jenni R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32(12):1446-1456.
5. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270-276.
6. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Longterm follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493-500.
7. Spirito P, Autore C. Apical hypertrophic cardiomyopathy or left ventricular non-compaction? A difficult differential diagnosis [editorial]. Eur Heart J. 2007;28(16):1923-1924.
8. Oechslin E, Jenni R. Non-compaction of the left ventricular myocardium—From clinical observation to the discovery of a new disease. Eur Cardiol Review. 2005;1(1):23-24.
9. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101-105.
10. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;53(15):e1-e90.
Left ventricular noncompaction (LVNC) is a rare disorder that is variably classified as a primary genetic cardiomyopathy (CM) by the American Heart Association.1 It is mostly believed to be a congenital abnormality, characterized by the arrest of the typical embryonic myocardial maturation process with the subsequent retention of the trabecular myocardial structure, which defines the early embryonic heart.2
During very early embryonic development, the left ventricular (LV)myocardium is composed of a loose network of fibers separated by deep recesses, which link it with the LV cavity. At 8 weeks of prenatal development, gradual compaction of these fibers occurs, and LVNC is thought to result from the arrest of this normal process.2,3 Significant variability in myocardial involvement exists, ranging from panventricular to isolated apical involvement, likely related to time of arrest of this maturation process.4 The decreased contractile capability and inadequate epicardial coronary system communication of this trabecular endocardium is thought to lead to the clinical manifestations of LVNC.1-7
This report describes the case of a 45-year-old male soldier who presented with a unique case of heart failure, diagnosed via cardiac magnetic resonance imaging (MRI).
Case Study
The patient presented to the San Antonio Military Medical Center emergency department in mid-2011 with increasing dyspnea for several weeks. He also reported significant lower-extremity and scrotal edema. Although the patient had been previously healthy, his recent medical history was remarkable for a severe combat injury suffered while on duty with the U.S. Army in Afghanistan: He was involved in an explosion from an improvised explosive device in August 2009. He was medically evacuated to the U.S., where he required multiple hospitalizations and surgeries. Prior to his current presentation, the patient had been briefly hospitalized for hospital-acquired pneumonia. During this hospitalization, he first noted abnormal swelling of his legs, a finding that was initially attributed to the large sodium load he had received with his IV antibiotics.
DIAGNOSIS
The patient’s vital signs on presentation were notable for 100/83 mm Hg blood pressure, 103 beats per minute (bpm) heart rate, and 18/min respiratory rate with a saturation of 100% on 4 liters of oxygen by nasal cannula. He was conversant but tachypneic and had to pause frequently to catch his breath. His neck veins were notably distended with jugular venous pulsations visible to the angle of the jaw with the patient at 30 degrees. His heart sounds were normal without an S3, but his lungs were notable for bilateral crackles over the lower- to mid-lung fields. He had profound bilateral upper and lower extremity and scrotal pitting edema. He had no lymphadenopathy or skin rashes.
On presentation, the patient’s laboratory results were remarkable for a 444 pg/mL brain natriuretic peptide. A chest X-ray revealed bilateral basilar opacities. An electrocardiogram showed normal sinus rhythm (70 bpm), with normal axis and poor R-wave progression across the precordium. An echocardiogram was performed and notable for a moderately dilated left ventricle with severely depressed systolic function of 10% to 15%, and elevated pulmonary artery pressures. Subsequently, the patient was referred for a coronary angiography, which showed no evidence of coronary atherosclerosis. A cardiac MRI was then performed to evaluate for nonischemic CM, which revealed prominent trabeculations in both ventricles, but most notably in the left ventricle, consistent with a diagnosis of LVNC.
The patient was treated with diuretics, beta-blockers, and an angiotensin-converting enzyme (ACE) inhibitor with improvement in his heart failure symptoms. He was started on systemic anticoagulation with warfarin for his severely depressed LV function. His hospital course was complicated by frequent, nonsustained ventricular tachycardia (VT), and he was referred to the electrophysiology service for implantation of an automated intracardiac cardioverter/defibrillator (AICD) for primary prevention of sudden cardiac death. His clinical course was otherwise unremarkable, and he was discharged after 8 days with complete resolution of his symptoms.
Discussion
The clinical presentation of LVNC is typically due to complications of ventricular dysfunction, including heart failure, arrhythmias, and cardioembolic events. Retrospective studies have shown much variability in the frequency of these complications, likely due to selection bias in earlier studies. These earlier studies had suggested a frequency of heart failure > 50%, but recent studies have shown a more modest frequency of 30% to 35% of affected patients.
Even greater variance has been found in the frequency of arrhythmias, but most studies have shown a frequency of at least 20% for VT. Poor blood flow in the deep intertrabecular recesses in patients with LVNC is additionally thought to lead to a predisposition for mural thrombus formation with an elevated frequency of systemic embolic events, ranging from 5% to 20% among previous studies.1-4,6,8
Much debate remains regarding the genetic association of this condition. The unique character of the resulting myocardium suggests a distinct CM, but the significant genetic heterogeneity with sarcomere protein gene mutations associated with several other CMs, including hypertrophic and dilated CM, suggests that LVNC may simply exist on a phenotypic continuum with these other conditions.4 Inheritance shows additional similarities to these other known CMs with autosomal-
dominant and X-linked modes of transmission shown with familial forms in about 25% of patients.5,7 This has led many to believe that screening of first-degree relatives of clinically affected patients may be appropriate.
The prevalence of LVNC in adults referred for echocardiography is about 0.014% to 1.3%.5 A recent increase in the rate of recognition has raised concerns of possible overdiagnosis, with attempts now made to develop specific imaging diagnostic criteria. Diagnosis of LVNC is most commonly suspected (but can be missed) on echocardiography using 2-D and color Doppler imaging modalities. Echocardiographic findings supporting the diagnosis of LVNC suggested by Oechslin and colleague include:
• Presence of multiple trabeculations, particularly in the LV apex and free wall;
• Multiple deep trabeculation recesses in communication with the LV cavity, usually seen on color Doppler imaging;
• A 2-layered structure of the endomyocardium with ratio of end systolic, noncompacted endocardial layer to compacted epicardial layer > 2 in adults; and
• Absence of other congenital or acquired heart disease, particularly those causing LV outflow obstruction.8
Another proposed standardized method for identifying LVNC via echocardiography by Chin and colleagues focuses on trabeculae at the LV apex on the parasternal short axis and apical views.2,3 LVNC is defined by a ratio of X/Y of ≤ 0.5, where X is the distance from the epicardial surface to the trough of the trabecular recess, and Y is the distance from the epicardial surface to the peak of the trabeculations.
Cardiac MRI is now a more common mode of imaging used for diagnosis of LVNC and often has better imaging characteristics than those of echocardiography. Using a ratio of noncompacted to compacted CM in diastole > 2.3 is suggestive of LVNC with sensitivity and specificity of 86% and 99%.9
The management of LVNC focuses primarily on treatment of complications, including heart failure, rhythm disturbances, and thromboembolic events. Treatment of heart failure is typically the same as for other CMs and includes medical therapy with salt restriction, diuretics, beta-blockers, and ACE inhibitors. In addition, exercise training, as tolerated, is beneficial to improve clinical status.3,10 Electrophysiology studies are often performed in these patients, and implantation of an AICD is typically done in cases of documented, sustained VT, presyncope with inducible VT or severally depressed ejection fraction of < 35%.4,10 Deep intertrabecular recesses and impaired blood flow increase the risk of thrombus formation. Hence, anticoagulation with warfarin (international normalized ratio target 2.3) for those with an impaired LV ejection fraction (< 40%) should be considered for the prevention of cardioembolic events.3,4,6,10
Summary
An active-duty solider with a history of battlefield trauma and multiple hospitalizations was admitted for symptomatic heart failure with cardiac MRI suggestive of LVNC. This condition is a phenotypic result of genetic heterogeneity with significant variability in clinical presentation and a predisposition for heart failure, ventricular arrhythmias, and systemic embolic events. The etiology of this patient’s clinical presentation remains unclear, and additional research is needed to understand whether his recent trauma and multiple hospitalizations played a role in the manifestation of his disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Left ventricular noncompaction (LVNC) is a rare disorder that is variably classified as a primary genetic cardiomyopathy (CM) by the American Heart Association.1 It is mostly believed to be a congenital abnormality, characterized by the arrest of the typical embryonic myocardial maturation process with the subsequent retention of the trabecular myocardial structure, which defines the early embryonic heart.2
During very early embryonic development, the left ventricular (LV)myocardium is composed of a loose network of fibers separated by deep recesses, which link it with the LV cavity. At 8 weeks of prenatal development, gradual compaction of these fibers occurs, and LVNC is thought to result from the arrest of this normal process.2,3 Significant variability in myocardial involvement exists, ranging from panventricular to isolated apical involvement, likely related to time of arrest of this maturation process.4 The decreased contractile capability and inadequate epicardial coronary system communication of this trabecular endocardium is thought to lead to the clinical manifestations of LVNC.1-7
This report describes the case of a 45-year-old male soldier who presented with a unique case of heart failure, diagnosed via cardiac magnetic resonance imaging (MRI).
Case Study
The patient presented to the San Antonio Military Medical Center emergency department in mid-2011 with increasing dyspnea for several weeks. He also reported significant lower-extremity and scrotal edema. Although the patient had been previously healthy, his recent medical history was remarkable for a severe combat injury suffered while on duty with the U.S. Army in Afghanistan: He was involved in an explosion from an improvised explosive device in August 2009. He was medically evacuated to the U.S., where he required multiple hospitalizations and surgeries. Prior to his current presentation, the patient had been briefly hospitalized for hospital-acquired pneumonia. During this hospitalization, he first noted abnormal swelling of his legs, a finding that was initially attributed to the large sodium load he had received with his IV antibiotics.
DIAGNOSIS
The patient’s vital signs on presentation were notable for 100/83 mm Hg blood pressure, 103 beats per minute (bpm) heart rate, and 18/min respiratory rate with a saturation of 100% on 4 liters of oxygen by nasal cannula. He was conversant but tachypneic and had to pause frequently to catch his breath. His neck veins were notably distended with jugular venous pulsations visible to the angle of the jaw with the patient at 30 degrees. His heart sounds were normal without an S3, but his lungs were notable for bilateral crackles over the lower- to mid-lung fields. He had profound bilateral upper and lower extremity and scrotal pitting edema. He had no lymphadenopathy or skin rashes.
On presentation, the patient’s laboratory results were remarkable for a 444 pg/mL brain natriuretic peptide. A chest X-ray revealed bilateral basilar opacities. An electrocardiogram showed normal sinus rhythm (70 bpm), with normal axis and poor R-wave progression across the precordium. An echocardiogram was performed and notable for a moderately dilated left ventricle with severely depressed systolic function of 10% to 15%, and elevated pulmonary artery pressures. Subsequently, the patient was referred for a coronary angiography, which showed no evidence of coronary atherosclerosis. A cardiac MRI was then performed to evaluate for nonischemic CM, which revealed prominent trabeculations in both ventricles, but most notably in the left ventricle, consistent with a diagnosis of LVNC.
The patient was treated with diuretics, beta-blockers, and an angiotensin-converting enzyme (ACE) inhibitor with improvement in his heart failure symptoms. He was started on systemic anticoagulation with warfarin for his severely depressed LV function. His hospital course was complicated by frequent, nonsustained ventricular tachycardia (VT), and he was referred to the electrophysiology service for implantation of an automated intracardiac cardioverter/defibrillator (AICD) for primary prevention of sudden cardiac death. His clinical course was otherwise unremarkable, and he was discharged after 8 days with complete resolution of his symptoms.
Discussion
The clinical presentation of LVNC is typically due to complications of ventricular dysfunction, including heart failure, arrhythmias, and cardioembolic events. Retrospective studies have shown much variability in the frequency of these complications, likely due to selection bias in earlier studies. These earlier studies had suggested a frequency of heart failure > 50%, but recent studies have shown a more modest frequency of 30% to 35% of affected patients.
Even greater variance has been found in the frequency of arrhythmias, but most studies have shown a frequency of at least 20% for VT. Poor blood flow in the deep intertrabecular recesses in patients with LVNC is additionally thought to lead to a predisposition for mural thrombus formation with an elevated frequency of systemic embolic events, ranging from 5% to 20% among previous studies.1-4,6,8
Much debate remains regarding the genetic association of this condition. The unique character of the resulting myocardium suggests a distinct CM, but the significant genetic heterogeneity with sarcomere protein gene mutations associated with several other CMs, including hypertrophic and dilated CM, suggests that LVNC may simply exist on a phenotypic continuum with these other conditions.4 Inheritance shows additional similarities to these other known CMs with autosomal-
dominant and X-linked modes of transmission shown with familial forms in about 25% of patients.5,7 This has led many to believe that screening of first-degree relatives of clinically affected patients may be appropriate.
The prevalence of LVNC in adults referred for echocardiography is about 0.014% to 1.3%.5 A recent increase in the rate of recognition has raised concerns of possible overdiagnosis, with attempts now made to develop specific imaging diagnostic criteria. Diagnosis of LVNC is most commonly suspected (but can be missed) on echocardiography using 2-D and color Doppler imaging modalities. Echocardiographic findings supporting the diagnosis of LVNC suggested by Oechslin and colleague include:
• Presence of multiple trabeculations, particularly in the LV apex and free wall;
• Multiple deep trabeculation recesses in communication with the LV cavity, usually seen on color Doppler imaging;
• A 2-layered structure of the endomyocardium with ratio of end systolic, noncompacted endocardial layer to compacted epicardial layer > 2 in adults; and
• Absence of other congenital or acquired heart disease, particularly those causing LV outflow obstruction.8
Another proposed standardized method for identifying LVNC via echocardiography by Chin and colleagues focuses on trabeculae at the LV apex on the parasternal short axis and apical views.2,3 LVNC is defined by a ratio of X/Y of ≤ 0.5, where X is the distance from the epicardial surface to the trough of the trabecular recess, and Y is the distance from the epicardial surface to the peak of the trabeculations.
Cardiac MRI is now a more common mode of imaging used for diagnosis of LVNC and often has better imaging characteristics than those of echocardiography. Using a ratio of noncompacted to compacted CM in diastole > 2.3 is suggestive of LVNC with sensitivity and specificity of 86% and 99%.9
The management of LVNC focuses primarily on treatment of complications, including heart failure, rhythm disturbances, and thromboembolic events. Treatment of heart failure is typically the same as for other CMs and includes medical therapy with salt restriction, diuretics, beta-blockers, and ACE inhibitors. In addition, exercise training, as tolerated, is beneficial to improve clinical status.3,10 Electrophysiology studies are often performed in these patients, and implantation of an AICD is typically done in cases of documented, sustained VT, presyncope with inducible VT or severally depressed ejection fraction of < 35%.4,10 Deep intertrabecular recesses and impaired blood flow increase the risk of thrombus formation. Hence, anticoagulation with warfarin (international normalized ratio target 2.3) for those with an impaired LV ejection fraction (< 40%) should be considered for the prevention of cardioembolic events.3,4,6,10
Summary
An active-duty solider with a history of battlefield trauma and multiple hospitalizations was admitted for symptomatic heart failure with cardiac MRI suggestive of LVNC. This condition is a phenotypic result of genetic heterogeneity with significant variability in clinical presentation and a predisposition for heart failure, ventricular arrhythmias, and systemic embolic events. The etiology of this patient’s clinical presentation remains unclear, and additional research is needed to understand whether his recent trauma and multiple hospitalizations played a role in the manifestation of his disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
2. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507-513.
3. Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187-192.
4. Oechslin E, Jenni R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32(12):1446-1456.
5. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270-276.
6. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Longterm follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493-500.
7. Spirito P, Autore C. Apical hypertrophic cardiomyopathy or left ventricular non-compaction? A difficult differential diagnosis [editorial]. Eur Heart J. 2007;28(16):1923-1924.
8. Oechslin E, Jenni R. Non-compaction of the left ventricular myocardium—From clinical observation to the discovery of a new disease. Eur Cardiol Review. 2005;1(1):23-24.
9. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101-105.
10. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;53(15):e1-e90.
1. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
2. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507-513.
3. Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187-192.
4. Oechslin E, Jenni R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32(12):1446-1456.
5. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270-276.
6. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Longterm follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493-500.
7. Spirito P, Autore C. Apical hypertrophic cardiomyopathy or left ventricular non-compaction? A difficult differential diagnosis [editorial]. Eur Heart J. 2007;28(16):1923-1924.
8. Oechslin E, Jenni R. Non-compaction of the left ventricular myocardium—From clinical observation to the discovery of a new disease. Eur Cardiol Review. 2005;1(1):23-24.
9. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101-105.
10. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology Foundation; American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;53(15):e1-e90.