User login
Is chest radiography routinely needed after thoracentesis?
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
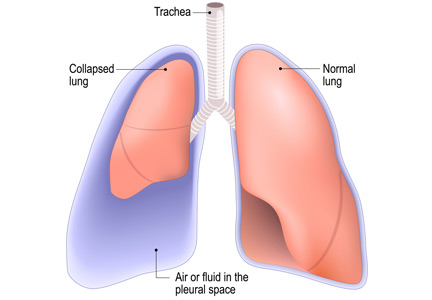
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
When does S aureus bacteremia require transesophageal echocardiography?
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
When should brain imaging precede lumbar puncture in cases of suspected bacterial meningitis?
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
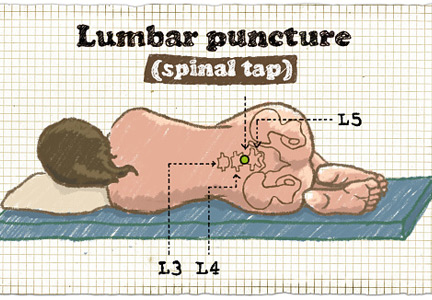
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
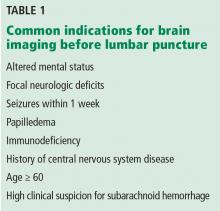
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Wilson disease
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
To the Editor: We read the IM Board Review article by Hanouneh et al in the February issue of the Journal with great interest.1 The authors described an interesting case of a young woman presenting with what initially seemed to be jaundice of acute onset, with rapid progression to acute encephalopathy and worsening liver failure. The patient was eventually diagnosed with fulminant Wilson disease and, thankfully, underwent successful liver transplant. We thank the authors for their in-depth review of the common causes of acute liver failure, the general approach to management, and the tailored treatment of Wilson disease in such settings.
However, we believe that several aspects merit further attention. First, on initial presentation and investigation, it would have been important to consider cholestatic hepatobiliary pathologic processes (eg, choledocholithiasis, cholangitis, primary biliary cirrhosis, primary sclerosing cholangitis), given the characteristic liver panel results.
Second, the authors rightly pointed out that hemolytic anemia is common in patients with acute liver failure secondary to Wilson disease. However, it is important to keep in mind that additional testing should include Coombs testing (typically negative in Wilson disease) and examination of the peripheral smear to exclude other etiologies, since such conditions as thrombotic thrombocytopenic purpura may present with multiorgan failure as well.2
Third, the authors report that Kayser-Fleischer rings are pathognomonic for Wilson disease. However, many reports in peer-reviewed medical journals suggest that this may not be the case and the overall clinical picture should be
considered.3
Fourth, while the authors focus their attention on liver transplant, several other treatments deserve mentioning. We agree that liver transplant is considered the only lifesaving treatment. But in certain situations, molecular absorbent recirculation systems and hemodialysis may provide temporary support while awaiting transportation to a liver transplant center or actual liver transplant.4
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.
- Hanouneh MA, Garber A, Tavill AS, Zein NN, Hanouneh IA. A tale of two sisters with liver disease. Cleve Clin J Med 2016; 83:109–115.
- Nguyen TC, Cruz MA, Carcillo JA. Thrombocytopenia-associated multiple organ failure and acute kidney injury. Crit Care Clin 2015; 31:661–674.
- Frommer D, Morris J, Sherlock S, Abrams J, Newman S. Kayser-Fleischer-like rings in patients without Wilson’s disease. Gastroenterology 1977; 72:1331–1335.
- Hamlyn AN, Gollan JL, Douglas AP, Sherlock S. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J 1977; 2:660–663.