User login
Does use of continuous or flash glucose monitors decrease hypoglycemia episodes in T2D?
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
EVIDENCE-BASED REVIEW:
NO. In adults with insulin-treated type 2 diabetes (T2D), continuous glucose monitoring (CGM) and flash glucose monitoring (FGM) do not decrease symptomatic hypoglycemia episodes (strength of recommendation [SOR], B) but do lower time in hypoglycemia (SOR, C; disease-oriented evidence).
CGM, in which glucose levels are sent automatically in numeric and graphic format to a patient’s smart device for their potential action, did not change the hypoglycemic event rate (SOR, B; 2 prospective studies). CGM significantly reduced hypoglycemia duration in an 8-month randomized controlled trial (RCT; SOR, C) but not in a 1-year prospective study (SOR, C).
FGM, in which glucose levels are sent on demand to a device, did not significantly reduce hypoglycemic episodes (SOR, B; 1 small RCT and 1 prospective study). Hypoglycemia duration was reduced significantly with FGM in a 6-month RCT (SOR, B) but not in a 1-year prospective study (SOR, B).
What barriers delay treatment in patients with hepatitis C?
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
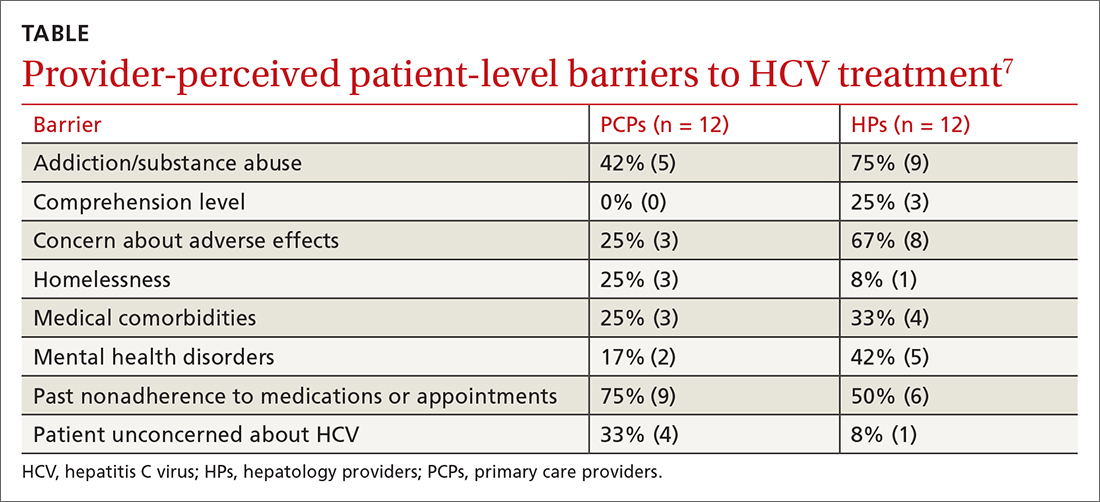
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.
Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE-BASED ANSWER:
Multiple patient-specific and provider-perceived factors delay initiation of treatment in patients with hepatitis C. Patient-specific barriers to initiation of treatment for hepatitis C virus (HCV) include age, race, gender, economic status, insurance status, and comorbidities such as HIV coinfection, psychiatric illness, and other psychosocial factors.
Provider-perceived patient factors include substance abuse history, older age, psychiatric illness, medical comorbidities, treatment adverse effect risks, and factors that might limit adherence (eg, comprehension level).
Study limitations included problems with generalizability of the populations studied and variability in reporting or interpreting data associated with substance or alcohol use disorders
Is the incidence of depressive disorders increased following cerebral concussion?
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
EVIDENCE-BASED ANSWER
Yes, in some populations. Youth and adolescents with self-reported history of concussion had increased risk of depressive disorders (strength of recommendation [SOR]: B, based on a prospective cohort study and a retrospective cohort study). Evidence was inconsistent for college athletes. Athletes with ≥ 3 concussions exhibited more depressive disorders, but no association was observed for those with 1 or 2 concussions compared to nonconcussion injuries (SOR: B, based on a cross-sectional study, a small prospective cohort study, and a case-control study).
In semiprofessional and professional athletes, evidence was variable and may be sport related. Retired rugby players with a history of concussion showed no increase in depression compared to controls with no concussion history (SOR: B, based on a case-control study). Retired football players with previous concussions displayed increased incidence of depression, especially after ≥ 3 concussions (SOR: B, based on a prospective cohort study and a small case-control study).
There is a significant risk of bias in these studies because of their reliance on self-reported concussions, differing definitions of depression, and possible unmeasured confounders in the study designs, making a causative relationship between concussion and depression unclear.