User login
Rapidly Enlarging Neoplasm on the Face
The Diagnosis: Atypical Fibroxanthoma
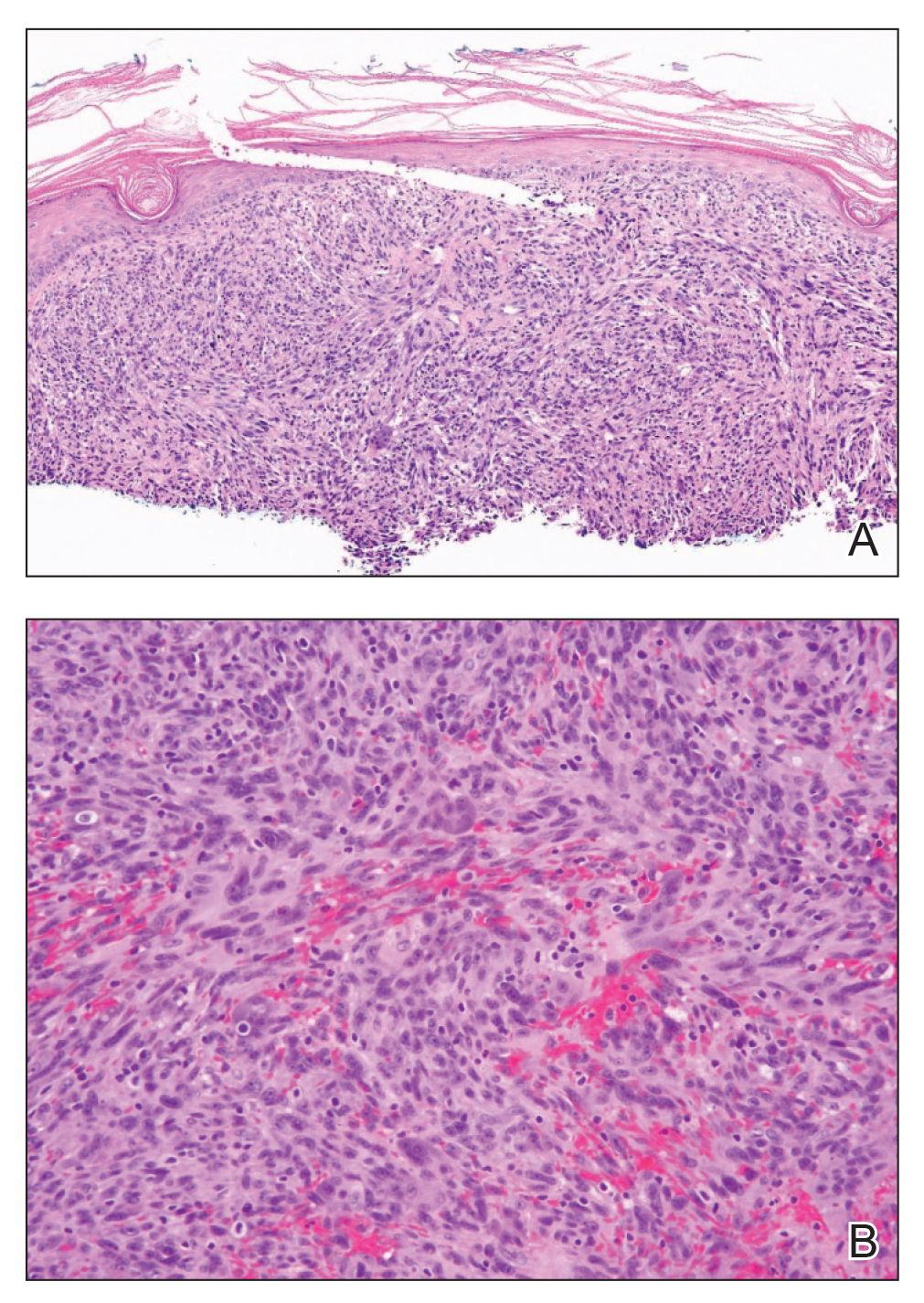
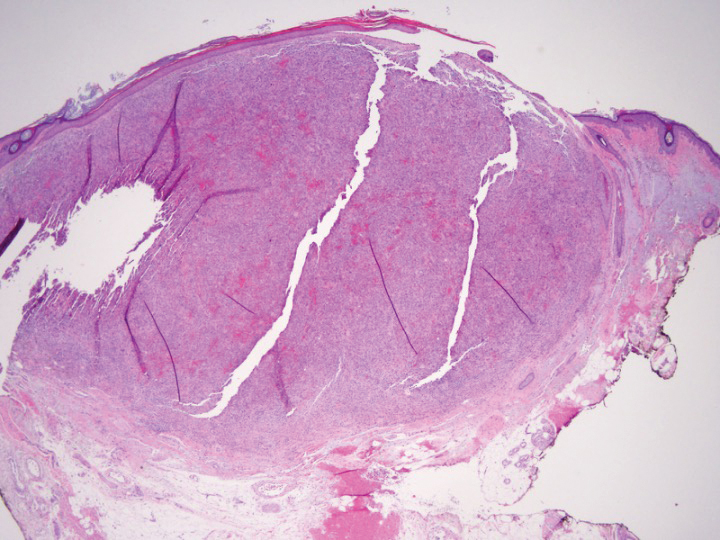
Shave biopsy showed the superficial aspect of a highly cellular tumor composed of pleomorphic spindle cells exhibiting storiform growth and increased mitotic activity (Figure 1). The tumor stained positive for factor XIIIa, CD163, CD68, and smooth muscle actin (mild), and negative for high-molecular-weight cytokeratin (HMW-CK), p63, S-100, and melan-A. Subsequent excision with 0.5-cm margins was performed, and histopathology showed a well-circumscribed tumor contained within the dermis with a histologic scar at the outer margin (Figure 2). There was no lymphovascular or perineural invasion by tumor cells. Re-excision with 0.3-cm margins demonstrated no residual scar or tumor, and external radiation was deferred due to clear surgical margins.
Atypical fibroxanthoma (AFX) belongs to a group of spindle cell neoplasms that can be diagnostically challenging, as they often lack specific morphologic features on examination or routine histology. These neoplasms--of which the differential includes malignant fibrous histiocytoma, spindle cell squamous cell carcinoma (SCC), desmoplastic melanoma, and leiomyosarcoma--may each appear as a rapidly enlarging solitary plaque or nodule on sun-damaged skin on the head and neck or less commonly on the trunk, arms, or legs. Histologically, the cells of AFX exhibit notable pleomorphism with frequent atypical mitotic figures and nonspecific surrounding dermal changes. Subcutaneous and lymphovascular or perineural invasion of tumor cells can point away from the diagnosis of AFX; however, these features are likely to be missed in small superficial shave biopsies.1,2 Therefore, immunohistochemistry (IHC) and adequate tumor sampling are essential in the accurate diagnosis of AFX and other spindle cell neoplasms.
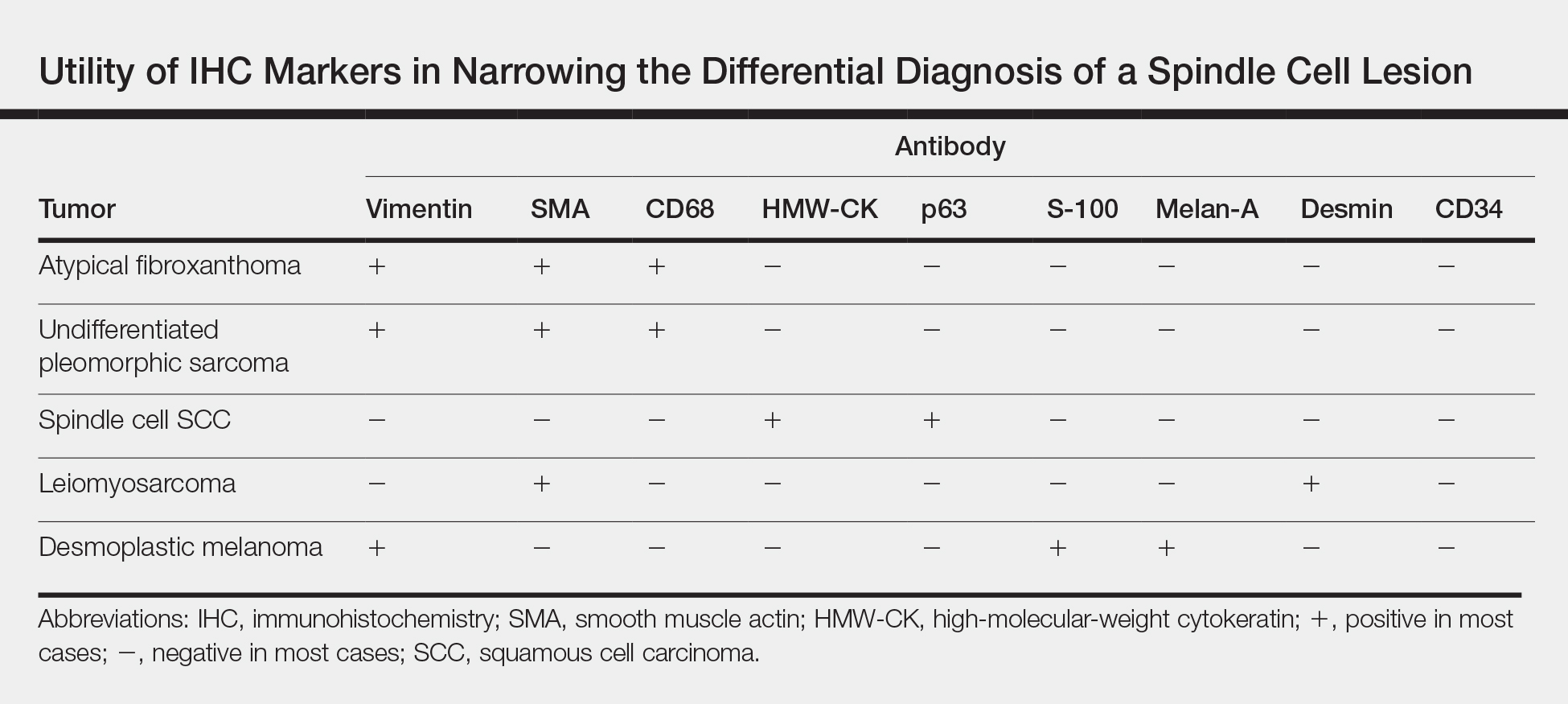
Several IHC markers have been employed in differentiating AFX from other spindle cell neoplasms.3-8 Positive stains for AFX include factor XIIIa (10%-25%), vimentin (>99%), CD10 (95%-100%), procollagen (87%), CD99 (35%-73%), CD163 (37%-79%), smooth muscle actin (50%), CD68 (>50%), and CD31 (43%). Other stains, such as HMW-CK, S-100, p63, desmin, CD34, and melan-A, typically are negative in AFX but are actively expressed in other pleomorphic spindle cell tumors. The Table summarizes the utility of these various markers in narrowing the differential diagnosis of a spindle cell lesion. Selection of an appropriate panel of IHC markers is critical for accurate diagnosis of AFX and exclusion of more aggressive, poorly differentiated spindle cell neoplasms. Key IHC markers include S-100 (negative in AFX; positive in desmoplastic melanoma), HMW-CK (negative in AFX; positive in spindle cell SCC), and p63 (negative in AFX; positive in spindle cell SCC). Benoit et al9 reported a case of a poorly differentiated spindle cell SCC misdiagnosed as AFX based on a limited IHC panel that was negative for pancytokeratin and S-100. Later, a more comprehensive IHC panel including HMW-CK and p63 confirmed spindle cell SCC, but by this time, a delay in therapy had allowed the tumor to metastasize, which ultimately proved fatal to the patient.9
In addition to incomplete IHC evaluation, accurate diagnosis of spindle cell tumors also may be obscured by inadequate tumor sampling. The cells of AFX tumors often are well circumscribed and dermally based, and an excisional biopsy is the preferred biopsy procedure for AFX. A tumor invading into subcutaneous tissue or into lymphovascular or perineural structures suggests a more aggressive, poorly differentiated spindle cell neoplasm.1,3 For example, the tumor cells of malignant fibrous histiocytoma, which belongs to the undifferentiated pleomorphic sarcoma group, may appear identical to those of AFX on histology, and the 2 tumors display similar IHC profiles.3 Malignant fibrous histiocytoma, however, extends into the subcutaneous space and portends a notably worse prognosis compared to AFX. Malignant fibrous histiocytoma tumors therefore require more aggressive treatment strategies such as external beam radiation therapy, whereas AFX can be safely treated with surgical removal alone. In our patient, complete visualization of tumor margins solidified the diagnosis of AFX and spared our patient from unnecessary radiation therapy. Overall, AFX has a good prognosis and metastasis is rare, particularly when good margin control is achieved.10
Our case highlights the importance of clinicopathologic correlation, including appropriate IHC analysis and adequate tumor sampling in the diagnostic workup of a pleomorphic spindle cell neoplasm. Although these tumors are well studied, their notable degree of clinical and histologic heterogeneity may pose a diagnostic challenge to even experienced dermatologists and require careful consideration of the potential pitfalls in diagnosis.
- Iorizzo LJ, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Lopez L, Velez R. Atypical fibroxanthoma. Arch Pathol Lab Med. 2016;140:376-379.
- Hussein MR. Atypical fibroxanthoma: new insights. Expert Rev Anticancer Ther. 2014;14:1075-1088.
- Gleason BC, Calder KB, Cibull TL, et al. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543-547.
- Pouryazdanparast P, Yu L, Cutland JE, et al. Diagnostic value of CD163 in cutaneous spindle cell lesions. J Cutan Pathol. 2009;36:859-864.
- Beer TW. CD163 is not a sensitive marker for identification of atypical fibroxanthoma. J Cutan Pathol. 2012;39:29-32.
- Longacre TA, Smoller BR, Rouse RV. Atypical fibroxanthoma. multiple immunohistologic profiles. Am J Surg Pathol. 1993;17:1199-1209.
- Altman DA, Nickoloff BD, Fivenson DP. Differential expression of factor XIIa and CD34 in cutaneous mesenchymal tumors. J Cutan Pathol. 1993;20:154-158.
- Benoit A, Wisell J, Brown M. Cutaneous spindle cell carcinoma misdiagnosed as atypical fibroxanthoma based on immunohistochemical stains. JAAD Case Rep. 2015;1:392-394.
- New D, Bahrami S, Malone J, et al. Atypical fibroxanthoma with regional lymph node metastasis: report of a case and review of the literature. Arch Dermatol. 2010;146:1399-1404.
The Diagnosis: Atypical Fibroxanthoma
Shave biopsy showed the superficial aspect of a highly cellular tumor composed of pleomorphic spindle cells exhibiting storiform growth and increased mitotic activity (Figure 1). The tumor stained positive for factor XIIIa, CD163, CD68, and smooth muscle actin (mild), and negative for high-molecular-weight cytokeratin (HMW-CK), p63, S-100, and melan-A. Subsequent excision with 0.5-cm margins was performed, and histopathology showed a well-circumscribed tumor contained within the dermis with a histologic scar at the outer margin (Figure 2). There was no lymphovascular or perineural invasion by tumor cells. Re-excision with 0.3-cm margins demonstrated no residual scar or tumor, and external radiation was deferred due to clear surgical margins.
Atypical fibroxanthoma (AFX) belongs to a group of spindle cell neoplasms that can be diagnostically challenging, as they often lack specific morphologic features on examination or routine histology. These neoplasms--of which the differential includes malignant fibrous histiocytoma, spindle cell squamous cell carcinoma (SCC), desmoplastic melanoma, and leiomyosarcoma--may each appear as a rapidly enlarging solitary plaque or nodule on sun-damaged skin on the head and neck or less commonly on the trunk, arms, or legs. Histologically, the cells of AFX exhibit notable pleomorphism with frequent atypical mitotic figures and nonspecific surrounding dermal changes. Subcutaneous and lymphovascular or perineural invasion of tumor cells can point away from the diagnosis of AFX; however, these features are likely to be missed in small superficial shave biopsies.1,2 Therefore, immunohistochemistry (IHC) and adequate tumor sampling are essential in the accurate diagnosis of AFX and other spindle cell neoplasms.
Several IHC markers have been employed in differentiating AFX from other spindle cell neoplasms.3-8 Positive stains for AFX include factor XIIIa (10%-25%), vimentin (>99%), CD10 (95%-100%), procollagen (87%), CD99 (35%-73%), CD163 (37%-79%), smooth muscle actin (50%), CD68 (>50%), and CD31 (43%). Other stains, such as HMW-CK, S-100, p63, desmin, CD34, and melan-A, typically are negative in AFX but are actively expressed in other pleomorphic spindle cell tumors. The Table summarizes the utility of these various markers in narrowing the differential diagnosis of a spindle cell lesion. Selection of an appropriate panel of IHC markers is critical for accurate diagnosis of AFX and exclusion of more aggressive, poorly differentiated spindle cell neoplasms. Key IHC markers include S-100 (negative in AFX; positive in desmoplastic melanoma), HMW-CK (negative in AFX; positive in spindle cell SCC), and p63 (negative in AFX; positive in spindle cell SCC). Benoit et al9 reported a case of a poorly differentiated spindle cell SCC misdiagnosed as AFX based on a limited IHC panel that was negative for pancytokeratin and S-100. Later, a more comprehensive IHC panel including HMW-CK and p63 confirmed spindle cell SCC, but by this time, a delay in therapy had allowed the tumor to metastasize, which ultimately proved fatal to the patient.9
In addition to incomplete IHC evaluation, accurate diagnosis of spindle cell tumors also may be obscured by inadequate tumor sampling. The cells of AFX tumors often are well circumscribed and dermally based, and an excisional biopsy is the preferred biopsy procedure for AFX. A tumor invading into subcutaneous tissue or into lymphovascular or perineural structures suggests a more aggressive, poorly differentiated spindle cell neoplasm.1,3 For example, the tumor cells of malignant fibrous histiocytoma, which belongs to the undifferentiated pleomorphic sarcoma group, may appear identical to those of AFX on histology, and the 2 tumors display similar IHC profiles.3 Malignant fibrous histiocytoma, however, extends into the subcutaneous space and portends a notably worse prognosis compared to AFX. Malignant fibrous histiocytoma tumors therefore require more aggressive treatment strategies such as external beam radiation therapy, whereas AFX can be safely treated with surgical removal alone. In our patient, complete visualization of tumor margins solidified the diagnosis of AFX and spared our patient from unnecessary radiation therapy. Overall, AFX has a good prognosis and metastasis is rare, particularly when good margin control is achieved.10
Our case highlights the importance of clinicopathologic correlation, including appropriate IHC analysis and adequate tumor sampling in the diagnostic workup of a pleomorphic spindle cell neoplasm. Although these tumors are well studied, their notable degree of clinical and histologic heterogeneity may pose a diagnostic challenge to even experienced dermatologists and require careful consideration of the potential pitfalls in diagnosis.
The Diagnosis: Atypical Fibroxanthoma
Shave biopsy showed the superficial aspect of a highly cellular tumor composed of pleomorphic spindle cells exhibiting storiform growth and increased mitotic activity (Figure 1). The tumor stained positive for factor XIIIa, CD163, CD68, and smooth muscle actin (mild), and negative for high-molecular-weight cytokeratin (HMW-CK), p63, S-100, and melan-A. Subsequent excision with 0.5-cm margins was performed, and histopathology showed a well-circumscribed tumor contained within the dermis with a histologic scar at the outer margin (Figure 2). There was no lymphovascular or perineural invasion by tumor cells. Re-excision with 0.3-cm margins demonstrated no residual scar or tumor, and external radiation was deferred due to clear surgical margins.
Atypical fibroxanthoma (AFX) belongs to a group of spindle cell neoplasms that can be diagnostically challenging, as they often lack specific morphologic features on examination or routine histology. These neoplasms--of which the differential includes malignant fibrous histiocytoma, spindle cell squamous cell carcinoma (SCC), desmoplastic melanoma, and leiomyosarcoma--may each appear as a rapidly enlarging solitary plaque or nodule on sun-damaged skin on the head and neck or less commonly on the trunk, arms, or legs. Histologically, the cells of AFX exhibit notable pleomorphism with frequent atypical mitotic figures and nonspecific surrounding dermal changes. Subcutaneous and lymphovascular or perineural invasion of tumor cells can point away from the diagnosis of AFX; however, these features are likely to be missed in small superficial shave biopsies.1,2 Therefore, immunohistochemistry (IHC) and adequate tumor sampling are essential in the accurate diagnosis of AFX and other spindle cell neoplasms.
Several IHC markers have been employed in differentiating AFX from other spindle cell neoplasms.3-8 Positive stains for AFX include factor XIIIa (10%-25%), vimentin (>99%), CD10 (95%-100%), procollagen (87%), CD99 (35%-73%), CD163 (37%-79%), smooth muscle actin (50%), CD68 (>50%), and CD31 (43%). Other stains, such as HMW-CK, S-100, p63, desmin, CD34, and melan-A, typically are negative in AFX but are actively expressed in other pleomorphic spindle cell tumors. The Table summarizes the utility of these various markers in narrowing the differential diagnosis of a spindle cell lesion. Selection of an appropriate panel of IHC markers is critical for accurate diagnosis of AFX and exclusion of more aggressive, poorly differentiated spindle cell neoplasms. Key IHC markers include S-100 (negative in AFX; positive in desmoplastic melanoma), HMW-CK (negative in AFX; positive in spindle cell SCC), and p63 (negative in AFX; positive in spindle cell SCC). Benoit et al9 reported a case of a poorly differentiated spindle cell SCC misdiagnosed as AFX based on a limited IHC panel that was negative for pancytokeratin and S-100. Later, a more comprehensive IHC panel including HMW-CK and p63 confirmed spindle cell SCC, but by this time, a delay in therapy had allowed the tumor to metastasize, which ultimately proved fatal to the patient.9
In addition to incomplete IHC evaluation, accurate diagnosis of spindle cell tumors also may be obscured by inadequate tumor sampling. The cells of AFX tumors often are well circumscribed and dermally based, and an excisional biopsy is the preferred biopsy procedure for AFX. A tumor invading into subcutaneous tissue or into lymphovascular or perineural structures suggests a more aggressive, poorly differentiated spindle cell neoplasm.1,3 For example, the tumor cells of malignant fibrous histiocytoma, which belongs to the undifferentiated pleomorphic sarcoma group, may appear identical to those of AFX on histology, and the 2 tumors display similar IHC profiles.3 Malignant fibrous histiocytoma, however, extends into the subcutaneous space and portends a notably worse prognosis compared to AFX. Malignant fibrous histiocytoma tumors therefore require more aggressive treatment strategies such as external beam radiation therapy, whereas AFX can be safely treated with surgical removal alone. In our patient, complete visualization of tumor margins solidified the diagnosis of AFX and spared our patient from unnecessary radiation therapy. Overall, AFX has a good prognosis and metastasis is rare, particularly when good margin control is achieved.10
Our case highlights the importance of clinicopathologic correlation, including appropriate IHC analysis and adequate tumor sampling in the diagnostic workup of a pleomorphic spindle cell neoplasm. Although these tumors are well studied, their notable degree of clinical and histologic heterogeneity may pose a diagnostic challenge to even experienced dermatologists and require careful consideration of the potential pitfalls in diagnosis.
- Iorizzo LJ, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Lopez L, Velez R. Atypical fibroxanthoma. Arch Pathol Lab Med. 2016;140:376-379.
- Hussein MR. Atypical fibroxanthoma: new insights. Expert Rev Anticancer Ther. 2014;14:1075-1088.
- Gleason BC, Calder KB, Cibull TL, et al. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543-547.
- Pouryazdanparast P, Yu L, Cutland JE, et al. Diagnostic value of CD163 in cutaneous spindle cell lesions. J Cutan Pathol. 2009;36:859-864.
- Beer TW. CD163 is not a sensitive marker for identification of atypical fibroxanthoma. J Cutan Pathol. 2012;39:29-32.
- Longacre TA, Smoller BR, Rouse RV. Atypical fibroxanthoma. multiple immunohistologic profiles. Am J Surg Pathol. 1993;17:1199-1209.
- Altman DA, Nickoloff BD, Fivenson DP. Differential expression of factor XIIa and CD34 in cutaneous mesenchymal tumors. J Cutan Pathol. 1993;20:154-158.
- Benoit A, Wisell J, Brown M. Cutaneous spindle cell carcinoma misdiagnosed as atypical fibroxanthoma based on immunohistochemical stains. JAAD Case Rep. 2015;1:392-394.
- New D, Bahrami S, Malone J, et al. Atypical fibroxanthoma with regional lymph node metastasis: report of a case and review of the literature. Arch Dermatol. 2010;146:1399-1404.
- Iorizzo LJ, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Lopez L, Velez R. Atypical fibroxanthoma. Arch Pathol Lab Med. 2016;140:376-379.
- Hussein MR. Atypical fibroxanthoma: new insights. Expert Rev Anticancer Ther. 2014;14:1075-1088.
- Gleason BC, Calder KB, Cibull TL, et al. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol. 2009;36:543-547.
- Pouryazdanparast P, Yu L, Cutland JE, et al. Diagnostic value of CD163 in cutaneous spindle cell lesions. J Cutan Pathol. 2009;36:859-864.
- Beer TW. CD163 is not a sensitive marker for identification of atypical fibroxanthoma. J Cutan Pathol. 2012;39:29-32.
- Longacre TA, Smoller BR, Rouse RV. Atypical fibroxanthoma. multiple immunohistologic profiles. Am J Surg Pathol. 1993;17:1199-1209.
- Altman DA, Nickoloff BD, Fivenson DP. Differential expression of factor XIIa and CD34 in cutaneous mesenchymal tumors. J Cutan Pathol. 1993;20:154-158.
- Benoit A, Wisell J, Brown M. Cutaneous spindle cell carcinoma misdiagnosed as atypical fibroxanthoma based on immunohistochemical stains. JAAD Case Rep. 2015;1:392-394.
- New D, Bahrami S, Malone J, et al. Atypical fibroxanthoma with regional lymph node metastasis: report of a case and review of the literature. Arch Dermatol. 2010;146:1399-1404.
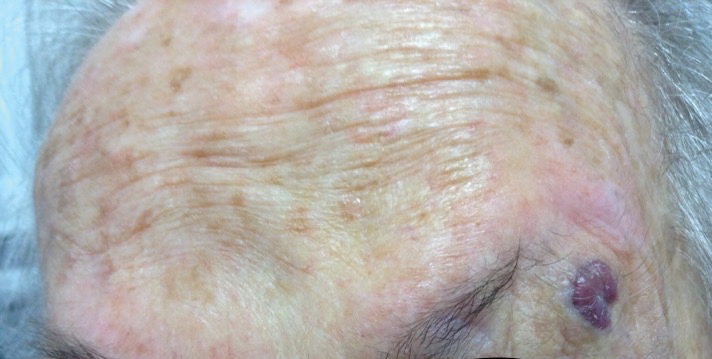
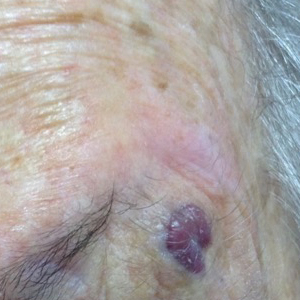
An 88-year-old woman presented for evaluation of an asymptomatic facial lesion that she first noticed 3 months prior, with rapid growth over the last month. Review of systems was negative, and she denied any history of connective tissue disease, skin cancer, or radiation to the head or neck area. Physical examination revealed a 1.5-cm, solitary, violaceous nodule on the left lateral eyebrow on a background of actinically damaged skin. The lesion was nontender and there were no similar lesions or palpable lymphadenopathy.