User login
‘Meth’ recovery: 3 steps to successful chronic management
Clinicians could become discouraged when confronting methamphetamine-dependent patients’ wide-ranging psychiatric symptoms.
These patients often present with:
- overlapping primary psychiatric syndromes and secondary substance abuse
- complex histories fraught with psychological trauma, limited social supports, and court involvement.
Treatment can be successful, however, and patients can change their addictive behaviors with a chronic disease management approach that targets the drug’s cognitive sequelae and psychiatric effects. Medications show limited benefit (Box 1),1-8 but behavioral treatments—including cognitive behavioral therapy (CBT) and motivational incentives—have proven efficacy in treating methamphetamine addiction.
This article discusses how to counteract methamphetamine’s negative cognitive effects and enable patients to engage in psychosocial treatment. Our discussion is informed by an extensive literature search and clinical experience from treating patients in the Midwest—at the geographic heart of the “meth” epidemic.
CASE REPORT: Overwhelmed and suicidal
Ms. D, age 27, presents to the emergency department with anxiety, dysphoria, and a plan to commit suicide by overdose. She feels overwhelmed by her 4-hour-a-day customer service job—a prerequisite for staying at the halfway house where she has lived for 2 months. She has a 13-year history of polysubstance dependence and is under court order to complete chemical dependence treatment or go to jail.
No medications are FDA-approved for treating methamphetamine dependence, and evidence supporting medication use in methamphetamine dependence is extremely limited. Research efforts are aimed at finding medications that might be neuroprotective, decrease craving, block reinforcement mechanisms, or affect other factors behind methamphetamine addiction and relapse.1 Most trials have been conducted in animal models or controlled laboratory evaluations of drug effects on methamphetamine-induced states.
Bupropion has shown slight treatment efficacy, possibly by decreasing neuronal damage and blocking reinforcement.2-4 Modafinil5 and baclofen6 may have potential, but evidence is lacking.
Some results have been unexpectedly negative. Sertraline might be contraindicated in methamphetamine dependence treatment, according to results of a randomized, placebo-controlled trial7 of sertraline and contingency management (Table 1). In a human laboratory study,8 topiramate accentuated—rather than diminished—subjective response to methamphetamine (Table 2).
Ms. D began using drugs at age 14 and has 3 convictions for driving under the influence of alcohol. An average student, she dropped out of high school but obtained a GED certificate. She first had psychiatric contact at age 16 and has been diagnosed at various times with attention deficit/hyperactivity disorder, bipolar disorder, and anxiety disorder. She also has been violently sexually assaulted while engaging in prostitution to support her drug habit.
Ms. D has been hospitalized multiple times—voluntarily and involuntarily—in dual diagnosis treatment centers. Her 5-year-old son no longer lives with her, and she has limited social supports beyond her parents, who live in a neighboring state.
Table 1
Antidepressant trials for treating methamphetamine dependence
| Drug | Investigation | Comments |
|---|---|---|
| Bupropion2-4 | Laboratory | Safety of bupropion with MAP |
| Laboratory | Reduced subjective effects and cue-induced craving | |
| Clinical trial | Trend toward reduced MAP use compared with placebo | |
| Sertraline7 | Clinical trial | Sertraline-treated subjects showed higher use of MAP compared with those receiving placebo and were less likely to complete treatment |
| MAP: methamphetamine | ||
3-step approach
For patients such as Ms. D, clinical evidence supports a 3-step approach to treating methamphetamine dependence:
- step 1: institute acute management and stabilization
- step 2: eliminate or decrease methamphetamine use to “move the frontal lobe back to the front”
- step 3: identify and target psychiatric and psychosocial comorbidities.
- help her eliminate or decrease methamphetamine use to allow neuronal systems to recover
- target maladaptive behaviors that hinder sobriety while providing motivational incentives to help her maintain a methamphetamine-free life.
How ‘meth’ affects cognition
Methamphetamine use has been associated with cognitive dysfunction at initial abstinence and even years later in some patients.10 Ms. D’s cognitive limitations in a fast-paced customer service job—even though hours are limited—lead to anxiety, dysphoria, and loss of self-esteem when she can’t manage patrons’ requests.
Methamphetamine has profound acute and chronic effects on the sympathetic nervous system, and dopaminergic, serotonergic, and noradrenergic neuronal networks. Most evidence of chronic neuronal effects comes from animal research and reflects toxic damage to dopaminergic and serotonergic neuronal systems. Postmortem human studies of direct neurotoxicity from chronic methamphetamine exposure show:
- decreased dopamine and tyrosine hydroxylase levels
- reduced concentrations of dopamine transporters.11
In chronic methamphetamine abusers, functional magnetic resonance imaging, proton magnetic resonance spectroscopy, and positron emission tomography show:
- changes in neurotransmitter, protein, brain metabolism, and transporter levels
- damage in multiple brain areas including the frontal region, basal ganglia, grey matter, corpus callosum, and striatum; smaller hippocampi; and cerebral vasculature changes.14-16
CASE CONTINUED: Does she understand?
After Ms. D is stabilized, her case manager expresses concern about her ability to follow through with treatment planning. He says, “I just don’t think she understands some of the things we discuss.” She then is referred for neuropsychological testing, which shows clear cognitive impairment. Specifically, she has a slowed rate of thinking, general cognitive ineficiency, deficits in learning and memory retention, and mild impulsivity.
Patients with a history of extensive methamphetamine abuse are ruled by the limbic system and may have higher cortical damage that complicates initiating, maintaining, and fully participating in treatment. Patients’ deficits in memory, executive functioning, attention, and cognitive speed may require you to simplify, repeat, and otherwise modify your treatment plan. You will need to provide clear instructions and consistent support—individually and psychosocially—and to recognize and reinforce patients’ treatment gains.
Even before using methamphetamine, patients may have had academic problems or learning disabilities that will compromise their ability to participate in treatment. Infection with HIV, syphilis, or hepatitis C can further hamper cognitive function.18
What treatments are effective?
Medications. Evidence is extremely limited, and no medications are approved to treat methamphetamine-addicted patients. Bupropion has shown some efficacy (Table 1),2-4,7 but other drugs such as sertraline and topiramate may aggravate rather than diminish methamphetamine dependence (Table 2).5,6,8,19
Behavioral treatments supply the evidence basis for methamphetamine dependence treatment. Cognitive behavioral therapy (CBT),20 contingency management (CM),21,22 and a manualized structured treatment—the Matrix Model23—all have proven efficacy.
CBT involves functional analysis and skills training. Patients are guided through analyzing their drug use and associated cognitions, emotions, and expectations and in identifying situations that trigger methamphetamine use or relapse. Skills training involves identifying, reinforcing, and practicing coping skills to help the patient avoid drug use and reinforce the ability to refuse use.
CM is based on operant conditioning—the use of consequences to modify behavior. It involves establishing a “contingent” relationship between a desired behavior/outcome (such as methamphetamine-free urinalysis) and delivering a positive reinforcing event to promote abstinence:
- Vouchers, privileges, or small amounts of money linked to healthy behaviors serve as incentives for negative urine testing.
- Rewards increase as periods of confirmed abstinence lengthen and are reset to smaller rewards if relapse occurs.
CM does not require extensive staff training and has been described as relatively simple to implement. CM also has been used successfully in urban gay and bisexual men with methamphetamine dependence (Box 2).18,25-29
Although CM’s efficacy is well-supported by clinical trials, we have encountered some resistance to the idea of “paying individuals to not use drugs” when training medical students, allied health staff, and residents. The National Institute on Drug Abuse (NIDA) supports the use of motivational incentives in treating substance abuse and offers support materials, resources, and training on this approach (see Related Resources).
Multiple studies show that CBT and CM are equally effective for treating chronic methamphetamine abuse at a 1-year follow-up, although CM may be more effective than CBT for acute treatment.
The Matrix model is a 4-month intensive, manualized treatment program that uses CBT, education on drug effects, positive reinforcement for intended behavioral change, and a 12-step approach.
Methamphetamine dependence outcomes based on the Matrix treatment model were compared with community treatment as usual in a project sponsored by The Center for Substance Abuse Treatment of the Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services.30 End-point outcomes were similar, but the Matrix treatment was more effective in early treatment, including decreased urinalyses positive for methamphetamine and increased abstinence.
Methamphetamine use is estimated to be 5 to 10 times more prevalent in U.S. urban gay and bisexual groups than in the general population25 and likely is contributing to rising human immunodeficiency virus (HIV) infection rates in men having sex with men (MSM).
Used to enhance sexual performance, libido, and mood, methamphetamine is associated with increased rates of unprotected anal sex and multiple partners in MSM.26 An HIV infection rate of 61% was reported in methamphetamine-dependent MSM seeking treatment in a Los Angeles clinical trial.27 Methamphetamine also results in high-risk sexual practices and multiple partners among heterosexual men and women.28
Although seroconverted men report using methamphetamine to alleviate HIV-associated depression, the combination of HIV infection and methamphetamine use may have powerful negative effects. Methamphetamine use is associated with HIV treatment nonadherence and also may suppress immune function.29 Cognitive impairments associated with HIV and methamphetamine use are additive and are further exacerbated by hepatitis C infection.18
Recommendation. Screen for methamphetamine use in MSM populations, and educate these patients about risks associated with methamphetamine use. In all patient groups who report using methamphetamine, provide counseling on high-risk sexual behavior, screen for sexually transmitted diseases, and ensure that patients are vaccinated against hepatitis A and B infection (see Related Resources). Most important, refer for medical treatment when indicated.
In patients such as Ms. D, the structure of court-ordered treatment can provide accountability, enforced abstinence, and mandated treatment resources. This, in turn, may give your patient a better chance to engage a recovering and better functioning frontal lobe to inhibit urges for methamphetamine use and manage stress.
Table 2
Other agents studied in methamphetamine dependence trials
| Drug | Investigation | Comment |
|---|---|---|
| Baclofen6 (GABAergic) | Clinical trial | No statistically significant effect compared with placebo; post hoc analysis showed ‘small’ treatment effects vs placebo |
| Gabapentin6 (GABAergic) | Clinical trial | No statistically signicant effect compared with placebo; post hoc analysis showed no treatment effects vs placebo |
| Topiramate8 (anticonvulsant) | Laboratory | Accentuated (rather than diminished) subjective effects of MAP |
| Aripiprazole19 (SGA) | Laboratory | Decreased subjective effects of amphetamine |
| Modafinil5 (wakefulness agent) | Clinical trial | Successful trial in cocaine dependence; potential option for MAP |
| MAP: methamphetamine; SGA: second-generation antipsychotic | ||
CASE CONTINUED: Racing thoughts and psychosis
Before hospital admission, Ms. D was being treated with gabapentin, 300 mg bid, and sustained-release bupropion, 150 mg/d, for anxiety and dysphoria. Previously, she has received multiple antidepressants and mood stabilizers with reportedly little effect.
Initially guarded, she at first denies psychotic symptoms but acknowledges their extent several days later. She describes periods of 6 months or more when she feels “lost.” The treatment team titrates quetiapine up to 200 mg/d and restarts duloxetine, 30 mg/d, for depressive symptoms, based on her past positive response to this antidepressant.
Methamphetamine abuse can cause and exacerbate psychiatric symptoms. Keep in mind 2 priorities as you approach these symptoms:
Aim for abstinence. Methamphetamine abuse produces a remarkable array of adverse effects. It causes dysphoria, anxiety, and psychosis during active use and in the interval after initial abstinence. Many of methamphetamine’s use and withdrawal symptoms resolve with time, however, and may not require pharmacologic treatment.31 Therefore, achieving abstinence and keeping patients in treatment is high priority.
Use behavioral approaches whenever feasible. Balance the need to use benzodiazepines for ongoing treatment of severe anxiety or agitation with the high risk of addiction or diversion in this group. Anxiety may resolve over time in association with sustained abstinence. Similarly, receiving treatment for methamphetamine dependence and maintaining abstinence appears to ease depressive symptoms, as shown by sustained improvements in Beck Depression Inventory scores at 1 year.32
Manage stress. Stress can worsen psychiatric symptoms, trigger methamphetamine abuse relapse and psychosis, and acutely and chronically augment methamphetamine’s toxic effects.33 You can help patients manage stress by:
- providing case management and CBT training
- advising them about proper sleep, nutrition, and medical care.
Targeting psychiatric symptoms
Step 3 in the chronic disease management approach to methamphetamine dependence is to identify and target psychiatric and psychosocial comorbidities. When approaching psychiatric symptoms, high priorities are to aim for abstinence and manage the patient’s stress (Box 3).31-33
In clinical practice, we find it difficult to diagnostically categorize and treat methamphetamine-abusing patients who show residual post-acute psychotic symptoms. Some appear to have no risk factors for primary psychotic illness, and their symptoms show an association with the severity of their past methamphetamine abuse.
Other patient presentations can be difficult to separate from family histories of psychotic illness. Research suggests that genetic risk factors may be associated with methamphetamine psychosis in some vulnerable patients.35
Unfortunately, no data exist to guide the use of antipsychotics to maintain symptom control. Some patients may need low-dose antipsychotics for maintenance treatment, and second-generation antipsychotics may have a theoretical advantage over first-generation antipsychotics. Use your clinical judgment in determining dosing and treatment duration, and in weighing risks and benefits of continued treatment.
Using imaging, researchers found aggression severity to be directly correlated with past total methamphetamine use and globally decreased serotonin transporter density.36 Serotonin transporter densities were 30% lower in methamphetamine users vs controls after >1 year of abstinence.
CASE CONTINUED: Discharge plans
Because of the severity of her psychiatric symptoms, Ms. D is unable to return to the halfway house after discharge. As her treatment team works to coordinate discharge placement, Ms. D continues to improve. Her psychotic and dysphoria symptoms resolve, and she shows increased spontaneity. These changes—attributed to supports during hospitalization, decreased stressors, and quetiapine treatment—continue until her discharge to a combined mental illness and chemical dependence program.
- Methamphetamine use and sexually transmitted diseases. Centers for Disease Control and Prevention. www.cdc.gov/std/DearColleagueRiskBehaviorMetUse8-18-2006.pdf.
- National Institute on Drug Abuse Blending Initiative. Promoting Awareness of Motivational Incentives (PAMI). www.drugabuse.gov/blending/PAMI.html.
- Aripiprazole • Abilify
- Baclofen • various
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Gabapentin • Neurontin
- Modafinil • Provigil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Topiramate • Topamax
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry 2005;162:1432-4.
2. Newton TF, Roache JD, De La Garza R, 2nd, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426-35.
3. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology 2006;31:1537-44.
4. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep 2006;8:345-54.
5. Umanoff DF. Trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005;30:2298; author reply 2299-300.
6. Heinzerling KG, Shoptaw S, Peck JA, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:177-84.
7. Shoptaw S, Huber A, Peck J, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:12-8.
8. Johnson BA, Roache JD, Ait-Daoud N, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol 2007;10:85-98.
9. Bostwick J, Lineberry T. The ‘meth’ epidemic: Managing acute psychosis, agitation, and suicide risk. Current Psychiatry 2006;5(11):46-62.
10. Simon SL, Dacey J, Glynn S, et al. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 2004;27:59-66.
11. Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996;2:699-703.
12. Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004;127:363-70.
13. Armstrong BD, Noguchi KK. The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABAergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 2004;25:905-14.
14. London ED, Berman SM, Voytek B, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry 2005;58:770-8.
15. London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 2004;61:73-84.
16. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15:317-25.
17. Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 2004;161:242-8.
18. Cherner M, Letendre S, Heaton RK, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 2005;64:1343-7.
19. Stoops WW. Aripiprazole as a potential pharmacotherapy for stimulant dependence: human laboratory studies with damphetamine. Exp Clin Psychopharmacol 2006;14:413-21.
20. Yen CF, Wu HY, Yen JY, Ko CH. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J Nerv Ment Dis 2004;192:788-91.
21. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry 2006;163:1993-9.
22. Shoptaw S, Klausner JD, Reback CJ, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health 2006;6:214.-
23. Shoptaw S, Rawson RA, McCann MJ, Obert JL. The Matrix model of outpatient stimulant abuse treatment: evidence of efficacy. J Addict Dis 1994;13:129-41.
24. Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction 2007;102:309-16.
25. Shoptaw S. Methamphetamine use in urban gay and bisexual populations. Top HIV Med 2006;14:84-7.
26. Bolding G, Hart G, Sherr L, Elford J. Use of crystal methamphetamine among gay men in London. Addiction 2006;101:1622-30.
27. Peck JA, Shoptaw S, Rotheram-Fuller E, et al. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. J Addict Dis 2005;24:115-32.
28. Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav 2004;29:807-10.
29. Mahajan SD, Hu Z, Reynolds JL, et al. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther 2006;10:257-69.
30. Rawson RA, Marinelli-Casey P, Anglin MD, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 2004;99:708-17.
31. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100:1320-9.
32. Peck JA, Reback CJ, Yang X, et al. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health 2005;82:i100-8.
33. Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience 2004;124:637-46.
34. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13:461-70.
35. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatr Genet 2006;16:133-8.
36. Sekine Y, Ouchi Y, Takei N, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 2006;63:90-100.
Clinicians could become discouraged when confronting methamphetamine-dependent patients’ wide-ranging psychiatric symptoms.
These patients often present with:
- overlapping primary psychiatric syndromes and secondary substance abuse
- complex histories fraught with psychological trauma, limited social supports, and court involvement.
Treatment can be successful, however, and patients can change their addictive behaviors with a chronic disease management approach that targets the drug’s cognitive sequelae and psychiatric effects. Medications show limited benefit (Box 1),1-8 but behavioral treatments—including cognitive behavioral therapy (CBT) and motivational incentives—have proven efficacy in treating methamphetamine addiction.
This article discusses how to counteract methamphetamine’s negative cognitive effects and enable patients to engage in psychosocial treatment. Our discussion is informed by an extensive literature search and clinical experience from treating patients in the Midwest—at the geographic heart of the “meth” epidemic.
CASE REPORT: Overwhelmed and suicidal
Ms. D, age 27, presents to the emergency department with anxiety, dysphoria, and a plan to commit suicide by overdose. She feels overwhelmed by her 4-hour-a-day customer service job—a prerequisite for staying at the halfway house where she has lived for 2 months. She has a 13-year history of polysubstance dependence and is under court order to complete chemical dependence treatment or go to jail.
No medications are FDA-approved for treating methamphetamine dependence, and evidence supporting medication use in methamphetamine dependence is extremely limited. Research efforts are aimed at finding medications that might be neuroprotective, decrease craving, block reinforcement mechanisms, or affect other factors behind methamphetamine addiction and relapse.1 Most trials have been conducted in animal models or controlled laboratory evaluations of drug effects on methamphetamine-induced states.
Bupropion has shown slight treatment efficacy, possibly by decreasing neuronal damage and blocking reinforcement.2-4 Modafinil5 and baclofen6 may have potential, but evidence is lacking.
Some results have been unexpectedly negative. Sertraline might be contraindicated in methamphetamine dependence treatment, according to results of a randomized, placebo-controlled trial7 of sertraline and contingency management (Table 1). In a human laboratory study,8 topiramate accentuated—rather than diminished—subjective response to methamphetamine (Table 2).
Ms. D began using drugs at age 14 and has 3 convictions for driving under the influence of alcohol. An average student, she dropped out of high school but obtained a GED certificate. She first had psychiatric contact at age 16 and has been diagnosed at various times with attention deficit/hyperactivity disorder, bipolar disorder, and anxiety disorder. She also has been violently sexually assaulted while engaging in prostitution to support her drug habit.
Ms. D has been hospitalized multiple times—voluntarily and involuntarily—in dual diagnosis treatment centers. Her 5-year-old son no longer lives with her, and she has limited social supports beyond her parents, who live in a neighboring state.
Table 1
Antidepressant trials for treating methamphetamine dependence
| Drug | Investigation | Comments |
|---|---|---|
| Bupropion2-4 | Laboratory | Safety of bupropion with MAP |
| Laboratory | Reduced subjective effects and cue-induced craving | |
| Clinical trial | Trend toward reduced MAP use compared with placebo | |
| Sertraline7 | Clinical trial | Sertraline-treated subjects showed higher use of MAP compared with those receiving placebo and were less likely to complete treatment |
| MAP: methamphetamine | ||
3-step approach
For patients such as Ms. D, clinical evidence supports a 3-step approach to treating methamphetamine dependence:
- step 1: institute acute management and stabilization
- step 2: eliminate or decrease methamphetamine use to “move the frontal lobe back to the front”
- step 3: identify and target psychiatric and psychosocial comorbidities.
- help her eliminate or decrease methamphetamine use to allow neuronal systems to recover
- target maladaptive behaviors that hinder sobriety while providing motivational incentives to help her maintain a methamphetamine-free life.
How ‘meth’ affects cognition
Methamphetamine use has been associated with cognitive dysfunction at initial abstinence and even years later in some patients.10 Ms. D’s cognitive limitations in a fast-paced customer service job—even though hours are limited—lead to anxiety, dysphoria, and loss of self-esteem when she can’t manage patrons’ requests.
Methamphetamine has profound acute and chronic effects on the sympathetic nervous system, and dopaminergic, serotonergic, and noradrenergic neuronal networks. Most evidence of chronic neuronal effects comes from animal research and reflects toxic damage to dopaminergic and serotonergic neuronal systems. Postmortem human studies of direct neurotoxicity from chronic methamphetamine exposure show:
- decreased dopamine and tyrosine hydroxylase levels
- reduced concentrations of dopamine transporters.11
In chronic methamphetamine abusers, functional magnetic resonance imaging, proton magnetic resonance spectroscopy, and positron emission tomography show:
- changes in neurotransmitter, protein, brain metabolism, and transporter levels
- damage in multiple brain areas including the frontal region, basal ganglia, grey matter, corpus callosum, and striatum; smaller hippocampi; and cerebral vasculature changes.14-16
CASE CONTINUED: Does she understand?
After Ms. D is stabilized, her case manager expresses concern about her ability to follow through with treatment planning. He says, “I just don’t think she understands some of the things we discuss.” She then is referred for neuropsychological testing, which shows clear cognitive impairment. Specifically, she has a slowed rate of thinking, general cognitive ineficiency, deficits in learning and memory retention, and mild impulsivity.
Patients with a history of extensive methamphetamine abuse are ruled by the limbic system and may have higher cortical damage that complicates initiating, maintaining, and fully participating in treatment. Patients’ deficits in memory, executive functioning, attention, and cognitive speed may require you to simplify, repeat, and otherwise modify your treatment plan. You will need to provide clear instructions and consistent support—individually and psychosocially—and to recognize and reinforce patients’ treatment gains.
Even before using methamphetamine, patients may have had academic problems or learning disabilities that will compromise their ability to participate in treatment. Infection with HIV, syphilis, or hepatitis C can further hamper cognitive function.18
What treatments are effective?
Medications. Evidence is extremely limited, and no medications are approved to treat methamphetamine-addicted patients. Bupropion has shown some efficacy (Table 1),2-4,7 but other drugs such as sertraline and topiramate may aggravate rather than diminish methamphetamine dependence (Table 2).5,6,8,19
Behavioral treatments supply the evidence basis for methamphetamine dependence treatment. Cognitive behavioral therapy (CBT),20 contingency management (CM),21,22 and a manualized structured treatment—the Matrix Model23—all have proven efficacy.
CBT involves functional analysis and skills training. Patients are guided through analyzing their drug use and associated cognitions, emotions, and expectations and in identifying situations that trigger methamphetamine use or relapse. Skills training involves identifying, reinforcing, and practicing coping skills to help the patient avoid drug use and reinforce the ability to refuse use.
CM is based on operant conditioning—the use of consequences to modify behavior. It involves establishing a “contingent” relationship between a desired behavior/outcome (such as methamphetamine-free urinalysis) and delivering a positive reinforcing event to promote abstinence:
- Vouchers, privileges, or small amounts of money linked to healthy behaviors serve as incentives for negative urine testing.
- Rewards increase as periods of confirmed abstinence lengthen and are reset to smaller rewards if relapse occurs.
CM does not require extensive staff training and has been described as relatively simple to implement. CM also has been used successfully in urban gay and bisexual men with methamphetamine dependence (Box 2).18,25-29
Although CM’s efficacy is well-supported by clinical trials, we have encountered some resistance to the idea of “paying individuals to not use drugs” when training medical students, allied health staff, and residents. The National Institute on Drug Abuse (NIDA) supports the use of motivational incentives in treating substance abuse and offers support materials, resources, and training on this approach (see Related Resources).
Multiple studies show that CBT and CM are equally effective for treating chronic methamphetamine abuse at a 1-year follow-up, although CM may be more effective than CBT for acute treatment.
The Matrix model is a 4-month intensive, manualized treatment program that uses CBT, education on drug effects, positive reinforcement for intended behavioral change, and a 12-step approach.
Methamphetamine dependence outcomes based on the Matrix treatment model were compared with community treatment as usual in a project sponsored by The Center for Substance Abuse Treatment of the Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services.30 End-point outcomes were similar, but the Matrix treatment was more effective in early treatment, including decreased urinalyses positive for methamphetamine and increased abstinence.
Methamphetamine use is estimated to be 5 to 10 times more prevalent in U.S. urban gay and bisexual groups than in the general population25 and likely is contributing to rising human immunodeficiency virus (HIV) infection rates in men having sex with men (MSM).
Used to enhance sexual performance, libido, and mood, methamphetamine is associated with increased rates of unprotected anal sex and multiple partners in MSM.26 An HIV infection rate of 61% was reported in methamphetamine-dependent MSM seeking treatment in a Los Angeles clinical trial.27 Methamphetamine also results in high-risk sexual practices and multiple partners among heterosexual men and women.28
Although seroconverted men report using methamphetamine to alleviate HIV-associated depression, the combination of HIV infection and methamphetamine use may have powerful negative effects. Methamphetamine use is associated with HIV treatment nonadherence and also may suppress immune function.29 Cognitive impairments associated with HIV and methamphetamine use are additive and are further exacerbated by hepatitis C infection.18
Recommendation. Screen for methamphetamine use in MSM populations, and educate these patients about risks associated with methamphetamine use. In all patient groups who report using methamphetamine, provide counseling on high-risk sexual behavior, screen for sexually transmitted diseases, and ensure that patients are vaccinated against hepatitis A and B infection (see Related Resources). Most important, refer for medical treatment when indicated.
In patients such as Ms. D, the structure of court-ordered treatment can provide accountability, enforced abstinence, and mandated treatment resources. This, in turn, may give your patient a better chance to engage a recovering and better functioning frontal lobe to inhibit urges for methamphetamine use and manage stress.
Table 2
Other agents studied in methamphetamine dependence trials
| Drug | Investigation | Comment |
|---|---|---|
| Baclofen6 (GABAergic) | Clinical trial | No statistically significant effect compared with placebo; post hoc analysis showed ‘small’ treatment effects vs placebo |
| Gabapentin6 (GABAergic) | Clinical trial | No statistically signicant effect compared with placebo; post hoc analysis showed no treatment effects vs placebo |
| Topiramate8 (anticonvulsant) | Laboratory | Accentuated (rather than diminished) subjective effects of MAP |
| Aripiprazole19 (SGA) | Laboratory | Decreased subjective effects of amphetamine |
| Modafinil5 (wakefulness agent) | Clinical trial | Successful trial in cocaine dependence; potential option for MAP |
| MAP: methamphetamine; SGA: second-generation antipsychotic | ||
CASE CONTINUED: Racing thoughts and psychosis
Before hospital admission, Ms. D was being treated with gabapentin, 300 mg bid, and sustained-release bupropion, 150 mg/d, for anxiety and dysphoria. Previously, she has received multiple antidepressants and mood stabilizers with reportedly little effect.
Initially guarded, she at first denies psychotic symptoms but acknowledges their extent several days later. She describes periods of 6 months or more when she feels “lost.” The treatment team titrates quetiapine up to 200 mg/d and restarts duloxetine, 30 mg/d, for depressive symptoms, based on her past positive response to this antidepressant.
Methamphetamine abuse can cause and exacerbate psychiatric symptoms. Keep in mind 2 priorities as you approach these symptoms:
Aim for abstinence. Methamphetamine abuse produces a remarkable array of adverse effects. It causes dysphoria, anxiety, and psychosis during active use and in the interval after initial abstinence. Many of methamphetamine’s use and withdrawal symptoms resolve with time, however, and may not require pharmacologic treatment.31 Therefore, achieving abstinence and keeping patients in treatment is high priority.
Use behavioral approaches whenever feasible. Balance the need to use benzodiazepines for ongoing treatment of severe anxiety or agitation with the high risk of addiction or diversion in this group. Anxiety may resolve over time in association with sustained abstinence. Similarly, receiving treatment for methamphetamine dependence and maintaining abstinence appears to ease depressive symptoms, as shown by sustained improvements in Beck Depression Inventory scores at 1 year.32
Manage stress. Stress can worsen psychiatric symptoms, trigger methamphetamine abuse relapse and psychosis, and acutely and chronically augment methamphetamine’s toxic effects.33 You can help patients manage stress by:
- providing case management and CBT training
- advising them about proper sleep, nutrition, and medical care.
Targeting psychiatric symptoms
Step 3 in the chronic disease management approach to methamphetamine dependence is to identify and target psychiatric and psychosocial comorbidities. When approaching psychiatric symptoms, high priorities are to aim for abstinence and manage the patient’s stress (Box 3).31-33
In clinical practice, we find it difficult to diagnostically categorize and treat methamphetamine-abusing patients who show residual post-acute psychotic symptoms. Some appear to have no risk factors for primary psychotic illness, and their symptoms show an association with the severity of their past methamphetamine abuse.
Other patient presentations can be difficult to separate from family histories of psychotic illness. Research suggests that genetic risk factors may be associated with methamphetamine psychosis in some vulnerable patients.35
Unfortunately, no data exist to guide the use of antipsychotics to maintain symptom control. Some patients may need low-dose antipsychotics for maintenance treatment, and second-generation antipsychotics may have a theoretical advantage over first-generation antipsychotics. Use your clinical judgment in determining dosing and treatment duration, and in weighing risks and benefits of continued treatment.
Using imaging, researchers found aggression severity to be directly correlated with past total methamphetamine use and globally decreased serotonin transporter density.36 Serotonin transporter densities were 30% lower in methamphetamine users vs controls after >1 year of abstinence.
CASE CONTINUED: Discharge plans
Because of the severity of her psychiatric symptoms, Ms. D is unable to return to the halfway house after discharge. As her treatment team works to coordinate discharge placement, Ms. D continues to improve. Her psychotic and dysphoria symptoms resolve, and she shows increased spontaneity. These changes—attributed to supports during hospitalization, decreased stressors, and quetiapine treatment—continue until her discharge to a combined mental illness and chemical dependence program.
- Methamphetamine use and sexually transmitted diseases. Centers for Disease Control and Prevention. www.cdc.gov/std/DearColleagueRiskBehaviorMetUse8-18-2006.pdf.
- National Institute on Drug Abuse Blending Initiative. Promoting Awareness of Motivational Incentives (PAMI). www.drugabuse.gov/blending/PAMI.html.
- Aripiprazole • Abilify
- Baclofen • various
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Gabapentin • Neurontin
- Modafinil • Provigil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Topiramate • Topamax
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Clinicians could become discouraged when confronting methamphetamine-dependent patients’ wide-ranging psychiatric symptoms.
These patients often present with:
- overlapping primary psychiatric syndromes and secondary substance abuse
- complex histories fraught with psychological trauma, limited social supports, and court involvement.
Treatment can be successful, however, and patients can change their addictive behaviors with a chronic disease management approach that targets the drug’s cognitive sequelae and psychiatric effects. Medications show limited benefit (Box 1),1-8 but behavioral treatments—including cognitive behavioral therapy (CBT) and motivational incentives—have proven efficacy in treating methamphetamine addiction.
This article discusses how to counteract methamphetamine’s negative cognitive effects and enable patients to engage in psychosocial treatment. Our discussion is informed by an extensive literature search and clinical experience from treating patients in the Midwest—at the geographic heart of the “meth” epidemic.
CASE REPORT: Overwhelmed and suicidal
Ms. D, age 27, presents to the emergency department with anxiety, dysphoria, and a plan to commit suicide by overdose. She feels overwhelmed by her 4-hour-a-day customer service job—a prerequisite for staying at the halfway house where she has lived for 2 months. She has a 13-year history of polysubstance dependence and is under court order to complete chemical dependence treatment or go to jail.
No medications are FDA-approved for treating methamphetamine dependence, and evidence supporting medication use in methamphetamine dependence is extremely limited. Research efforts are aimed at finding medications that might be neuroprotective, decrease craving, block reinforcement mechanisms, or affect other factors behind methamphetamine addiction and relapse.1 Most trials have been conducted in animal models or controlled laboratory evaluations of drug effects on methamphetamine-induced states.
Bupropion has shown slight treatment efficacy, possibly by decreasing neuronal damage and blocking reinforcement.2-4 Modafinil5 and baclofen6 may have potential, but evidence is lacking.
Some results have been unexpectedly negative. Sertraline might be contraindicated in methamphetamine dependence treatment, according to results of a randomized, placebo-controlled trial7 of sertraline and contingency management (Table 1). In a human laboratory study,8 topiramate accentuated—rather than diminished—subjective response to methamphetamine (Table 2).
Ms. D began using drugs at age 14 and has 3 convictions for driving under the influence of alcohol. An average student, she dropped out of high school but obtained a GED certificate. She first had psychiatric contact at age 16 and has been diagnosed at various times with attention deficit/hyperactivity disorder, bipolar disorder, and anxiety disorder. She also has been violently sexually assaulted while engaging in prostitution to support her drug habit.
Ms. D has been hospitalized multiple times—voluntarily and involuntarily—in dual diagnosis treatment centers. Her 5-year-old son no longer lives with her, and she has limited social supports beyond her parents, who live in a neighboring state.
Table 1
Antidepressant trials for treating methamphetamine dependence
| Drug | Investigation | Comments |
|---|---|---|
| Bupropion2-4 | Laboratory | Safety of bupropion with MAP |
| Laboratory | Reduced subjective effects and cue-induced craving | |
| Clinical trial | Trend toward reduced MAP use compared with placebo | |
| Sertraline7 | Clinical trial | Sertraline-treated subjects showed higher use of MAP compared with those receiving placebo and were less likely to complete treatment |
| MAP: methamphetamine | ||
3-step approach
For patients such as Ms. D, clinical evidence supports a 3-step approach to treating methamphetamine dependence:
- step 1: institute acute management and stabilization
- step 2: eliminate or decrease methamphetamine use to “move the frontal lobe back to the front”
- step 3: identify and target psychiatric and psychosocial comorbidities.
- help her eliminate or decrease methamphetamine use to allow neuronal systems to recover
- target maladaptive behaviors that hinder sobriety while providing motivational incentives to help her maintain a methamphetamine-free life.
How ‘meth’ affects cognition
Methamphetamine use has been associated with cognitive dysfunction at initial abstinence and even years later in some patients.10 Ms. D’s cognitive limitations in a fast-paced customer service job—even though hours are limited—lead to anxiety, dysphoria, and loss of self-esteem when she can’t manage patrons’ requests.
Methamphetamine has profound acute and chronic effects on the sympathetic nervous system, and dopaminergic, serotonergic, and noradrenergic neuronal networks. Most evidence of chronic neuronal effects comes from animal research and reflects toxic damage to dopaminergic and serotonergic neuronal systems. Postmortem human studies of direct neurotoxicity from chronic methamphetamine exposure show:
- decreased dopamine and tyrosine hydroxylase levels
- reduced concentrations of dopamine transporters.11
In chronic methamphetamine abusers, functional magnetic resonance imaging, proton magnetic resonance spectroscopy, and positron emission tomography show:
- changes in neurotransmitter, protein, brain metabolism, and transporter levels
- damage in multiple brain areas including the frontal region, basal ganglia, grey matter, corpus callosum, and striatum; smaller hippocampi; and cerebral vasculature changes.14-16
CASE CONTINUED: Does she understand?
After Ms. D is stabilized, her case manager expresses concern about her ability to follow through with treatment planning. He says, “I just don’t think she understands some of the things we discuss.” She then is referred for neuropsychological testing, which shows clear cognitive impairment. Specifically, she has a slowed rate of thinking, general cognitive ineficiency, deficits in learning and memory retention, and mild impulsivity.
Patients with a history of extensive methamphetamine abuse are ruled by the limbic system and may have higher cortical damage that complicates initiating, maintaining, and fully participating in treatment. Patients’ deficits in memory, executive functioning, attention, and cognitive speed may require you to simplify, repeat, and otherwise modify your treatment plan. You will need to provide clear instructions and consistent support—individually and psychosocially—and to recognize and reinforce patients’ treatment gains.
Even before using methamphetamine, patients may have had academic problems or learning disabilities that will compromise their ability to participate in treatment. Infection with HIV, syphilis, or hepatitis C can further hamper cognitive function.18
What treatments are effective?
Medications. Evidence is extremely limited, and no medications are approved to treat methamphetamine-addicted patients. Bupropion has shown some efficacy (Table 1),2-4,7 but other drugs such as sertraline and topiramate may aggravate rather than diminish methamphetamine dependence (Table 2).5,6,8,19
Behavioral treatments supply the evidence basis for methamphetamine dependence treatment. Cognitive behavioral therapy (CBT),20 contingency management (CM),21,22 and a manualized structured treatment—the Matrix Model23—all have proven efficacy.
CBT involves functional analysis and skills training. Patients are guided through analyzing their drug use and associated cognitions, emotions, and expectations and in identifying situations that trigger methamphetamine use or relapse. Skills training involves identifying, reinforcing, and practicing coping skills to help the patient avoid drug use and reinforce the ability to refuse use.
CM is based on operant conditioning—the use of consequences to modify behavior. It involves establishing a “contingent” relationship between a desired behavior/outcome (such as methamphetamine-free urinalysis) and delivering a positive reinforcing event to promote abstinence:
- Vouchers, privileges, or small amounts of money linked to healthy behaviors serve as incentives for negative urine testing.
- Rewards increase as periods of confirmed abstinence lengthen and are reset to smaller rewards if relapse occurs.
CM does not require extensive staff training and has been described as relatively simple to implement. CM also has been used successfully in urban gay and bisexual men with methamphetamine dependence (Box 2).18,25-29
Although CM’s efficacy is well-supported by clinical trials, we have encountered some resistance to the idea of “paying individuals to not use drugs” when training medical students, allied health staff, and residents. The National Institute on Drug Abuse (NIDA) supports the use of motivational incentives in treating substance abuse and offers support materials, resources, and training on this approach (see Related Resources).
Multiple studies show that CBT and CM are equally effective for treating chronic methamphetamine abuse at a 1-year follow-up, although CM may be more effective than CBT for acute treatment.
The Matrix model is a 4-month intensive, manualized treatment program that uses CBT, education on drug effects, positive reinforcement for intended behavioral change, and a 12-step approach.
Methamphetamine dependence outcomes based on the Matrix treatment model were compared with community treatment as usual in a project sponsored by The Center for Substance Abuse Treatment of the Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services.30 End-point outcomes were similar, but the Matrix treatment was more effective in early treatment, including decreased urinalyses positive for methamphetamine and increased abstinence.
Methamphetamine use is estimated to be 5 to 10 times more prevalent in U.S. urban gay and bisexual groups than in the general population25 and likely is contributing to rising human immunodeficiency virus (HIV) infection rates in men having sex with men (MSM).
Used to enhance sexual performance, libido, and mood, methamphetamine is associated with increased rates of unprotected anal sex and multiple partners in MSM.26 An HIV infection rate of 61% was reported in methamphetamine-dependent MSM seeking treatment in a Los Angeles clinical trial.27 Methamphetamine also results in high-risk sexual practices and multiple partners among heterosexual men and women.28
Although seroconverted men report using methamphetamine to alleviate HIV-associated depression, the combination of HIV infection and methamphetamine use may have powerful negative effects. Methamphetamine use is associated with HIV treatment nonadherence and also may suppress immune function.29 Cognitive impairments associated with HIV and methamphetamine use are additive and are further exacerbated by hepatitis C infection.18
Recommendation. Screen for methamphetamine use in MSM populations, and educate these patients about risks associated with methamphetamine use. In all patient groups who report using methamphetamine, provide counseling on high-risk sexual behavior, screen for sexually transmitted diseases, and ensure that patients are vaccinated against hepatitis A and B infection (see Related Resources). Most important, refer for medical treatment when indicated.
In patients such as Ms. D, the structure of court-ordered treatment can provide accountability, enforced abstinence, and mandated treatment resources. This, in turn, may give your patient a better chance to engage a recovering and better functioning frontal lobe to inhibit urges for methamphetamine use and manage stress.
Table 2
Other agents studied in methamphetamine dependence trials
| Drug | Investigation | Comment |
|---|---|---|
| Baclofen6 (GABAergic) | Clinical trial | No statistically significant effect compared with placebo; post hoc analysis showed ‘small’ treatment effects vs placebo |
| Gabapentin6 (GABAergic) | Clinical trial | No statistically signicant effect compared with placebo; post hoc analysis showed no treatment effects vs placebo |
| Topiramate8 (anticonvulsant) | Laboratory | Accentuated (rather than diminished) subjective effects of MAP |
| Aripiprazole19 (SGA) | Laboratory | Decreased subjective effects of amphetamine |
| Modafinil5 (wakefulness agent) | Clinical trial | Successful trial in cocaine dependence; potential option for MAP |
| MAP: methamphetamine; SGA: second-generation antipsychotic | ||
CASE CONTINUED: Racing thoughts and psychosis
Before hospital admission, Ms. D was being treated with gabapentin, 300 mg bid, and sustained-release bupropion, 150 mg/d, for anxiety and dysphoria. Previously, she has received multiple antidepressants and mood stabilizers with reportedly little effect.
Initially guarded, she at first denies psychotic symptoms but acknowledges their extent several days later. She describes periods of 6 months or more when she feels “lost.” The treatment team titrates quetiapine up to 200 mg/d and restarts duloxetine, 30 mg/d, for depressive symptoms, based on her past positive response to this antidepressant.
Methamphetamine abuse can cause and exacerbate psychiatric symptoms. Keep in mind 2 priorities as you approach these symptoms:
Aim for abstinence. Methamphetamine abuse produces a remarkable array of adverse effects. It causes dysphoria, anxiety, and psychosis during active use and in the interval after initial abstinence. Many of methamphetamine’s use and withdrawal symptoms resolve with time, however, and may not require pharmacologic treatment.31 Therefore, achieving abstinence and keeping patients in treatment is high priority.
Use behavioral approaches whenever feasible. Balance the need to use benzodiazepines for ongoing treatment of severe anxiety or agitation with the high risk of addiction or diversion in this group. Anxiety may resolve over time in association with sustained abstinence. Similarly, receiving treatment for methamphetamine dependence and maintaining abstinence appears to ease depressive symptoms, as shown by sustained improvements in Beck Depression Inventory scores at 1 year.32
Manage stress. Stress can worsen psychiatric symptoms, trigger methamphetamine abuse relapse and psychosis, and acutely and chronically augment methamphetamine’s toxic effects.33 You can help patients manage stress by:
- providing case management and CBT training
- advising them about proper sleep, nutrition, and medical care.
Targeting psychiatric symptoms
Step 3 in the chronic disease management approach to methamphetamine dependence is to identify and target psychiatric and psychosocial comorbidities. When approaching psychiatric symptoms, high priorities are to aim for abstinence and manage the patient’s stress (Box 3).31-33
In clinical practice, we find it difficult to diagnostically categorize and treat methamphetamine-abusing patients who show residual post-acute psychotic symptoms. Some appear to have no risk factors for primary psychotic illness, and their symptoms show an association with the severity of their past methamphetamine abuse.
Other patient presentations can be difficult to separate from family histories of psychotic illness. Research suggests that genetic risk factors may be associated with methamphetamine psychosis in some vulnerable patients.35
Unfortunately, no data exist to guide the use of antipsychotics to maintain symptom control. Some patients may need low-dose antipsychotics for maintenance treatment, and second-generation antipsychotics may have a theoretical advantage over first-generation antipsychotics. Use your clinical judgment in determining dosing and treatment duration, and in weighing risks and benefits of continued treatment.
Using imaging, researchers found aggression severity to be directly correlated with past total methamphetamine use and globally decreased serotonin transporter density.36 Serotonin transporter densities were 30% lower in methamphetamine users vs controls after >1 year of abstinence.
CASE CONTINUED: Discharge plans
Because of the severity of her psychiatric symptoms, Ms. D is unable to return to the halfway house after discharge. As her treatment team works to coordinate discharge placement, Ms. D continues to improve. Her psychotic and dysphoria symptoms resolve, and she shows increased spontaneity. These changes—attributed to supports during hospitalization, decreased stressors, and quetiapine treatment—continue until her discharge to a combined mental illness and chemical dependence program.
- Methamphetamine use and sexually transmitted diseases. Centers for Disease Control and Prevention. www.cdc.gov/std/DearColleagueRiskBehaviorMetUse8-18-2006.pdf.
- National Institute on Drug Abuse Blending Initiative. Promoting Awareness of Motivational Incentives (PAMI). www.drugabuse.gov/blending/PAMI.html.
- Aripiprazole • Abilify
- Baclofen • various
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Gabapentin • Neurontin
- Modafinil • Provigil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Topiramate • Topamax
- Trazodone • Desyrel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry 2005;162:1432-4.
2. Newton TF, Roache JD, De La Garza R, 2nd, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426-35.
3. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology 2006;31:1537-44.
4. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep 2006;8:345-54.
5. Umanoff DF. Trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005;30:2298; author reply 2299-300.
6. Heinzerling KG, Shoptaw S, Peck JA, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:177-84.
7. Shoptaw S, Huber A, Peck J, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:12-8.
8. Johnson BA, Roache JD, Ait-Daoud N, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol 2007;10:85-98.
9. Bostwick J, Lineberry T. The ‘meth’ epidemic: Managing acute psychosis, agitation, and suicide risk. Current Psychiatry 2006;5(11):46-62.
10. Simon SL, Dacey J, Glynn S, et al. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 2004;27:59-66.
11. Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996;2:699-703.
12. Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004;127:363-70.
13. Armstrong BD, Noguchi KK. The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABAergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 2004;25:905-14.
14. London ED, Berman SM, Voytek B, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry 2005;58:770-8.
15. London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 2004;61:73-84.
16. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15:317-25.
17. Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 2004;161:242-8.
18. Cherner M, Letendre S, Heaton RK, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 2005;64:1343-7.
19. Stoops WW. Aripiprazole as a potential pharmacotherapy for stimulant dependence: human laboratory studies with damphetamine. Exp Clin Psychopharmacol 2006;14:413-21.
20. Yen CF, Wu HY, Yen JY, Ko CH. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J Nerv Ment Dis 2004;192:788-91.
21. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry 2006;163:1993-9.
22. Shoptaw S, Klausner JD, Reback CJ, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health 2006;6:214.-
23. Shoptaw S, Rawson RA, McCann MJ, Obert JL. The Matrix model of outpatient stimulant abuse treatment: evidence of efficacy. J Addict Dis 1994;13:129-41.
24. Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction 2007;102:309-16.
25. Shoptaw S. Methamphetamine use in urban gay and bisexual populations. Top HIV Med 2006;14:84-7.
26. Bolding G, Hart G, Sherr L, Elford J. Use of crystal methamphetamine among gay men in London. Addiction 2006;101:1622-30.
27. Peck JA, Shoptaw S, Rotheram-Fuller E, et al. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. J Addict Dis 2005;24:115-32.
28. Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav 2004;29:807-10.
29. Mahajan SD, Hu Z, Reynolds JL, et al. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther 2006;10:257-69.
30. Rawson RA, Marinelli-Casey P, Anglin MD, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 2004;99:708-17.
31. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100:1320-9.
32. Peck JA, Reback CJ, Yang X, et al. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health 2005;82:i100-8.
33. Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience 2004;124:637-46.
34. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13:461-70.
35. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatr Genet 2006;16:133-8.
36. Sekine Y, Ouchi Y, Takei N, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 2006;63:90-100.
1. Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry 2005;162:1432-4.
2. Newton TF, Roache JD, De La Garza R, 2nd, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426-35.
3. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology 2006;31:1537-44.
4. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep 2006;8:345-54.
5. Umanoff DF. Trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005;30:2298; author reply 2299-300.
6. Heinzerling KG, Shoptaw S, Peck JA, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:177-84.
7. Shoptaw S, Huber A, Peck J, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006;85:12-8.
8. Johnson BA, Roache JD, Ait-Daoud N, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol 2007;10:85-98.
9. Bostwick J, Lineberry T. The ‘meth’ epidemic: Managing acute psychosis, agitation, and suicide risk. Current Psychiatry 2006;5(11):46-62.
10. Simon SL, Dacey J, Glynn S, et al. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 2004;27:59-66.
11. Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996;2:699-703.
12. Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004;127:363-70.
13. Armstrong BD, Noguchi KK. The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABAergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 2004;25:905-14.
14. London ED, Berman SM, Voytek B, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry 2005;58:770-8.
15. London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 2004;61:73-84.
16. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15:317-25.
17. Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 2004;161:242-8.
18. Cherner M, Letendre S, Heaton RK, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology 2005;64:1343-7.
19. Stoops WW. Aripiprazole as a potential pharmacotherapy for stimulant dependence: human laboratory studies with damphetamine. Exp Clin Psychopharmacol 2006;14:413-21.
20. Yen CF, Wu HY, Yen JY, Ko CH. Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J Nerv Ment Dis 2004;192:788-91.
21. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry 2006;163:1993-9.
22. Shoptaw S, Klausner JD, Reback CJ, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health 2006;6:214.-
23. Shoptaw S, Rawson RA, McCann MJ, Obert JL. The Matrix model of outpatient stimulant abuse treatment: evidence of efficacy. J Addict Dis 1994;13:129-41.
24. Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction 2007;102:309-16.
25. Shoptaw S. Methamphetamine use in urban gay and bisexual populations. Top HIV Med 2006;14:84-7.
26. Bolding G, Hart G, Sherr L, Elford J. Use of crystal methamphetamine among gay men in London. Addiction 2006;101:1622-30.
27. Peck JA, Shoptaw S, Rotheram-Fuller E, et al. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. J Addict Dis 2005;24:115-32.
28. Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav 2004;29:807-10.
29. Mahajan SD, Hu Z, Reynolds JL, et al. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther 2006;10:257-69.
30. Rawson RA, Marinelli-Casey P, Anglin MD, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 2004;99:708-17.
31. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100:1320-9.
32. Peck JA, Reback CJ, Yang X, et al. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health 2005;82:i100-8.
33. Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience 2004;124:637-46.
34. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13:461-70.
35. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatr Genet 2006;16:133-8.
36. Sekine Y, Ouchi Y, Takei N, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 2006;63:90-100.
The ‘meth’ epidemic: Managing acute psychosis, agitation, and suicide risk
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
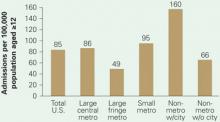
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
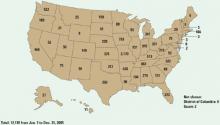
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Methamphetamine abuse has spread to every region of the United States in the past 10 years (Table 1).1 Its long-lasting, difficult-to-treat medical effects destroy lives and create psychiatric and physical comorbidities that confound clinicians in emergency rooms and community practice settings.
This first in a series of two articles describes methamphetamine’s growing use and offers guidance to identify abusers and manage acute “meth” intoxication. Methamphetamine-abusing patients can appear in any area of acute psychiatric practice—during emergency department (ED) evaluations, medical-surgical consultations, and inpatient psychiatric admissions. Using case examples, we describe key clinical principles to help you assess patients in each of these settings.
Table 1
10-year growth in hospitalization rates
for methamphetamine/amphetamine use*
| Year | ||
|---|---|---|
| State | 1993 | 2003 |
| U.S. national rate | 13 | 56 |
| Northeast | ||
| Connecticut | 1 | 4 |
| Maine | 2 | 5 |
| Massachusetts | <1 | 2 |
| New Hampshire | <1 | 2 |
| New Jersey | 3 | 2 |
| New York | 2 | 4 |
| Pennsylvania | 3 | 2 |
| Rhode Island | 2 | 2 |
| Vermont | 5 | 4 |
| South | ||
| Alabama | 1 | 45 |
| Arkansas | 13 | 130 |
| Delaware | 2 | 2 |
| District of Columbia | — | 2 |
| Florida | 2 | 7 |
| Georgia | 3 | 39 |
| Kentucky | — | 20 |
| Louisiana | 4 | 21 |
| Maryland | 1 | 3 |
| Mississippi | — | 23 |
| North Carolina | <1 | 4 |
| Oklahoma | 19 | 117 |
| South Carolina | 1 | 9 |
| Tennessee | † | 6 |
| Texas | 7 | 17 |
| Virginia | 1 | 4 |
| West Virginia | <1 | — |
| Midwest | ||
| Illinois | 1 | 19 |
| Indiana | 3 | 28 |
| Iowa | 13 | 213 |
| Kansas | 15 | 65 |
| Michigan | 2 | 7 |
| Minnesota | 8 | 100 |
| Missouri | 7 | 84 |
| Nebraska | 8 | 117 |
| North Dakota | 3 | 44 |
| Ohio | 3 | 3 |
| South Dakota | 5 | 90 |
| Wisconsin | <1 | 5 |
| West | ||
| Alaska | 4 | 13 |
| Arizona | — | 36 |
| California | 66 | 212 |
| Colorado | 18 | 86 |
| Hawaii | 52 | 241 |
| Idaho | 20 | 72 |
| Montana | 30 | 133 |
| Nevada | 59 | 176 |
| New Mexico | 7 | 10 |
| Oregon | 98 | 251 |
| Utah | 16 | 186 |
| Washington | 18 | 143 |
| Wyoming | 15 | 209 |
| * Per 100,000 population aged 12 or older, with methamphetamine/amphetamine use as the primary diagnosis. Percentages in boldface exceed the national rate for that year. | ||
| † <0.05% | ||
| –No data available | ||
| Source: Reference 1 | ||
Scourge of the Heartland
A stimulant first synthesized in Japan,2 methamphetamine is the primary drug of abuse in Asia3 and the leading drug threat in the United States, according to U.S. law enforcement officials.4 Although most methamphetamine used in the United States is manufactured in “super-labs” along the U.S.-Mexican border,4 the drug is also easily made from common ingredients in small-scale home laboratories.
These smaller domestic “meth labs” have devastated rural communities and altered demographic patterns of methamphetamine abuse (Figure 1).5 Two aspects of rural life—relative isolation and availability of ingredients for production—proved critical in the initial spread of methamphetamine production and use in the United States. As a result, production by smaller labs is being targeted by state and federal law enforcement officers, who have had some success in eradicating this scourge (Box 1, Figure 2).6-9
Figure 1 Substance abuse treatment admission rates
for methamphetamine-related diagnoses
Substance abuse treatment centers in rural areas had the highest admission rates for methamphetamine/amphetamine-related diagnoses in 2004. Admission rates in nonmetropolitan regions containing cities with populations >10,000 were triple those of suburbs, nearly twice those of big cities, and twice the U.S. average.
Figure 2 Methamphetamine clandestine laboratory incidents,* 2005
* Incidents include chemicals, contaminated glass, and equipment used in methamphetamine production found by law enforcement agencies at laboratories or dump sites.
Source: Drug Enforcement Administration database, reference 9Box 1
Dangerous recipes. The ease with which methamphetamine can be “cooked” in a home kitchen from ingredients available in pharmacies and hardware stores has contributed to the drug’s rapid spread. Meth users produce their own “fixes” using recipes readily available on the Internet and passed on by other “cooks.”6 The combination of inexperienced or intoxicated cooks, homemade equipment, and highly flammable ingredients results in frequent fires and explosions, often with injuries to home occupants and emergency responders.7
Meth labs have been estimated to produce 6 pounds of toxic waste for each 1 pound of methamphetamine produced. Composed of acid, lye, and phosphorus, this waste typically is dumped into ditches, rivers, yards, and drains. The fine-particulate methamphetamine residue generated during home production settles on exposed household surfaces, leading to absorption by children and others who come into contact with it.6,8
Disastrous results. Methamphetamine cooking has caused a social, environmental, and medical disaster—particularly in the Midwest (Figure 2), although the situation has improved in the past 2 years. Many states have passed laws restricting and monitoring sales of the methamphetamine ingredients ephedrine and pseudoephedrine. A change in U.S. law prohibiting pseudoephedrine imports in bulk from Canada has decreased domestic “superlab” production.9 Although these laws appear to have slowed U.S. manufacturing, the drug is still readily available, predominantly smuggled in from large-scale producers in Mexico.
Symptoms of ‘Meth’ Use
Physiologic effects. Methamphetamine is taken because it induces euphoria, anorexia, and increased energy, sexual stimulation, and alertness. Initial use evolves into abuse because of the drug’s highly addictive properties. Available in multiple forms and carrying a variety of labels (see Related resources), methamphetamine causes CNS release of monoamines—particularly dopamine—and damages dopaminergic neurons in the striatum and serotonergic neurons in the frontal lobes, striatum, and hippocampus.10,11
Through sympathetic nervous system activation, methamphetamine can cause reversible or irreversible damage to organ systems (Table 2).12-17
Table 2
Physiologic signs of methamphetamine abuse
| Vital signs | Tachycardia |
| Hypertension | |
| Pyrexia | |
| Laboratory abnormalities | Metabolic acidosis |
| Evidence of rhabdomyolysis | |
| Organ damage | Cardiomyopathy |
| Acute coronary syndrome | |
| Pulmonary edema | |
| Stigmata of chronic use | Premature aging |
| Cachexia | |
| Discolored and fractured teeth | |
| Skin lesions from stereotypical scratching related to formication (“meth bugs”) and/or compulsive picking | |
| Source: References 12-17 | |
Psychiatric effects. Methamphetamine abusers frequently report depressive symptoms, including irritability, anxiety, social isolation, and suicidal ideation.10,18 These patients may show:
- signs of psychosis, including paranoia, hallucinations, and homicidal thoughts
- neurocognitive changes, including poor attention, impaired verbal memory, and decreased executive functioning.19
Agitation is frequent, and its severity appears to correlate directly with methamphetamine blood levels.20 Violent behavior is common. In 1,016 previous users, 40% of men and 46% of women described difficulty controlling their behavior when under methamphetamine’s influence.18
In acute clinical practice, differentiating a primary thought disorder from methamphetamine-induced psychosis is challenging—especially when a patient shows signs of both.21 Methamphetamine also can contribute profoundly to depressive and anxiety disorders. Users may experience residual psychotic symptoms years after the original abuse ends, particularly when stressed. Their positive and negative symptoms are strikingly similar to those seen in schizophrenia.21
Longitudinal illness course, recent history, collateral information, and laboratory and physical data may all inform clinical presentations and comorbidity.
Emergent Evaluation
Gathering data. Police bring Mr. J, age 22, to the ED after his parents said he talked about killing himself and the mother of his 4-year-old child. Police report that Mr. J’s parents said he and his friends abuse methamphetamine, but no first-hand information is available.
Disheveled and uncooperative, Mr. J threatens to harm ED staff. His speech is pressured, and he appears to be responding to internal stimuli. Vital signs include temperature 37.8° C, pulse rate 105 bpm, blood pressure 140/85 mm Hg, and respiration rate 18 breaths per minute.
Mr. J refuses to provide blood or urine for drug screening or to provide a history to the ED physician. He attempts to walk out and is placed in restraints after he tries to punch the ED security officer.
Options for containing uncooperative and agitated patients such as Mr. J are extremely limited, and the overriding concern with violently intoxicated patients is to minimize damage to self, others, and property. Methamphetamine abusers have a propensity for impulsivity and violence;18 many are brought to the hospital by police and have criminal histories.1 In emergent evaluation, begin by searching patients and their belongings for weapons.
Because laboratory results and patient history are not immediately available, methamphetamine abuse often is not included in the initial differential diagnosis—particularly for patients with pre-existing primary affective or psychotic disorders. It is critical to remember that methamphetamine abuse might be complicating a patient’s psychiatric presentation.
Managing agitation. When agitation is prominent, secure the patient in a quiet room to reduce stimulation. Have on hand adequate staffing and benzodiazepines, antipsychotics, or both.
In theory, using an antipsychotic to control methamphetamine-induced agitation is problematic because synergy between the two agents might adversely affect cardiac function.15 On the other hand, acute treatment of agitation often leads to salutary declines in pulse rate, blood pressure, respiration rate, and body temperature.
Benzodiazepines vs neuroleptics. Evidence for acute treatment of agitation is limited,22 especially when agitation was induced by methamphetamine. A randomized, controlled comparison of lorazepam and droperidol (a neuroleptic not routinely prescribed for psychosis) suggested that droperidol could be used safely to control agitation in ED patients, with methamphetamine toxicity.23 Droperidol provided more rapid and effective sedation than lorazepam.
Droperidol use has decreased dramatically since 2001, however, when the FDA ordered a black-box warning about potential for cardiac dysrhythmias.24-25 After that warning, the American College of Emergency Physicians26 examined the evidence to identify the most effective pharmacologic treatment for agitation of unknown etiology. Its recommendation—felt to represent “moderate” clinical certainty—was monotherapy with either:
- a benzodiazepine (lorazepam or midazolam)
- or a conventional antipsychotic (droperidol or haloperidol).
The level of certainty for combining a benzodiazepine with an antipsychotic was lower. In our experience, psychiatrists tend to favor haloperidol and lorazepam over droperidol and midazolam.
Evidence on treating methamphetamine-induced agitation is limited (Box 2).22-26 Before you prescribe any medication, keep in mind its side effect profile, the patient’s age and physical condition, and the possibility that other substances might be contributing to emergent presentations.
We have repeatedly and effectively treated acutely agitated patients in the ED with haloperidol and lorazepam without observing adverse effects. Psychiatrists generally favor haloperidol and lorazepam over droperidol and midazolam. When a patient can cooperate with treatment, we recommend an ECG to rule out prolonged QTc interval, an uncommon complication. Telemetry and a cardiology consultation are indicated with a QTc interval >450 msec or >25% over previous ECGs, particularly if you plan to continue haloperidol treatment.27
Physical examination. Because methamphetamine use can cause substantial physical morbidity, we recommend a thorough physical exam aimed at identifying its stigmata (Table 2). Look especially for injuries resulting from violence, and test for sexually-transmitted diseases. Drug testing in the ED is essential to diagnosis and for planning treatment (Box 3).28,29
Medical-Surgical Consultation
Ensuring safety. Ms. A, age 41, is admitted to the trauma surgery service after a motor vehicle accident in which she was the driver. She has long-standing methamphetamine dependence and is severely agitated. Urine drug testing is positive for methamphetamine, marijuana, and alcohol. Her alcohol serum level of 165 mg/dL exceeds the legal threshold for intoxication.
Tibial and fibular fractures sustained in the car accident require open reduction and internal fixation. On the postsurgical floor 2 days later, Ms. A remains “extremely irritable, dysphoric, and suicidal,” according to the trauma surgery consultation. Staff is concerned about her boyfriend’s behavior: “We think he’s using drugs and might be bringing her drugs.”
Understanding Ms. A’s behavior requires us to consider a broad range of diagnostic contributors, including:
- untreated withdrawal from alcohol or other drugs
- delirium from ongoing effects of the trauma or corrective operation
- inadequate pain control, particularly given her history of substance dependence
- psychiatric comorbidity.
Management includes:
- monitoring for withdrawal and treating it if symptoms emerge
- identifying and minimizing medical factors contributing to confusion, and medicating agitation with psychotropics
- providing adequate analgesia, mindful that dosing may need to be aggressive—particularly if the abused substances include narcotics
- assessing for pre-existing and methamphetamine-induced psychiatric disorders.
If the patient is cognitively able to cooperate, perform a thorough suicide assessment and provide initial supportive and cognitive-behavioral therapy to target suicidal behavior. Consider one-to-one monitoring, depending on the potential for deliberate self-injury, and guard against impulsive actions occurring in a drug- or treatment-induced delirium that could endanger the patient or staff.
A one-to-one monitor also can watch for smuggled contraband. When hospitalized, patients who are chronic substance abusers are prone to continue using illicit substances smuggled in by associates, such as the boyfriend in this case. Consider further testing for illicit drugs if you suspect smuggling.
Acute Psychiatric Inpatient
Initial diagnosis and treatment planning. Miss G, age 23 and homeless, is admitted directly to the inpatient psychiatric unit from an urgent care clinic. She reports being “depressed and suicidal.” An intermittent methamphetamine abuser, she says she last used the drug the previous day.
Miss G reveals that she is on probation for forged checks and drug use. She believes she failed a random urinalysis given earlier in the day as a condition of her probation, and she fears being sent back to jail. Her history includes childhood sexual abuse and emotional abuse in a relationship that ended the previous year.
Take the long-term view. Emergency room physicians and psychiatrists often disagree about drug testing in the ED. Emergency medicine physicians argue that the yield is low and results do not affect short-term ED management. However, we believe that drug testing is essential during the initial evaluation and that, at a minimum, urine toxicology screening must be performed to aid diagnosis and subsequent treatment planning.
A positive toxicology screen provides nearly irrefutable evidence with which to confront a resistant patient who is likely to be involved with the criminal justice system. In a study by Perrone et al,28 the patient history combined with drug testing was most likely to identify substance abuse. Overreliance on either the history or testing alone was flawed.
Objective data. In our experience, patients with legal problems often deny drug abuse. A toxicology screen provides objective data on concomitant use of other substances abused by many methamphetamine users to temper methamphetamine-related insomnia, anxiety, and overstimulation. Hair testing, a promising tool being investigated, may allow more substance abuse to be detected and possibly determine the level of use.29
Physical examination shows multiple erythematous excoriations on her arms from repetitive picking at her skin, poor dentition, and cachexia. She reports multiple recent sexual partners without using condoms. She cannot remember when she last menstruated, and she doesn’t recall ever being tested for sexually transmitted disease.
As in any medical setting involving methamphetamine abusers, acute management of psychiatric inpatients includes careful attention to methamphetamine-related physical conditions—in Miss G’s case possible sexually transmitted diseases, pregnancy, cellulitis, and dental disease.
Mood and anxiety disorders. Methamphetamine users may present with depressive symptoms and suicidality.18,30 In a study of Taiwanese methamphetamine abusers who had recently quit the drug, depressive symptoms were common on cessation but often resolved without antidepressants within 2 to 3 weeks.30 Evidence on antidepressant use in the methamphetamine-dependent patient is limited, and the existing studies have yielded conflicting results (as we will detail in part 2 of this article).
For patients previously diagnosed with mood or anxiety disorders, do not restart psychotropics until you have considered how methamphetamine use is contributing to the immediate presentation. We recommend initial observation for several weeks before starting an antidepressant if there is no pre-methamphetamine history of mood or anxiety symptoms.
Psychosocial treatments. Involve social services in assessing the patient’s need for community resources. Miss G’s ability to benefit from these programs will depend on her cognitive capacity, education level, trauma history, and comorbid psychiatric illness.
For patients who relapse to methamphetamine use, previous successful treatment and abstinence may be a hopeful prognostic sign and warrant referral to a program for recidivists. The patient’s legal status may limit some options in the community but open others in the criminal justice system.
Methamphetamine users often have multiple problems that require attention. For example, compared with other mothers under investigation by child welfare services in California, methamphetamine-abusing mothers were younger and less educated on average, less likely to have had substance-abuse treatment, and more likely to have criminal records.31 These findings underscore the challenge of coordinating a response that integrates separate and complex systems—psychiatric/substance abuse treatment, child welfare, and criminal justice.
- Methresources. Web site pooling information from multiple agencies for communities, law enforcement, and policy makers. www.methresources.gov.
- Methamphetamine. National drug threat assessment, with information on methamphetamine production, trafficking, and patterns of use. U.S. Department of Justice. Drug Enforcement Administration. www.dea.gov/concern/18862/meth.htm.
- Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. www.oas.samhsa.gov/2k6/methTX/methTX.pdf.
Drug brand names
- Droperidol • Inapsine
- Haloperidol • Haldol
- Lorazepam • Ativan
- Midazolam • Versed
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
1. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Trends in methamphetamine/amphetamine admissions to treatment: 1993-2003. The DASIS Report 2006; Issue 9. Available at: http://www.oas.samhsa.gov/2k6/methTX/methTX.htm. Accessed September 4, 2006.
2. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45 year history and the current situation. In: Klee H, ed. Amphetamine misuse: international perspectives on current trends Amsterdam: Harwood Academic Publishers; 1997:199-214.
3. Greenfeld KT. The need for speed. Time (Asia ed) February 26, 2001. Available at: http://www.time.com/time/asia/news/magazine/0,9754,100581,00.html. Accessed September 4, 2006.
4. Williams P. Meth trade moves south of the border. Tighter restrictions reduce U.S. meth labs, so Mexican drug lords fill gap. NBC News August 25, 2006. Available at: http://www.msnbc.msn.com/id/14500890/. Accessed September 4, 2006.
5. Substance Abuse and Mental Health Services Administration Drug and Alcohol Services Information System. Methamphetamine/amphetamine treatment admissions in urban and rural areas: 2004. The DASIS Report 2006; Issue 27. Available at: http://www.oas.samhsa.gov/2k6/methRuralTX/methRuralTX.htm. Accessed September 4, 2006.
6. Research Overview: Methamphetamine Production Precursor Chemicals and Child Endangerment Albuquerque, NM: New Mexico Sentencing Commission; January 2004. Available at: http://www.nmsc.state.nm.us/DWIDrugReports.htm. Accessed October 1, 2006.
7. Santos AP, Wilson AK, Hornung CA, et al. Methamphetamine laboratory explosions: a new and emerging burn injury. J Burn Care Rehabil 2005;26(3):228-32.
8. Martyny JW, Arbuckle SL, McCammon CS, et al. Chemical exposures associated with clandestine methamphetamine laboratories Denver, CO: The National Jewish Medical and Research Center. Available at: http://www.njc.org/pdf/chemical_exposures.pdf. Accessed September 4, 2006.
9. U.S. Department of Justice. Drug Enforcement Administration. Maps of methamphetamine lab incidents. Available at: http://www.usdoj.gov/dea/concern/map_lab_seizures.html. Accessed September 4, 2006.
10. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs 2000;32(2):137-41.
11. National Institutes of Health National Institute on Drug Abuse. Methamphetamine abuse and addiction. NIH Publication No 02-4210. Research Report Series January 2002. Available at: http://www.nida.nih.gov/ResearchReports/Methamph/Methamph.html. Accessed October 1, 2006.
12. Burchell SA, Ho HC, Yu M, Margulies DR. Effects of methamphetamine on trauma patients: a cause of severe metabolic acidosis? Crit Care Med 2000;28(6):2112-5.
13. Richards JR, Johnson EB, Stark RW, Derlet RW. Methamphetamine abuse and rhabdomyolysis in the ED: a 5-year study. Am J Emerg Med 1999;17(7):681-5.
14. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol 2003;41(7):981-6.
15. Turnipseed SD, Richards JR, Kirk JD, et al. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 2003;24(4):369-73.
16. Nestor TA, Tamamoto WI, Kam TH, Schultz T. Acute pulmonary oedema caused by crystalline methamphetamine. Lancet 1989;2(8674):1277-8.
17. Venker D. Crystal methamphetamine and the dental patient. Iowa Dent J 1999;85(4):34.-
18. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004;13(2):181-90.
19. Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 2003;15(3):317-25.
20. Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesia. Am J Addict 2004;13(5):461-70.
21. Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 2000;914:1-12.
22. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry 2006;67(suppl10):13-21.
23. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med 1998;16(4):567-73.
24. Shale JH, Shale CM, Mastin WD. Safety of droperidol in behavioural emergencies. Expert Opin Drug Saf 2004;3(4):369-78.
25. Jacoby JL, Fulton J, Cesta M, Heller M. After the black box warning: dramatic changes in ED use of droperidol. Am J Emerg Med 2005;23(2):196.-Letter.
26. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 2006;47(1):79-99.
27. American Psychiatric Association Practice guideline for the treatment of patients with delirium. Practice guidelines for the treatment of psychiatric disorders, compendium 2004 Washington, DC: American Psychiatric Publishing; 29-66.
28. Perrone J, De Roos F, Jayaraman S, Hollander JE. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med 2001;19(1):49-51.
29. Mieczkowski T. Hair analysis for detection of psychotropic drug use [letter]. Mayo Clin Proc 2006;81(4):568-9.
30. McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320-9.
31. Grella CE, Hser YI, Huang YC. Mothers in substance abuse treatment: differences in characteristics based on involvement with child welfare services. Child Abuse Negl 2006;30(1):55-73.
Military sexual trauma: How to identify and treat a unique form of PTSD
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
U.S. troops returning home: Are you prepared?
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.