User login
Commentary: Topical Treatments for AD and Possible Lifestyle Adjustments, July 2024
In this real-life study, Patruno and colleagues found that dupilumab worked well but more slowly in patients with a higher body mass index (BMI). On the basis of these findings, if patients are not in a hurry, the standard dose of dupilumab should eventually work, regardless of BMI. If patients are in a hurry to see improvement, perhaps dose escalation could be considered for patients with a high BMI, or perhaps topical triamcinolone could be used to speed time-to–initial resolution in the high-BMI population.
In the very well-done study by Silverberg and colleagues, tapinarof was effective, well tolerated, and generally safe for atopic dermatitis in adults and children. Great! Topical tapinarof should soon be another good option for our patients with atopic dermatitis. How valuable will it be? We already have topical corticosteroids that are very effective for atopic dermatitis, and we have multiple other nonsteroidal topical agents, including topical calcineurin inhibitors and topical ruxolitinib.
Perhaps the biggest limitation of all these treatments is poor adherence to topical treatment. I'm not sure how effective even highly effective nonsteroidal topicals will be for patients who did not respond to topical steroids when the primary reason for topical steroid failure is poor treatment adherence. I'd love to see the development of a once-a-week or once-a-month topical therapy that would address the poor-adherence hurdle.
Abrocitinib is an effective treatment for improving atopic dermatitis. Although atopic dermatitis is a chronic condition requiring long-term management, we'd like to minimize exposure to the drug to avoid side effects. Thyssen and colleagues described the effectiveness of two maintenance treatment regimens: continuing 200 mg/d or reducing the dose to 100 mg/d. Both regimens prevented flares more than did placebo. This study also provided information on safety of the maintenance regimens. Rates of herpetic infections were low across all the groups, but unlike the two treatment groups, there were no cases of herpes simplex infection in the patients in the placebo arm.
In this real-life study, Patruno and colleagues found that dupilumab worked well but more slowly in patients with a higher body mass index (BMI). On the basis of these findings, if patients are not in a hurry, the standard dose of dupilumab should eventually work, regardless of BMI. If patients are in a hurry to see improvement, perhaps dose escalation could be considered for patients with a high BMI, or perhaps topical triamcinolone could be used to speed time-to–initial resolution in the high-BMI population.
In the very well-done study by Silverberg and colleagues, tapinarof was effective, well tolerated, and generally safe for atopic dermatitis in adults and children. Great! Topical tapinarof should soon be another good option for our patients with atopic dermatitis. How valuable will it be? We already have topical corticosteroids that are very effective for atopic dermatitis, and we have multiple other nonsteroidal topical agents, including topical calcineurin inhibitors and topical ruxolitinib.
Perhaps the biggest limitation of all these treatments is poor adherence to topical treatment. I'm not sure how effective even highly effective nonsteroidal topicals will be for patients who did not respond to topical steroids when the primary reason for topical steroid failure is poor treatment adherence. I'd love to see the development of a once-a-week or once-a-month topical therapy that would address the poor-adherence hurdle.
Abrocitinib is an effective treatment for improving atopic dermatitis. Although atopic dermatitis is a chronic condition requiring long-term management, we'd like to minimize exposure to the drug to avoid side effects. Thyssen and colleagues described the effectiveness of two maintenance treatment regimens: continuing 200 mg/d or reducing the dose to 100 mg/d. Both regimens prevented flares more than did placebo. This study also provided information on safety of the maintenance regimens. Rates of herpetic infections were low across all the groups, but unlike the two treatment groups, there were no cases of herpes simplex infection in the patients in the placebo arm.
In this real-life study, Patruno and colleagues found that dupilumab worked well but more slowly in patients with a higher body mass index (BMI). On the basis of these findings, if patients are not in a hurry, the standard dose of dupilumab should eventually work, regardless of BMI. If patients are in a hurry to see improvement, perhaps dose escalation could be considered for patients with a high BMI, or perhaps topical triamcinolone could be used to speed time-to–initial resolution in the high-BMI population.
In the very well-done study by Silverberg and colleagues, tapinarof was effective, well tolerated, and generally safe for atopic dermatitis in adults and children. Great! Topical tapinarof should soon be another good option for our patients with atopic dermatitis. How valuable will it be? We already have topical corticosteroids that are very effective for atopic dermatitis, and we have multiple other nonsteroidal topical agents, including topical calcineurin inhibitors and topical ruxolitinib.
Perhaps the biggest limitation of all these treatments is poor adherence to topical treatment. I'm not sure how effective even highly effective nonsteroidal topicals will be for patients who did not respond to topical steroids when the primary reason for topical steroid failure is poor treatment adherence. I'd love to see the development of a once-a-week or once-a-month topical therapy that would address the poor-adherence hurdle.
Abrocitinib is an effective treatment for improving atopic dermatitis. Although atopic dermatitis is a chronic condition requiring long-term management, we'd like to minimize exposure to the drug to avoid side effects. Thyssen and colleagues described the effectiveness of two maintenance treatment regimens: continuing 200 mg/d or reducing the dose to 100 mg/d. Both regimens prevented flares more than did placebo. This study also provided information on safety of the maintenance regimens. Rates of herpetic infections were low across all the groups, but unlike the two treatment groups, there were no cases of herpes simplex infection in the patients in the placebo arm.
Commentary: Interrelationships Between AD and Other Conditions, June 2024
Traidl and colleagues report that obesity was linked to worse AD in German patients. The authors hit the nail on the head with their conclusions: "In this large and well-characterized AD patient cohort, obesity is significantly associated with physician- and patient-assessed measures of AD disease severity. However, the corresponding effect sizes were low and of questionable clinical relevance." What might account for the small difference in disease severity? Adherence to treatment is highly variable among patients with AD. A small tendency toward worse adherence in patients with obesity could easily explain the small differences seen in disease severity.
Eichenfeld and colleagues report that topical ruxolitinib maintained good efficacy over a year in open-label use. Topical ruxolitinib is a very effective treatment for AD. If real-life AD patients on topical ruxolitinib were to lose efficacy over time, I'd consider the possibility that they've developed mutant Janus kinase (JAK) enzymes that are no longer responsive to the drug. Just kidding. I doubt that such mutations ever occur. If topical ruxolitinib in AD patients were to lose efficacy over time, I'd strongly consider the possibility that patients' adherence to the treatment is no longer as good as it was before. Long-term adherence to topical treatment can be abysmal. Adherence in clinical trials is probably a lot better than in clinical practice. When we see topical treatments that are effective in clinical trials failing in real-life patients with AD, it may be prudent to address the possibility of poor adherence.
I'd love to see a head-to-head trial of tralokinumab vs dupilumab in the treatment of moderate to severe AD. Lacking that, Torres and colleagues report an indirect comparison of the two drugs in patients also treated with topical steroids. This study, funded by the manufacturer of tralokinumab, reported that the two drugs have similar efficacy. How much of the efficacy was due to the topical steroid use is not clear to me. I'd still love to see a head-to-head trial of tralokinumab vs dupilumab to have a better, more confident sense of their relative efficacy.
Is AD associated with brain cancer, as reported by Xin and colleagues? I'm not an expert in their methodology, but they did find a statistically significant increased risk, with an odds ratio of 1.0005. I understand the odds ratio for smoking and lung cancer to be about 80. Even if the increased odds of 1.005 — no, wait, that's 1.0005 — is truly due to AD, this tiny difference doesn't seem meaningful in any way.
Traidl and colleagues report that obesity was linked to worse AD in German patients. The authors hit the nail on the head with their conclusions: "In this large and well-characterized AD patient cohort, obesity is significantly associated with physician- and patient-assessed measures of AD disease severity. However, the corresponding effect sizes were low and of questionable clinical relevance." What might account for the small difference in disease severity? Adherence to treatment is highly variable among patients with AD. A small tendency toward worse adherence in patients with obesity could easily explain the small differences seen in disease severity.
Eichenfeld and colleagues report that topical ruxolitinib maintained good efficacy over a year in open-label use. Topical ruxolitinib is a very effective treatment for AD. If real-life AD patients on topical ruxolitinib were to lose efficacy over time, I'd consider the possibility that they've developed mutant Janus kinase (JAK) enzymes that are no longer responsive to the drug. Just kidding. I doubt that such mutations ever occur. If topical ruxolitinib in AD patients were to lose efficacy over time, I'd strongly consider the possibility that patients' adherence to the treatment is no longer as good as it was before. Long-term adherence to topical treatment can be abysmal. Adherence in clinical trials is probably a lot better than in clinical practice. When we see topical treatments that are effective in clinical trials failing in real-life patients with AD, it may be prudent to address the possibility of poor adherence.
I'd love to see a head-to-head trial of tralokinumab vs dupilumab in the treatment of moderate to severe AD. Lacking that, Torres and colleagues report an indirect comparison of the two drugs in patients also treated with topical steroids. This study, funded by the manufacturer of tralokinumab, reported that the two drugs have similar efficacy. How much of the efficacy was due to the topical steroid use is not clear to me. I'd still love to see a head-to-head trial of tralokinumab vs dupilumab to have a better, more confident sense of their relative efficacy.
Is AD associated with brain cancer, as reported by Xin and colleagues? I'm not an expert in their methodology, but they did find a statistically significant increased risk, with an odds ratio of 1.0005. I understand the odds ratio for smoking and lung cancer to be about 80. Even if the increased odds of 1.005 — no, wait, that's 1.0005 — is truly due to AD, this tiny difference doesn't seem meaningful in any way.
Traidl and colleagues report that obesity was linked to worse AD in German patients. The authors hit the nail on the head with their conclusions: "In this large and well-characterized AD patient cohort, obesity is significantly associated with physician- and patient-assessed measures of AD disease severity. However, the corresponding effect sizes were low and of questionable clinical relevance." What might account for the small difference in disease severity? Adherence to treatment is highly variable among patients with AD. A small tendency toward worse adherence in patients with obesity could easily explain the small differences seen in disease severity.
Eichenfeld and colleagues report that topical ruxolitinib maintained good efficacy over a year in open-label use. Topical ruxolitinib is a very effective treatment for AD. If real-life AD patients on topical ruxolitinib were to lose efficacy over time, I'd consider the possibility that they've developed mutant Janus kinase (JAK) enzymes that are no longer responsive to the drug. Just kidding. I doubt that such mutations ever occur. If topical ruxolitinib in AD patients were to lose efficacy over time, I'd strongly consider the possibility that patients' adherence to the treatment is no longer as good as it was before. Long-term adherence to topical treatment can be abysmal. Adherence in clinical trials is probably a lot better than in clinical practice. When we see topical treatments that are effective in clinical trials failing in real-life patients with AD, it may be prudent to address the possibility of poor adherence.
I'd love to see a head-to-head trial of tralokinumab vs dupilumab in the treatment of moderate to severe AD. Lacking that, Torres and colleagues report an indirect comparison of the two drugs in patients also treated with topical steroids. This study, funded by the manufacturer of tralokinumab, reported that the two drugs have similar efficacy. How much of the efficacy was due to the topical steroid use is not clear to me. I'd still love to see a head-to-head trial of tralokinumab vs dupilumab to have a better, more confident sense of their relative efficacy.
Is AD associated with brain cancer, as reported by Xin and colleagues? I'm not an expert in their methodology, but they did find a statistically significant increased risk, with an odds ratio of 1.0005. I understand the odds ratio for smoking and lung cancer to be about 80. Even if the increased odds of 1.005 — no, wait, that's 1.0005 — is truly due to AD, this tiny difference doesn't seem meaningful in any way.
Commentary: Studies Often Do Not Answer Clinical Questions in AD, May 2024
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
Commentary: Choosing Treatments of AD, and Possible Connection to Learning Issues, April 2024
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.
Not everyone with AD treated with dupilumab gets clear or almost clear in clinical trials. The study by Cork and colleagues looked to see whether those patients who did not get to clear or almost clear were still having clinically meaningful improvement. To test this, the investigators looked at patients who still had mild or worse disease and then at the proportion of those patients at week 16 who achieved a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms, and quality of life: ≥50% improvement in Eczema Area and Severity Index or ≥4-point reduction in worst scratch/itch numerical rating scale, or ≥6-point reduction in Children's Dermatology Life Quality Index/Infants' Dermatitis Quality of Life Index. Significantly more patients, both clinically and statistically significantly more, receiving dupilumab vs placebo achieved the composite endpoint (77.7% vs 24.6%; P < .0001).
The "success rate" reported in clinical trials underestimates how often patients can be successfully treated with dupilumab. I don't need a complicated composite outcome to know this. I just use the standardized 2-point Patient Global Assessment measure. I ask patients, "How are you doing?" If they say "Great," that's success. If they say, "Not so good," that's failure. I think about 80% of patients with AD treated with dupilumab have success based on this standard.
Hand dermatitis can be quite resistant to treatment. Even making a diagnosis can be challenging, as psoriasis and dermatitis of the hands looks so similar to me (and when I used to send biopsies and ask the pathologist whether it's dermatitis or psoriasis, invariably the dermatopathologist responded "yes"). The study by Kamphuis and colleagues examined the efficacy of abrocitinib in just over 100 patients with hand eczema who were enrolled in the BioDay registry. Such registries are very helpful for assessing real-world results. The drug seemed reasonably successful, with only about 30% discontinuing treatment. About two thirds of the discontinuations were due to inefficacy and about one third to an adverse event.
I think there's real value in prescribing the treatments patients want. Studies like the one by Ameen and colleagues, using a discrete-choice methodology, allows one to determine patients' average preferences. In this study, the discrete-choice approach found that patients prefer safety over other attributes. Some years ago, my colleagues and I queried patients to get a sense of their quantitative preferences for different treatments. Our study also found that patients preferred safety over other attributes. However, when we asked them to choose among different treatment options, they didn't choose the safest one. I think they believe that they prefer safety, but I'm not sure they really do. In any case, the average preference of the entire population of people with AD isn't really all that important when we've got just one patient sitting in front of us. It's that particular patient's preference that should drive the treatment plan.
Commentary: Drug Comparisons and Contact Allergy in AD, February 2024
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
Commentary: JAK Inhibitors and Comorbidities in AD, December 2023
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Commentary: New and old treatments for AD, November 2023
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Flohr and colleagues present the results of a controlled trial of cyclosporine vs methotrexate for severe atopic dermatitis ("Efficacy and Safety of Ciclosporin Versus Methotrexate in the Treatment of Severe Atopic Dermatitis in Children and Young People"). Cyclosporine worked faster, yet methotrexate was a bit more effective in the long run. Both treatments had considerable side effects; 10% and 14% had serious events with cyclosporine and methotrexate, respectively. My only quibble is with the first word of the abstract background section; the authors call cyclosporine and methotrexate "conventional" systemic drugs for atopic dermatitis. At this point, considering safety and efficacy, I would consider drugs like dupilumab to be the "conventional" systemic treatment for atopic dermatitis.
Wan and colleagues ("Neuropsychiatric Disorders in Adults With Atopic Dermatitis") present an exceptionally well-done study with a huge patient population. The study compared about 600,000 adults with atopic dermatitis vs over 2,000,000 adults without the disease. A sample size like that offers a lot of power to detect very small differences between groups. The researchers report higher rates of anxiety and depression in patients with atopic dermatitis compared to those without. Are those differences clinically meaningfully different? The rates of depression were 14 and 17 cases per 1000 patient-years for those without and those with severe atopic dermatitis, respectively. That's a difference of 3 per 1000 patient-years. So maybe roughly 300 patients with atopic dermatitis would need to be seen to observe one patient with depression due to atopic dermatitis (assuming that the observed differences in rates between those with and those without atopic dermatitis were due to the dermatitis). The authors conclude, "Clinicians should inquire about mental health in patients with AD." I don't think their data support such a conclusion. We'd need to see a cost-effectiveness study to know if that's an intervention that we should do. Given the very small difference between the rates in those with and those without atopic dermatitis, it might be reasonable to conclude that we should inquire about mental health in patients with atopic dermatitis about as much as we should in patients without atopic dermatitis.
Some years ago, there was an over-the-counter topical product for psoriasis based on a banana peel extract. I think it was marketed as "FDA approved" for psoriasis (which was legal to say because the product also contained tar) and as being as effective as topical calcipotriene as published in the Journal of Investigational Dermatology (JID). I went to look for the article; the "publication" was the abstract of a poster presentation. The study followed a very small study population for a short period of time. The study was, I believe, underpowered to detect differences between the banana peel extract and the vitamin D analog. Those data were presented as a poster, the poster abstracts were printed in JID, and, voilà, the product was marketed as being as effective as topical calcipotriene as published in JID.
Sowlati and colleagues ("Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children") randomly assigned 42 patients to receive either a xyloglucan/pea protein topical therapy or hydrocortisone. The participants were followed for 2 weeks. Both groups improved. We don't know whether they improved more than they would have with moisturizer. This study doesn't make me excited about prescribing the xyloglucan/pea protein topical.
The study by Mohamed and colleagues comparing tacrolimus and hydrocortisone reminds me that we have an effective generic topical anti-inflammatory for our patients with atopic dermatitis. Given the safety of topical tacrolimus, I prefer prescribing the 0.1% ointment for all my patients, though I give the lower concentration, approved for children, if the insurer makes me.
Simpson and colleagues' post hoc analysis of tralokinumab tells us that, with continued use, some patients who don't respond well initially will have greater improvement. But what I'd really like to see is a head-to-head study comparing tralokinumab vs dupilumab. Dupilumab seems to have stronger efficacy based on their reported trial numbers, but a head-to-head trial would give us greater confidence in their relative benefits.
I have trouble getting excited about this study by Cork and colleagues ("Dupilumab Safety and Efficacy in a Phase III Open-Label Extension Trial in Children 6-11 Years of Age With Severe Atopic Dermatitis"). I feel very comfortable with dupilumab already.
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
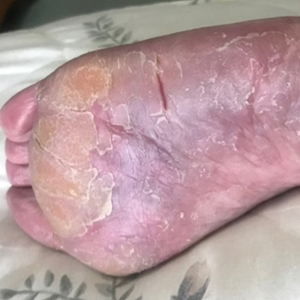
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
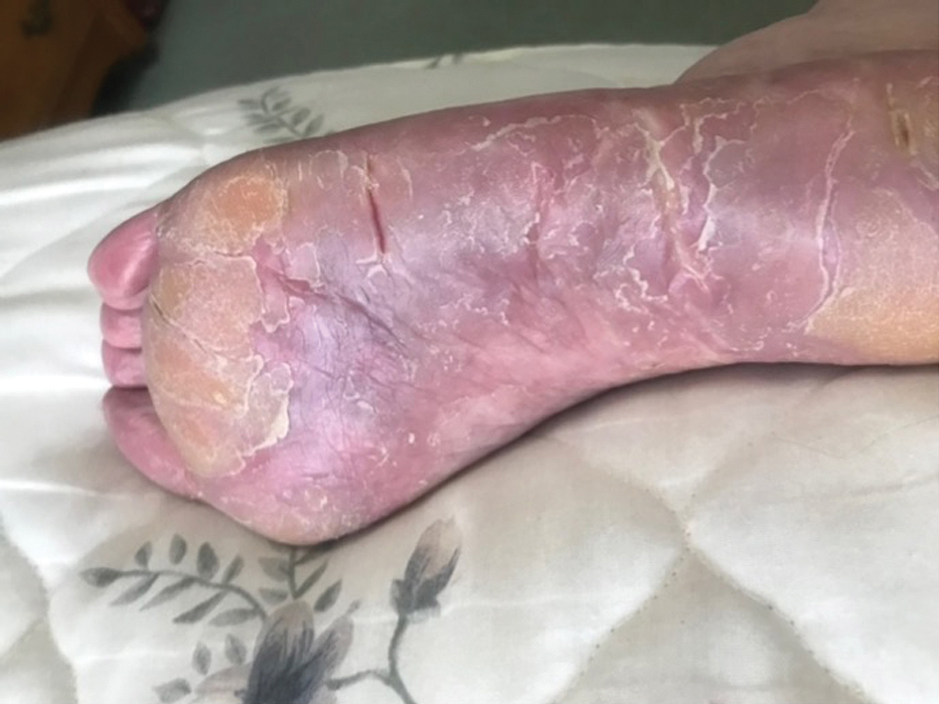
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
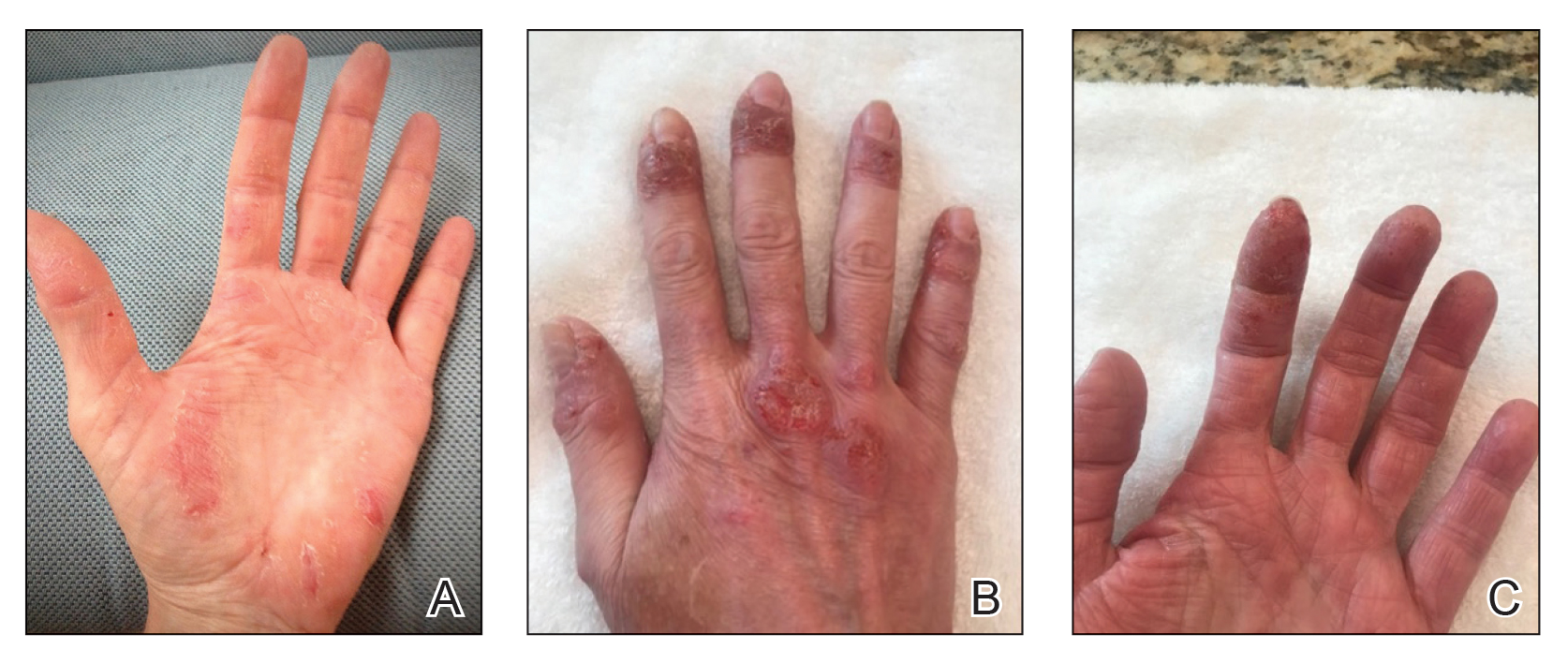
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
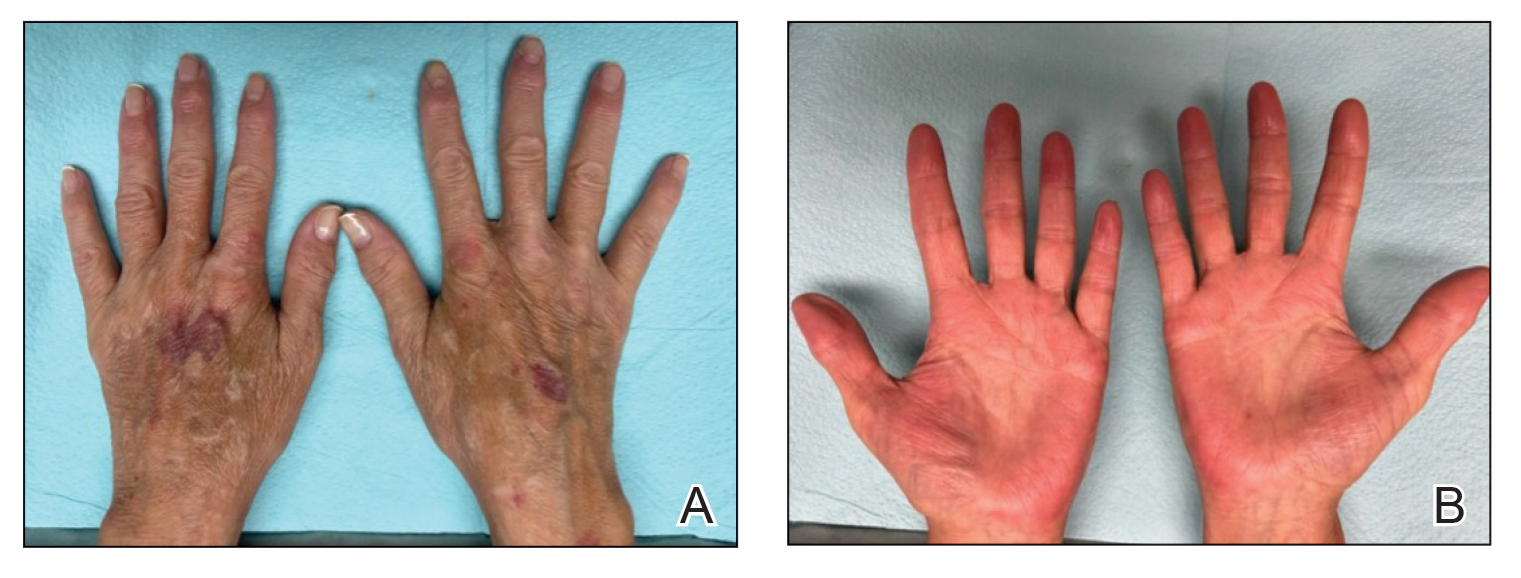
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Commentary: Are "significant" results necessarily clinically meaningful? October 2023
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
Commentary: Newer Drugs for AD Plus Dupilumab and Other Issues, September 2023
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059