User login
Abdominal pain and bloody diarrhea in a 32-year-old woman
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
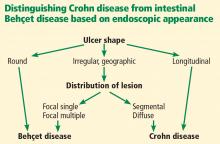
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
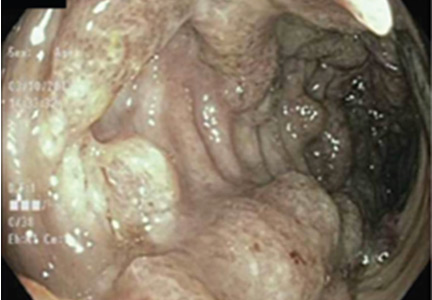
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
A 32-year-old woman presented to our emergency department with chest pain and painful ulcerations on her arms, abdomen, back, groin, axillae, and in her mouth. She first noticed the ulcers 7 days earlier.
She also reported bloody diarrhea, which had started 2 years earlier, with 10 or more bowel movements daily. She described her stools as semiformed and associated with urgency and painful abdominal cramps.
Medical history
Her medical history included obstructive sleep apnea and morbid obesity. She had first presented 2 years earlier to another hospital with diarrhea, abdominal pain, and rectal bleeding. At that time, results of esophagogastroduodenoscopy and colonoscopy were reported as normal. Later, she became pregnant, and her symptoms went away. She had a normal pregnancy and delivery.
About 1 year postpartum, her abdominal pain and bloody diarrhea recurred. Colonoscopy showed severe sigmoid inflammation with small, shallow ulcerations and friable mucosa interrupted by areas of normal mucosa. Histopathologic study of the colonic mucosa indicated mild to moderate chronic active colitis consisting of focal areas of cryptitis with occasional crypt abscess formation. She was diagnosed with Crohn colitis based on the endoscopic appearance, histopathology, and clinical presentation. The endoscope, however, could not be advanced beyond the sigmoid colon, which suggested stenosis. She was started on 5-aminosalicylic acid (5-ASA) but developed visual hallucinations, and the medication was stopped.
Her symptoms continued, and she developed worsening rectal bleeding and anemia that required hospitalization and blood transfusions. Another colonoscopy performed 1 month before this emergency department visit had shown multiple mucosal ulcerations, but again, the colonoscope could not be advanced beyond the sigmoid colon. She was started on oral corticosteroids, which provided only minimal clinical improvement.
Her current medications included atenolol (for sinus tachycardia), prednisone (initial dose 60 mg/day tapered to 20 mg/day at presentation), and ciprofloxacin.
Her family history was unknown because she had been adopted.
About 1 week before presentation, she had noticed ulcers developing on her arms, abdomen, back, groin, oral mucosa, and axillae. The ulcers were large and painful, with occasional spontaneous bleeding. She also reported pustules and ulcerations at sites of previous skin punctures, consistent with pathergy.
Findings on presentation
- Temperature 99.5°F (37.5°C)
- Heart rate 124 beats per minute
- Respiratory rate 22 breaths per minute
- Oxygen saturation 100% on room air
- Blood pressure 128/81 mm Hg
- Body mass index 67 kg/m2 (morbidly obese).
She had multiple greyish-white patches and erosions over the soft palate, tongue, and upper and lower lip mucosa, erythematous pustules in the axillae bilaterally, and large erythematous, sharply demarcated ulcerations with a fibrinous base bilaterally covering her arms, thighs, groin, and abdomen.
Blood testing showed multiple abnormal results (Table 1). Urinalysis revealed a urine protein concentration of 100 mg/dL (reference range 0), more than 25 white blood cells per high-power field (reference range < 5), 6 to 10 red blood cells per high-power field (0–3), and more than 10 casts per low-power field (0), which suggested a urinary tract infection with hematuria.
Computed tomography (CT) of the abdomen and pelvis with intravenous and oral contrast showed diffuse fatty infiltration of the liver and wall thickening of the rectum and sigmoid colon.
She was admitted to the medical intensive care unit for potential septic shock. Intravenous vancomycin and ciprofloxacin were started (the latter owing to penicillin allergy).
CAUSES OF DIARRHEA AND SKIN CHANGES
1. What is the most likely diagnosis in our patient?
- Ulcerative colitis
- Crohn disease
- Behçet disease
- Intestinal tuberculosis
- Herpes simplex virus infection
- Cytomegalovirus infection
All of the above can cause diarrhea in combination with mucocutaneous lesions and other manifestations.
Ulcerative colitis and Crohn disease: Mucocutaneous findings
Extraintestinal manifestations of inflammatory bowel diseases (Crohn disease, ulcerative colitis, and Behçet disease) include arthritis, ocular involvement, mucocutaneous manifestations, and liver involvement in the form of primary sclerosing cholangitis. Less common extraintestinal manifestations include vascular, renal, pulmonary, cardiac, and neurologic involvement.
Mucocutaneous findings are observed in 5% to 10% of patients with ulcerative colitis and 20% to 75% of patients with Crohn disease.1–3 The most common are erythema nodosum and pyoderma gangrenosum.4
Yüksel et al5 reported that of 352 patients with inflammatory bowel disease, 7.4% had erythema nodosum and 2.3% had pyoderma gangrenosum. Erythema nodosum was significantly more common in patients with Crohn disease than in those with ulcerative colitis, and its severity was linked with higher disease activity. Lesions frequently resolved when bowel disease subsided.
Lebwohl and Lebwohl6 reported that pyoderma gangrenosum occurred in up to 20% of patients with Crohn disease and up to 10% of those with ulcerative colitis. It is not known whether pyoderma gangrenosum correlates with intestinal disease severity.
Other mucocutaneous manifestations of inflammatory bowel disease include oral aphthous ulcers, acute febrile neutrophilic dermatosis (Sweet syndrome), and metastatic Crohn disease. Aphthous ulcers in the oral cavity, often observed in both Crohn disease and ulcerative colitis, cannot be differentiated on clinical examination from herpes simplex virus (HSV) type 1-induced or idiopathic mucous membrane ulcers. The most common ulcer locations are the lips and buccal mucosa. If biopsied (seldom required), noncaseating granulomas can be identified that are comparable with intestinal mucosal granulomas found in Crohn disease.7
Behçet disease has similar signs
Oral aphthous ulcers are also the most frequent symptom in Behçet disease, occurring in 97% to 100% of cases.8 They most commonly affect the tongue, lips, buccal mucosa, and gingiva.
Cutaneous manifestations include erythema nodosum-like lesions, which present as erythematous painful nodules over pretibial surfaces of the lower limbs but can also affect the arms and thighs; they can also present as papulopustular rosacea eruptions composed of papules, pustules, and noninflammatory comedones, most commonly on the chest, back, and shoulders.8,9
Pathergy, ie, skin hyperresponse to minor trauma such as a bump or bruise, is a typical trait of Behçet disease. A positive pathergy test (ie, skin hyperreactivity to a needlestick or intracutaneous injection) has a specificity of 98.4% in patients with Behçet disease.10
Interestingly, there appears to be a regional difference in the susceptibility to pathergy. While a pathergy response in patients with Behçet disease is rare in the United States and the United Kingdom, it is very common in Japan, Turkey, and Israel.11
Patient demographics also distinguish Behçet disease from Crohn disease. The prevalence of Behçet disease is highest along the Silk Road from the Mediterranean Basin to East Asia and lowest in North America and Northern Europe.12 The mean age at onset is around the third and fourth decades. In males, the prevalence is highest in Mediterranean, Middle Eastern, and Asian countries. In females, the prevalence is highest in the United States, Northern Europe, and East Asia.10
Tuberculosis
Tubercular skin lesions can present in different forms.13 Lupus vulgaris, the most common, occurs after primary infection and presents as translucent brown nodules, mainly over the face and neck. So-called scrofuloderma is common at the site of a lymph node. It appears as a gradually enlarging subcutaneous nodule followed by skin breaks and ulcerations. Tuberculosis verrucosa cutis, also known as warty tuberculosis, is common in developing countries and presents as warty plaque over the hands, knees, and buttocks.14 Tuberculids are skin reactions to systemic tuberculosis infection.
Herpes simplex virus
Mucocutaneous manifestations of herpes simplex virus affect the oral cavity (gingivostomatitis, pharyngitis, and lip border lesions), the entire integumentary system, the eyes (HSV-1), and the genital region (HSV-2). The classic presentation is systemic symptoms (fever and malaise) associated with multiple vesicles on an erythematous base in a distinct region of skin. The virus can remain latent with reactivation occurring because of illness, immunosuppression, or stress. Pruritus and pain precede the appearance of these lesions.
Cytomegalovirus
Primary cytomegalovirus infection is subclinical in almost all cases unless the patient is immunocompromised, and it presents similarly to mononucleosis induced by Epstein-Barr virus. The skin manifestations are nonspecific and can include macular, maculopapular, morbilliform, and urticarial rashes, but usually not ulcerations.15
OUR PATIENT: BEHÇET DISEASE OR CROHN DISEASE?
In our patient, oral mucosal aphthous ulcers and the location of pustular skin lesions, in addition to pathergy, were highly suggestive of Behçet disease. However, Crohn disease with mucocutaneous manifestations remained in the differential diagnosis.
Because there is significant overlap between these diseases, it is important to know the key distinguishing features. Oral aphthous ulcers, pathergy, uveitis, skin and genital lesions, and neurologic involvement are much more common in Behçet disease than in Crohn disease.16,17 Demographic information was not helpful in this case, given that the patient was adopted.
FURTHER WORKUP
2. What should be the next step in the work-up?
- CT enterography
- Skin biopsy
- Colonoscopy with biopsy
- C-reactive protein, erythrocyte sedimentation rate, and fecal calprotecting testing
The endoscopic appearance and histopathology of the affected tissues are crucial for the diagnosis. Differentiating between Crohn disease and Behçet disease can be particularly challenging because of significant overlap between the intestinal and extraintestinal manifestations of the two diseases, especially the oral lesions and arthralgias. Thus, both colonoscopy with biopsy of the intestinal lesions and biopsy of a cutaneous ulceration should be pursued.
No single test or feature is pathognomonic for Behçet disease. Although many diagnostic criteria have been established, those of the International Study Group (Table 2) are the most widely used.18 Their sensitivity for Behçet disease has been found to be 92%, and their specificity 97%.19
Both CT enterography and inflammatory markers would depict inflammation, but since this is present in both Crohn disease and Behçet disease, these tests would not be helpful in this situation.
Endoscopic appearance of Crohn disease and Behçet disease
Intestinal Behçet disease, like Crohn disease, is an inflammatory bowel disease occurring throughout the gastrointestinal tract (small and large bowel). Both are chronic diseases with a waxing and waning course and have similar extraintestinal manifestations. Typical endoscopic lesions are deep, sharply demarcated (“punched-out”), round ulcers. The intestinal Behçet disease and Crohn disease ulcer phenotype and distribution can look the same, and in both entities, rectal sparing and “skip lesions” have been described.20–22
Nevertheless, findings on endoscopy have been analyzed to try to differentiate between Crohn disease and Behçet disease.
In 2009, Lee et al23 published a simple and accurate strategy for distinguishing the two diseases endoscopically. The authors reviewed 250 patients (115 with Behçet disease, 135 with Crohn disease) with ulcers on colonoscopy and identified 5 endoscopic findings indicative of intestinal Behçet disease:
- Round ulcers
- Focal single or focal multiple distribution of ulcers
- Fewer than 6 ulcers
- Absence of a “cobblestone” appearance
- Absence of aphthous lesions.
The two most accurate factors were absence of a cobblestone appearance (sensitivity 100%) and round ulcer shape (specificity 97.5 %). When more than one factor was present, specificity increased but sensitivity decreased.
Using a classification and regression tree analysis, the investigators created an algorithm that endoscopically differentiates between Crohn disease and Behçet disease (Figure 1) with an accuracy of 92 %.23
Histopathologic analysis of both colonic and skin lesions can provide additional clues to the correct diagnosis. Vasculitis suggests Behçet disease, whereas granulomas suggest Crohn disease.
CASE CONTINUED: SKIN BIOPSY AND COLONOSCOPY
Punch biopsy of the skin was performed on the right anterior thigh. Histopathologic analysis revealed acanthotic epidermis, a discrete full-thickness necrotic ulcer with a neutrophilic base, granulation tissue, and vasculitic changes. There were no vasculitic changes or granulomas outside the ulcer base. Cytomegalovirus staining was negative. An interferon-gamma release assay for tuberculosis was negative. Eye examination results were normal.
Colonoscopy showed multiple deep, round, and confluent ulcers with a punched-out appearance and fissures with normal intervening mucosa in the entire examined colon (Figure 2). The terminal ileal mucosa was normal. Colonic biopsies were consistent with cryptitis and rare crypt abscesses. Vasculitis was not identified.
Although the histologic changes were nonspecific, at this point we considered Behçet disease to be more likely than Crohn disease, given the typical endoscopic appearance and skin changes.
TREATING INTESTINAL BEHÇET DISEASE
3. Which is not considered a standard treatment for intestinal Behçet disease?
- Mesalamine (5-ASA)
- Corticosteroids
- Immunosuppressants
- Mycophenolate mofetil
- Surgery
Overall, data on the management of intestinal Behçet disease are limited. The data that do exist have shown that 5-ASA, corticosteroids, immunosuppressants, and surgery are options, but not mycophenolate mofetil.
Consensus recommendations from the Japanese IBD Research Group,24 published in 2007, included 5-ASA, corticosteroids, immunosuppressants, enteral and total parenteral nutrition, and surgical resection. In 2014, the group published a second consensus statement, adding anti-tumor necrosis factor (TNF) agents as standard therapy for this disease.22
Mycophenolate mofetil has not been shown to be effective in the treatment of mucocutaneous Behçet disease,25 although it may be effective in the treatment of its neurologic manifestations.26 Data regarding its efficacy in intestinal Behçet disease are sparse.
Differences in treatment for Crohn and Behçet disease
Although the treatment options are comparable for Behçet disease and Crohn disease, certain features differ.
Doses of 5-ASA and immunnosuppressive agents are typically higher in Crohn disease. For example, the optimal dose of 5-ASA is up to 3 g/day for Behçet disease but up to 4.8 g/day for Crohn disease.
Standard dosing for azathioprine is 50 to 100 mg/day for Behçet disease but 2 to 2.5 mg/kg/day (eg, 168 to 210 mg/day for a 185-lb patient) for Crohn disease.
In addition, evidence supporting the use of biologic agents such as anti-TNF agents or vedolizumab is more abundant in Crohn disease.
Finally, data on monitoring drug levels of immunomodulators or biologics are available only for patients with Crohn disease, not Behçet disease. Thus, an accurate diagnosis is important.
CASE CONTINUED: EMERGENCY LAPAROTOMY
Our patient continued to experience abdominal pain and bloody diarrhea despite receiving corticosteroids intravenously in high doses. We were also considering anti-TNF therapy.
At this point, CT of her abdomen and pelvis was repeated and showed free intraperitoneal air consistent with a perforation of the transverse colon.
She underwent emergency exploratory laparotomy. Intraoperative findings included pneumoperitoneum but no gross peritoneal contamination, extensive colitis with a contained splenic flexure perforation, and normal small-bowel features without evidence of enteritis. Subtotal colectomy, implantation of the rectal stump into the subcutaneous tissue, and end-ileostomy were performed.
After 23 days of recovery in the hospital, she was discharged on oral antibiotics and 4 weeks of steroid taper.
PROGNOSIS OF INTESTINAL BEHÇET DISEASE
4. What can the patient expect from her intestinal Behçet disease in the future?
- The disease is cured after resection of the diseased segments
- Behçet disease is a progressive lifelong disorder that can recur after surgery
Like Crohn disease, Behçet disease should be considered a lifelong progressive disorder, even after surgical resection of diseased segments.
It is unclear which patients will have a complicated disease course and need treatment with stronger immunosuppression. In patients with intestinal Behçet disease whose disease is in remission on thiopurine therapy, the 1-year relapse rate has been reported as 5.8%, and the 5-year relapse rate 51.7%.27,28 After surgical resection, the 5-year recurrence rate was 47.2%, and 30.6% of patients needed repeat surgery.29 Predictors of poor prognosis were younger age, higher erythrocyte sedimentation rate, higher C-reactive protein level, low albumin level at diagnosis, and a high disease-activity index for intestinal Behçet disease.30
The Korean IBD Study Group has developed and validated a disease activity index for intestinal Behçet disease.28 The index has a list of weighted scores for 8 symptoms, which provides for a more objective assessment of disease activity for determining the best treatment approach.
CASE CONTINUED
The patient has continued with her follow-up care and appointments in gastroenterology, rheumatology, and dermatology clinics. She still complains of intermittent abdominal pain, occasional bleeding at the rectal stump, intermittent skin lesions mainly in the form of pustular lesions, and intermittent joint pain. If symptoms persist, anti-TNF therapy is an option.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol 1981; 5:689–695.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol 2008; 22:1033–1043.
- Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol 2008; 26:265–273.
- Tavarela Veloso F. Skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20(suppl 4):50–53.
- Yüksel I, Basar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:546–550.
- Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis 1998; 4:142–148.
- Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011; 7:235–241.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet’s syndrome: facts and controversies. Clin Dermatol 2013; 31:352–361.
- Lee ES, Bangz D, Lee S. Dermatologic manifestation of Behçet’s disease. Yonsei Med J 1997; 38:380–389.
- Davatchi F, Chams-Davatchi C, Ghodsi Z, et al. Diagnostic value of pathergy test in Behçet’s disease according to the change of incidence over the time. Clin Rheumatol 2011; 30:1151–1155.
- Friedman-Birnbaum R, Bergman R, Aizen E. Sensitivity and specificity of pathergy test results in Israeli patients with Behçet’s disease. Cutis 1990; 45:261–264.
- Mahr A, Maldini C. Epidemiology of Behçet’s disease. Rev Med Interne 2014; 35:81–89. French.
- Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis. Am J Clin Dermatol 2002; 3:319–328.
- Padmavathy L, Lakshmana Rao L, Ethirajan N, Ramakrishna Rao M, Subrahmanyan EN, Manohar U. Tuberculosis verrucosa cutis (TBVC)—foot with miliary tuberculosis. Indian J Tuberc 2007; 54:145–148.
- Drago F, Aragone MG, Lugani C, Rebora A. Cytomegalovirus infection in normal and immunocompromised humans. A review. Dermatology 2000; 200:189–195.
- Yazısız V. Similarities and differences between Behçet’s disease and Crohn’s disease. World J Gastrointest Pathophysiol 2014; 5:228–238.
- Chin AB, Kumar AS. Behçet colitis. Clin Colon Rectal Surg 2015; 28:99–102.
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet 1990; 335:1078–1080.
- Davatchi F. Diagnosis/classification criteria for Behcet’s disease. Patholog Res Int 2012; 2012:607921.
- Chang DK, Kim JJ, Choi H, et al. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc 2007; 66(suppl):S96–S98.
- Hamdulay SS, Cheent K, Ghosh C, Stocks J, Ghosh S, Haskard DO. Wireless capsule endoscopy in the investigation of intestinal Behçet’s syndrome. Rheumatology (Oxford) 2008; 47:1231–1234.
- Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFa monoclonal antibodies. J Gastroenterol 2014; 49:156–162.
- Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 2009; 41:9–16.
- Kobayashi K, Ueno F, Bito S, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet’s disease using a modified Delphi approach. J Gastroenterol 2007; 42:737–745.
- Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 2001; 203:322–324.
- Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011; 29(suppl 67):S64–S67.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2012; 18:750–757.
- Cheon JH, Han DS, Park JY, et al; Korean IBD Study Group. Development, validation, and responsiveness of a novel disease activity index for intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:605–613.
- Jung YS, Yoon JY, Lee JH, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behçet’s disease. Inflamm Bowel Dis 2011; 17:1594–1602.
- Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behçet’s disease during the first five years. Dig Dis Sci 2013; 58:496–503.
Detecting and managing hereditary colorectal cancer syndromes in your practice
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
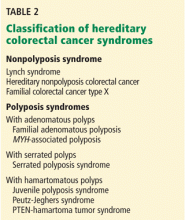
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
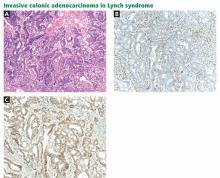
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
KEY POINTS
- Hereditary colorectal cancer syndromes carry a substantial risk of intestinal and extraintestinal tumors.
- Affected patients need increased cancer surveillance and may benefit from prophylactic surgery.
- Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history.
- Patients suspected of having hereditary colorectal cancer syndromes should be referred for genetic counseling and, if appropriate, for genetic testing.