User login
Updates in Perioperative Medicine
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event <1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P<0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, <24% of patients had known coronary artery disease, and <5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P<0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P<0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P<0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event <1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P<0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, <24% of patients had known coronary artery disease, and <5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P<0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P<0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P<0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
Given the rapid expansion of the field of perioperative medicine, clinicians need to remain apprised of the current evidence to ensure optimization of patient care. In this update, we review 10 key articles from the perioperative literature, with the goal of summarizing the most clinically important evidence over the past year. This summary of recent literature in perioperative medicine is derived from the Update in Perioperative Medicine sessions presented at the 10th Annual Perioperative Medicine Summit and the Society of General Internal Medicine 38th Annual Meeting. A systematic search strategy was used to identify pertinent articles, and the following were selected by the authors based on their relevance to the clinical practice of perioperative medicine.
PERIOPERATIVE CARDIOVASCULAR CARE
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e278e333.
Background
The American College of Cardiology/American Heart Association (ACC/AHA) perioperative guideline provides recommendations for the evaluation and management of cardiovascular disease in patients undergoing noncardiac surgery.
Findings
The new guideline combines the evaluation of surgery‐ and patient‐specific risk in the algorithm for preoperative cardiovascular evaluation into a single step and recommends the use of 1 of 3 tools: the Revised Cardiac Risk Index (RCRI),[1] National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator,[2] or the NSQIP‐derived myocardial infarction and cardiac arrest calculator.[3] Estimation of risk is also simplified by stratification into only 2 groups: low risk (risk of major adverse cardiac event <1%) and elevated risk (1% risk). Coronary evaluation can be considered for patients with elevated cardiac risk and poor functional capacity, but is advised only if the results would alter perioperative management. For example, a patient with very high risk who has evidence of ischemia on stress testing may choose to forego surgery. Preoperative coronary revascularization is only indicated for patients meeting criteria in the nonsurgical setting.
For patients with previous percutaneous coronary intervention, the ACC/AHA has not changed its recommendations to optimally delay surgery for at least 30 days after bare‐metal stenting and at least 1 year after drug‐eluting stent (DES) placement. However, in patients with a DES placed 6 to 12 months previously, surgery can be performed if the risks of surgical delay outweigh the risks of DES thrombosis. After any type of coronary stenting, dual antiplatelet therapy should be continued uninterrupted through the first 4 to 6 weeks and even later whenever feasible. If not possible, aspirin therapy should be maintained through surgery unless bleeding risk is too high.
The guideline recommends perioperative continuation of ‐blockers in patients taking them chronically. Preoperative initiation of ‐blocker therapy may be considered for patients with myocardial ischemia on stress testing or 3 RCRI factors and should be started far enough in advance to allow determination of patient's tolerance prior to surgery.
Cautions
Many recommendations are based on data from nonrandomized trials or expert opinion, and the data in areas such as perioperative ‐blockade continue to evolve.
Implications
The ACC/AHA guideline continues to be a critically valuable resource for hospitalists providing perioperative care to noncardiac surgery patients.
Wijeysundera DN, Duncan D, Nkonde‐Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines.
J Am Coll Cardiol. 2014;64(22):24062425.
Background
Various clinical trials have reported conflicting results regarding the efficacy and safety of perioperative ‐blockers resulting in guideline committees changing their recommendations. Because of questions raised regarding the scientific integrity of the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography)‐I[4] and DECREASE‐IV[5] trials as well as the dosing of ‐blockers in POISE (PeriOperative Ischemic Evaluation) study,[6] this systematic review was performed in conjunction with the ACC/AHA guideline update[7] to evaluate the data with and without these trials.
Findings
Sixteen randomized control trials (RCTs) (n=12,043) and 1 cohort study (n=348) were included in the analysis. Perioperative ‐blockers were associated with a reduction in nonfatal myocardial infarction (MI) (relative risk [RR]: 0.69; 95% confidence interval [CI]: 0.58‐0.82; P<0.001) but an increase in bradycardia (RR: 2.61; 95% CI: 2.18‐3.12), hypotension (RR: 1.47; 95% CI: 1.34‐1.6), and nonfatal strokes (RR: 1.76; 95% CI: 1.07‐2.91; P=0.02). The POISE trial was the only one demonstrating a statistically significant increase in stroke.
The major discrepancy between the DECREASE trials and the other RCTs was related to mortalitya reduction in both cardiovascular and all‐cause death in DECREASE but an increased risk of all‐cause death in the other trials.
Cautions
Because of its size, the POISE trial heavily influences the results, particularly for mortality and stroke. Including the DECREASE trials reduces the otherwise increased risk for death to a null effect. Exclusion of the POISE and DECREASE trials leaves few data to make conclusions about safety and efficacy of perioperative ‐blockade. Several cohort studies have found metoprolol to be associated with worse outcomes than with atenolol or bisoprolol (which were preferred by the European Society of Cardiology guidelines).[8]
Implications
Perioperative ‐blockade started within 1 day of noncardiac surgery was associated with fewer nonfatal MIs but at the cost of an increase in hypotension, bradycardia, and a possible increase in stroke and death. Long‐term ‐blockade should be continued perioperatively, whereas the decision to initiate a ‐blocker should be individualized. If starting a ‐blocker perioperatively, it should be done 2 days before surgery.
Botto F, Alonso‐Coello P, Chan MT, et al.; on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology. 2014;120(3):564578.
Background
Many patients sustain myocardial injury in the perioperative period as evidenced by troponin elevations, but most do not meet diagnostic criteria for MI. Myocardial injury after noncardiac surgery (MINS) is defined as prognostically relevant myocardial injury due to ischemia that occurs within 30 days after noncardiac surgery. This international, prospective cohort study of 15,065 patients 45 years old who underwent in‐patient noncardiac surgery determined diagnostic criteria, characteristics, predictors, and 30‐day outcomes of MINS.
Findings
The diagnostic criterion for MINS was a peak troponin T level 0.03 ng/mL judged to be due to an ischemic etiology. Twelve independent predictors of MINS were identified including age 75 years, known cardiovascular disease or risk factors, and surgical factors. MINS was an independent predictor of 30‐day mortality (adjusted hazard ratio [HR]: 3.87; 95% CI: 2.96‐5.08). Age >75 years, ST elevation, or new left bundle branch block, and anterior ischemic findings were independent predictors of 30‐day mortality among patients with MINS.
Cautions
Although screening high‐risk surgical patients without signs or symptoms of ischemia with postoperative troponins will increase the frequency of diagnosing MINS, evidence for an effective treatment has not yet been established. The ACC/AHA guidelines state that routine screening is of uncertain benefit for this reason.
Implications
Because MINS is common and carries a poor 30‐day prognosis, clinical trials are needed to determine when to obtain postoperative troponins and how to prevent and treat this complication.[9] Some observational data from POISE suggest that aspirin and statins can reduce the risk of 30‐day mortality in patients with postoperative MIs.
Devereaux PJ, Mrkobrada M, Sessler DI, et al. for the POISE‐2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):14941503.
Devereaux PJ, Sessler DI, Leslie K, et al. for the POISE‐2 Investigators. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014; 370(16):15041513.
Background
Medical risk reduction with aspirin and other agents in perioperative patients remains controversial. The POISE‐2 trial is a blinded RCT examining the effects of aspirin and clonidine on outcomes in >10,000 noncardiac surgery patients at risk of cardiovascular complications. The aspirin arm of the study included the initiation group and the continuation stratum, as well as placebo. Patients in the clonidine portion of the trial received 0.2 mg of clonidine or placebo daily for the same time periods.
Findings
The primary outcome was a composite of death or nonfatal MI within 30 days of surgery. Outcomes were similar in patients initiated or continued on aspirin. No difference was seen between aspirin or placebo in the primary outcome (7.0% vs 7.1%; HR: 0.86; 95% CI: 0.86‐1.15; P=0.92). There were no differences in rates of MI, venous thromboembolism, or stroke. Major bleeding rates were higher in aspirin versus placebo‐treated patients (4.6% vs 3.8%; HR: 1.23; 95% CI: 1.01‐1.49; P=0.04).
Clonidine did not alter the composite outcome of death or nonfatal MI (7.3% vs 6.8%; HR: 1.08; 95% CI: 0.93‐1.26; P=0.29). Clinically significant hypotension, bradycardia, and nonfatal cardiac arrest were more common in clonidine‐treated patients, although no difference was detected in stroke rates.
Cautions
Although patients in the trial had cardiovascular risk factors, <24% of patients had known coronary artery disease, and <5% had coronary stents. Conclusions based on this trial regarding perioperative management of antiplatelet therapy should not include patients with coronary artery stents.
Implications
Aspirin started before surgery and continued perioperatively did not decrease the rate of death or nonfatal MI but increased the risk of major bleeding. Perioperative management of aspirin needs to be undertaken in the context of cardiac and bleeding risks. Clonidine also did not improve outcomes and increased the risk of bradycardia and hypotension. Current guidelines recommend against using alpha‐2 agonists for prevention of perioperative cardiac events7; however, patients already on alpha‐2 agonists should not stop them abruptly.
PERIOPERATIVE PULMONARY CARE
Mutter TC, Chateau D, Moffatt M, et al. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707718.
Background
An increasing body of literature associates obstructive sleep apnea (OSA) with an increased risk of postoperative complications. Despite evidence of risk, potential benefits of preoperative diagnosis and treatment of OSA remain unclear.
Findings
Using databases to identify patients prescribed continuous positive airway pressure (CPAP) therapy, the study compared postoperative outcomes of patients who underwent surgery any time after polysomnography (PSG) and CPAP prescription (diagnosed OSA [DOSA]) and those who had surgery during the 5 years preceding their PSG (undiagnosed OSA [UOSA]). These patients were matched with patients who underwent the same procedure for the same indication and had no insurance claims for PSG or diagnosis of sleep‐disordered breathing.
After multivariate analysis, OSA of any type was associated with increased pulmonary complications (odds ratio [OR]: 2.08; 95% CI: 1.35‐2.19). However, no significant differences in respiratory outcomes were noted between DOSA patients (N=2640) and those with UOSA (N=1571). DOSA patients did have fewer cardiovascular complications than UOSA patients (OR: 0.34; 95% CI: 0.15‐0.77). Only severe OSA (apnea‐hypopnea index >30) was associated with increased pulmonary and cardiovascular complications.
Cautions
Although this study suggests an association between preoperative diagnosis and treatment of OSA and reduced cardiovascular complications, the results are not definitive due to the inability to control for all confounding variables in a retrospective study utilizing an administrative database.
Implications
OSA is an important risk factor for postoperative complications, and this study suggests that preoperative treatment with CPAP is associated with reduced risk of cardiovascular complications, particularly in patients with severe OSA. Future controlled trials should focus on the risk‐reduction potential of preoperative diagnosis and treatment of OSA.
Mazo V, Sabat S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219231.
Background
In 2010, Canet et al. published a novel risk index, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index, to provide a quantitative estimate of the risk of postoperative pulmonary complications (PPCs).[10]
In the current report, Mazo and colleagues studied the ARISCAT index in a broader sample to characterize its accuracy in predicting PPC risk. The ARISCAT index is derived from clinical risk factors: (1) age, (2) preoperative oxygen saturation, (3) respiratory infection in the prior month, (4) anemia, (5) surgical site, (6) duration of surgery, and (7) emergency surgery, with varying weights based on the strength of the association in a multivariable analysis. This score can be calculated via addition of these weighted risk factors, with a score>45 equal to high risk for PPC.
Findings
Examining 5099 patients from 63 European hospitals, the authors definition of PPC included respiratory failure, pulmonary infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. PPC rates were as follows: low risk (3.39%), intermediate risk (12.98%), and high risk (38.01%). The positive likelihood ratio for PPC among the highest risk group was 7.12. The C statistic for fit was 0.80. Observed PPC rates were higher than predicted for the low (3.39% vs 0.87%) and intermediate (12.98% vs 7.82%) risk groups.
Cautions
The calibration slopes were less than ideal in all subsamples, with the Western European sample performing better than the other geographic areas; suggesting that the coefficients on the ARISCAT index may benefit from recalibration to match specific populations.
Implications
This is the first major pulmonary risk index that has been externally validated. Its use of readily available clinical information, simplicity, and accuracy in estimating PPC risk make it an important addition to the toolkit during a preoperative evaluation.
PERIOPERATIVE ATRIAL FIBRILLATION/ANTICOAGULATION
Gialdini G, Nearing K, Bhave P, et al. Perioperative atrial fibrillation and the long term risk of ischemic stroke. JAMA. 2014;312(6):616622.
Background
New‐onset atrial fibrillation (AF) is the most common perioperative arrhythmia.[11] However, little is known regarding the long‐term risks of ischemic stroke in patients who develop perioperative AF. This retrospective cohort study examined adults with no preexisting history of AF, hospitalized for surgery, and discharged free of cerebrovascular disease between 2007 and 2011 (n=1,729,360).
Findings
Of the eligible patients, 1.43% (95% CI: 1.41%‐1.45%) developed perioperative AF, and 0.81% (95% CI: 0.79%‐0.82%) had a stroke up to 1 year after discharge. Perioperative AF was associated with subsequent stroke after both cardiac (HR: 1.3; 95% CI: 1.1‐1.6) and noncardiac surgery (HR: 2; 95% CI: 1.7‐2.3). The association with stroke was stronger for perioperative AF after noncardiac versus cardiac surgery (P<0.001 for interaction).
Cautions
This is a retrospective cohort study, using claims data to identify AF and stroke. Data on duration of the perioperative AF episodes or use of antithrombotic therapies were not available.
Implications
The association found between perioperative AF and long‐term risk of ischemic stroke may suggest that perioperative AF, especially after noncardiac surgery, should be treated aggressively in terms of thromboembolic risk; however, further data will be required to validate this association.
Van Diepen S, Youngson E, Ezekowitz J, McAlister F. Which risk score best predicts perioperative outcomes in nonvalvular atrial fibrillation patients undergoing noncardiac surgery? Am Heart J. 2014;168(1):6067.
Background
Patients with nonvalvular AF (NVAF) are at increased risk for adverse perioperative outcomes after noncardiac surgery.[12] The RCRI is commonly used to predict perioperative cardiovascular events for all patients, including those with NVAF, though AF is not part of this risk assessment. The goal of this retrospective cohort study was to examine the prognostic utility of already existing NVAF risk indices, including the CHADS2 (Congestive heart failure, Hypertension, Age 75 years, Diabetes mellitus, prior stroke or transient ischemic attack), CHA2DS2‐VASc (Congestive heart failure; Hypertension; Age 75 years; Diabetes mellitus; Stroke, TIA, or thromboembolism [TE]; Vascular disease; Age 65 to 74 years; Sex category [female]), and R2CHADS2 (Renal dysfunction, Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA) for perioperative outcomes in patients undergoing noncardiac surgery.
Findings
A population dataset of NVAF patients (n=32,160) who underwent noncardiac surgery was examined, with outcome measures including 30‐day mortality, stroke, TIA, or systemic embolism. The incidence of the 30‐day composite outcome was 4.2% and the C indices were 0.65 for the RCRI, 0.67 for CHADS2, 0.67 for CHA2DS2‐VASc, and 0.68 for R2CHADS2. The Net Reclassification Index (NRI), a measure evaluating the improvement in prediction performance gained by adding a marker to a set of baseline predictors, was calculated. All NVAF scores performed better than the RCRI for predicting mortality risk (NRI: 12.3%, 8.4%, and 13.3% respectively, all P<0.01).
Cautions
Patients in the highest risk category by RCRI appear to have an unadjusted higher 30‐day mortality risk (8%) than that predicted by the other 3 scores (5%, 5.6%, and 5%), indicating that these risk scores should not completely supplant the RCRI for risk stratification in this population. In addition, the overall improvement in predictive capacity of the CHADS2, CHA2DS2‐VASc, and R2CHADS2, although superior to the RCRI, is modest.
Implications
These findings indicate that the preoperative risk stratification for patients with NVAF can be improved by utilizing the CHADS2, CHA2DS2‐VASc, or R2CHADS2 scores when undergoing noncardiac surgery. For patients with NVAF identified as high risk for adverse outcomes, this assessment can be integrated into the preoperative discussion on the risks/benefits of surgery.
Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488494
Background
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with AF. Many AF patients on long‐term anticoagulation undergo procedures requiring temporary interruption of OAC. Although guidelines have been published on when and how to initiate bridging therapy, they are based on observational data. Thus, it remains unclear which patients should receive bridging anticoagulation.
Findings
This is a US registry of outpatients with AF with temporary interruptions of OAC for a procedure. Of 7372 patients treated with OAC, 2803 overall interruption events occurred in 2200 patients (30%). Bridging anticoagulants were used in 24% (n=665). Bleeding events were more common in bridged than nonbridged patients (5.0% vs 1.3%; adjusted OR: 3.84; P<0.0001). The overall composite end point of myocardial infarction, stroke or systemic embolism, major bleeding, hospitalization, or death within 30 days was significantly higher in patients receiving bridging (13% vs 6.3%; adjusted OR: 1.94; P=0.0001). This statistically significant increase in the composite outcome, which includes cardiovascular events, is most likely in part secondary to inclusion of bleeding events. The recently published BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial did not find a statistically significant difference in cardiovascular events between bridged and nonbridged patients.[13]
Cautions
Although patients who were bridged appear to have had more comorbidities and a higher mean CHADS2 score than patients who were not bridged, it is difficult to determine which population of patients may be high risk enough to warrant bridging, as indicated by current American College of Chest Physicians guidelines, as this was not evaluated in this study
Implications
The use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates. The BRIDGE trial also found that forgoing bridging anticoagulation decreased the risk of major bleeding in patients with AF and was noninferior to bridging for the prevention of arterial TE.[13]
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
- , , , et al. Derivation and prospective evaluation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
- , , , et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.
- , , , et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387.
- , , , et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341(24):1789–1794.
- , , , et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Ann Surg. 2009;249(6):921–926.
- POISE Study Group, , , , et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- , , , et al. American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137.
- , , , et al. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non‐cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383–431.
- , , , et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119(5):1053–1063.
- , , , et al. ARISCAT Group: Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology. 2010;113:1338–1350.
- , . Noncardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28(10 suppl):N145–N150.
- , , , et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major cardiac surgery. Am Heart J. 2012;164(6):918–924.
- , , , et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833.
Case studies in perioperative management: Challenges, controversies, and common ground
CASE 1: RADICAL PROSTATECTOMY IN A MAN WITH ACUTE DEEP VEIN THROMBOSIS
A 69-year-old man is seen in the preoperative clinic 1 week before a scheduled radical prostatectomy. He has been diagnosed with femoral deep vein thrombosis (DVT) following a complaint of calf soreness.
Question 1.1: How would you treat him for his DVT?
A. Intravenous (IV) unfractionated heparin (UFH)
B. Low-molecular-weight heparin (LMWH)
C. Inferior vena cava (IVC) filter
D. Combination of pharmacologic therapy and then an IVC filter
Dr. Steven L. Cohn: The latest edition of the American College of Chest Physicians (ACCP) evidence-based guidelines on antithrombotic therapy recommends the use of therapeutic-dose subcutaneous LMWH over IV UFH for initial treatment of acute DVT in the outpatient or inpatient setting.1 Additionally, indications for an IVC filter include the prevention of pulmonary embolism (PE) in a patient with DVT who requires full-dose anticoagulation but cannot receive it, as would be the case here if the patient proceeds with surgery as scheduled. So if surgery will be postponed, the best option is LMWH; if surgery will not be postponed, the best answer is a combination of pharmacologic therapy with low-dose LMWH and an IVC filter, preferably a retrievable one.2
Question 1.2: You recommend postponing surgery, but the patient is worried about metastatic disease. For how long should surgery be postponed?
A. 2 weeks
B. 1 month
C. 2 months
D. 3 months
E. 6 months
Dr. Cohn: In the absence of anticoagulation therapy, the risk of venous thromboembolism (VTE) is approximately 40% (~1% per day) during the first month following an acute VTE and then declines markedly, to approximately 10%, during the second and third months following the acute event.3 Therefore, I would suggest that the patient wait at least 1 month after an acute DVT before undergoing surgery.
Dr. BobbieJean Sweitzer: This patient is in a hypercoagulable state, and the surgery itself will induce excess hypercoagulability. With a femoral DVT already present, his risk of VTE or PE is likely to be greater than 1% per day during the first month. If he does develop a PE, it may potentially be fatal.
Question 1.3: According to the patient, the surgeon and the internist discussed options, but the surgeon “doesn’t believe in filters” and the patient doesn’t want to postpone the procedure, despite your recommendation. Two weeks later he shows up for surgery having stopped his LMWH 3 days before. What would you do?
A. Cancel the surgery and restart full-dose LMWH
B. Proceed with prophylactic-dose LMWH
C. Proceed after giving a full therapeutic dose
D. Insert a filter and give DVT prophylaxis
Dr. Cohn: A bridging protocol should have been discussed with the surgeon and anesthesiologist before the procedure. Therapeutic levels of LMWH persist as long as 18 hours after discontinuation; therefore, the ACCP recommends interrupting LMWH 24 hours before surgery.4
Dr. Sweitzer: The lack of a bridging protocol in this case created a problem. The patient was afraid to continue anticoagulation after hearing the internist and surgeon disagree about the plan, and thus stopped it entirely, and he did not want to delay surgery because he was fearful of metastasis. The surgeon was adamant that IVC filters don’t work. The internist was concerned that the patient was at high risk for a PE. Even though the documented risk of postponing radical prostatectomy for a short time is inconsequential, I was convinced that the patient would not believe this if metastasis were to develop in the future.
Question 1.4: How would you have managed his anticoagulation perioperatively?
A. Stop LMWH 12 hours before surgery and restart at full dose 12 to 24 hours after surgery
B. Stop LMWH 24 hours before surgery and restart at full dose 24 hours after surgery
C. Stop LMWH 24 hours before surgery and restart prophylactic dosing 12 to 24 hours after surgery, and then full-dose LMWH in 48 to 72 hours
D. Stop LMWH 24 hours before surgery and restart at full dose 72 hours after surgery
Dr. Cohn: The correct timing for stopping LMWH is 24 hours before surgery. As for how to resume anticoagulation in patients at high risk for VTE or those undergoing major surgery, the latest ACCP guidelines recommend the following4:
- Reinitiation of anticoagulation 12 to 24 hours postoperatively, assuming adequate hemostasis in patients not at high risk for bleeding
- Use of a prophylactic dose or no anticoagulation for up to 72 hours if the patient is at high risk for bleeding.
These recommendations are a departure from previous practice, in which we routinely restarted anticoagulation 6 to 12 hours postoperatively.
Dr. Sweitzer: According to guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA),5 if twice-daily LMWH is stopped 24 hours ahead of time (as long as patients have normal renal function), it is safe to perform epidural or spinal anesthesia, if either is an option. If full-dose UFH is used, the partial thromboplastin time (PTT) is monitored and central neuraxial blockade may be done if the PTT is in the normal range, which typically is 2 to 6 hours after UFH is stopped.
Additionally, the platelet count should be checked every 3 days postoperatively while the patient is on UFH or LMWH. It may be just as important to monitor the platelet count preoperatively if the patient has been on UFH or LMWH for an extended duration, especially if a central neuraxial anesthetic technique is planned.
Dr. Cohn: The reason for monitoring the platelet count is the potential for heparin-induced thrombocytopenia in patients on UFH. I recently encountered a patient who developed postoperative heparin-induced thrombocytopenia with thrombosis while on LMWH, which is relatively uncommon compared with UFH.
Case resolution
After much discussion of the risk of a significant PE with the patient, family, urologist, and vascular surgeon, it is decided that a temporary IVC filter will be placed in the operating room immediately after induction of general anesthesia and before the prostatectomy. The operation is delayed about 1 hour to allow this option. The patient is successfully treated and has the IVC filter removed 1 month postoperatively.
CASE 2: RADICAL CYSTECTOMY IN ELDERLY MAN WITH CARDIAC RISK FACTORS
A 78-year-old obese Russian-speaking man is seen in the preoperative clinic prior to a scheduled radical cystectomy for highly invasive bladder cancer. He is a poor historian and argues with the several family members accompanying him, but it is determined that his medical history includes hypertension, diabetes mellitus, a myocardial infarction (MI) 5 years previously (in Russia), and stable angina that is determined to be class II.
He had no previous work-up and no electrocardiogram (ECG). His medications are aspirin, metoprolol, and metformin. His blood pressure is 190/100 mm Hg, heart rate 90 beats per minute, and body mass index 32. On examination, there is no murmur, S3 gallop, or rales. His blood glucose is 220 mg/dL, and his creatinine is slightly elevated (1.4 mg/dL). ECG verifies a prior MI.
Question 2.1: Which of the following additional tests should be ordered preoperatively?
A. Hemoglobin (Hb) A1c
B. Lipid profile
C. Both
D. Neither
Dr. Sweitzer: Because the surgery is not elective, no immediate benefit would be achieved by ordering either an HbA1c or a lipid profile. However, if you view the preoperative evaluation as an opportunity to manage risk factors over the long term, then it may be a good idea to order the lipid profile because this patient has rarely engaged the health care system. Likewise, the HbA1c can be ordered to set in place his long-term management. Sometimes we focus on the preoperative visit only in the context of the surgery, but if a test or intervention is appropriate and needed for long-term management, then it is appropriate to do now.
Dr. Cohn: There is no evidence to support using the preoperative HbA1c to alter management decisions. I would not postpone surgery based on the HbA1c value, as I would if his glucose level were 600 mg/dL. Most of the studies that have assessed postoperative complications based on preoperative HbA1c did not control for postoperative glucose levels. The incidence of complications varies based on the type of complication and the type of surgery.
Similarly, I would not use lipid values to guide management of this patient. Studies suggest that perioperative statin therapy may reduce postoperative morbidity and mortality in patients undergoing vascular surgery (see article by Poldermans on page S79 of this supplement), but our patient already has indications for a statin—a remote MI and diabetes—independent of what his lipid values are.
Question 2.2: How would you manage his elevated blood pressure (190/100 mm Hg)?
A. Discontinue metoprolol and start a different antihypertensive drug
B. Increase the metoprolol dose
C. Continue metoprolol and add a second drug
D. Observe him on his current regimen
Dr. Cohn: I would increase the dose of metoprolol and consider adding another drug, in view of his heart rate (90 beats per minute) and his cardiac status. Beta-blocker therapy should not be discontinued because doing so in the perioperative period is associated with an increased risk of adverse events such as cardiac death and MI.
Dr. Sweitzer: I would push up the metoprolol a bit to reduce the heart rate, knowing that beta-blockers are probably not the most efficacious antihypertensive agents. I would caution against starting an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) because he is scheduled to undergo a fairly significant procedure with expected blood loss and fluid shifts, and either of those agents in combination with a beta-blocker would be challenging to manage on the day of surgery.
Question 2.3: How would you manage his metformin perioperatively?
A. Discontinue it 48 hours preoperatively
B. Discontinue it 24 hours preoperatively
C. Withhold it on the morning of surgery
D. Continue it on the morning of surgery
Dr. Sweitzer: We routinely advise patients to hold all their oral diabetes medications the morning of surgery, primarily because many anesthesiologists are uncertain about the differing risks of hypoglycemia associated with the various oral agents.
Most of us will never see a patient who has lactic acidosis from metformin use. A systematic literature review and analysis found no increase in the risk of lactic acidosis with metformin compared with other oral hypoglycemics,6 so fear of lactic acidosis is not a valid reason to discontinue metformin. In fact, I think it is inappropriate to ever postpone or cancel surgery simply because the patient inadvertently took metformin on the morning of surgery. Some may argue that patients with renal insufficiency are at higher risk of lactic acidosis from metformin use on the morning of surgery, but keep in mind that renal insufficiency is a relative contraindication to metformin use in the first place. Unless the patient is scheduled for a bilateral nephrectomy, his or her renal function is not going to be acutely reduced enough to enable a morning dose of metformin to cause lactic acidosis.
Dr. Cohn: Additionally, in a recent study of patients undergoing coronary artery bypass graft surgery (CABG), there was no increased risk of in-hospital morbidity or mortality in patients who received metformin on the morning of surgery,7 although I typically stop it 24 hours before major surgery.
Question 2.4: With respect to statin therapy, which course would you choose preoperatively?
A. Start a statin at a low dose
B. Start a statin at an intermediate dose
C. Start a statin at a high dose
D. Do not start a statin
Dr. Cohn: The answer to this question is not clear cut. The reason not to start a prophylactic statin would be the lack of evidence of benefit in patients undergoing noncardiac, nonvascular surgery, although there is evidence of potential benefit in patients undergoing vascular surgery.* The arguments in favor of starting a statin are that this patient has independent indications for a statin and the planned surgery is a high-risk procedure.
(* Editor’s note: In the time since this summit, results of the DECREASE-IV trial were published [Dunkelgrun et al, Ann Surg 2009; 249:921–926], showing a statisically nonsignificant trend toward improved outcomes at 30 days with fluvastatin in intermediate-risk patients undergoing noncardiovascular surgery.)
In cohort studies, perioperative death rates have been lower in statin recipients than in those not taking a statin.8 In the Dutch Echographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III), which randomized noncardiac vascular surgery patients to perioperative fluvastatin or placebo, rates of MI and the composite end point of nonfatal MI or cardiovascular death were significantly lower in the statin group than in the placebo group.9
Question 2.5: Which of the following cardiac tests would you order preoperatively?
A. Exercise ECG
B. Dobutamine stress echocardiogram
C. Dipyridamole nuclear imaging
D. Coronary angiography
E. No further cardiac testing
Dr. Cohn: I wouldn’t do any cardiac testing since this patient needs surgery for his malignancy and the results of any testing would be highly unlikely to change management, in terms of canceling the surgery. This approach is consistent with the 2007 guidelines on perioperative cardiovascular evaluation for noncardiac surgery issued by the American College of Cardiology (ACC) and the American Heart Association (AHA).10
Dr. Sweitzer: I would differ on this question. This patient has not been evaluated adequately for his coronary artery disease. He has poor functional capacity that complicates assessment of his symptoms. He also has diabetes, so he is more likely to have silent myocardial ischemia. At age 78, he is understandably concerned about his survival: radical cystectomy is a major operation associated with significant blood loss, fluid shifts, and a long-term recuperative state. In this case, a cardiac evaluation may change management, not in terms of considering coronary revascularization before the surgery, but in terms of affecting the assessment of his chance of surviving this major operation, his life span following the operation, and his quality of life. For example, a highly positive dobutamine stress echocardiogram or certain wall motion abnormalities would suggest that he might not be protected even by optimal perioperative medical management.
Question 2.6: Which of the following would you do preoperatively to assess pulmonary risk?
A. Obtain pulmonary function tests
B. Order a sleep study
C. Both
D. Neither
Dr. Sweitzer: There is no evidence supporting routine pulmonary function tests for patients undergoing procedures other than lung resection. If obstructive sleep apnea were suspected, I would order a sleep study only if I had access to one quickly to avoid delaying the surgery. Cancer surgery should never be delayed to get a sleep study. However, if this patient were seen in the primary care clinic, I would order a sleep study and, if indicated, put him on continuous positive airway pressure (CPAP). Whether or not preoperative CPAP makes a difference hasn’t been shown. No randomized controlled trials have been conducted, but there are some suggestions that the risks of ischemia and atrial arrhythmias in patients with known coronary artery disease can be reduced with CPAP. It is not always easy to initiate CPAP postoperatively because the number of CPAP machines is limited and titration by a respiratory technician is required, which is typically done in a sleep lab.
How the case was actually managed
Neither an HbA1c measurement nor a lipid profile was ordered preoperatively, for lack of supportive evidence. The patient was continued on his beta-blocker and the dosage was increased sufficiently to control his blood pressure and heart rate. Metformin was continued, and statin therapy was begun preoperatively in light of the patient’s independent indications for it and the high-risk nature of the procedure. Stress testing was not ordered, in light of the lack of indication, given the patient’s stable angina. The patient refused a sleep study. The operation was lengthy and involved significant blood loss. The patient had a complicated postoperative course and ultimately died from multiorgan failure.
CASE 3: OPERATIONS OF VARIABLE RISK IN ELDERLY MAN WITH ACTIVE CARDIAC CONDITION
Scenario A: A 75-year-old man with diabetes, class III angina, and Q waves in inferior leads on his ECG is scheduled for elective femoropopliteal bypass surgery. His medications include isosorbide mononitrate (120 mg), amlodipine (10 mg), metoprolol controlled release (100 mg), atorvastatin (80 mg), insulin, and aspirin (81 mg). His heart rate is 64 beats per minute, blood pressure is controlled at 120/80 mm Hg, low-density lipoprotein cholesterol is 80 mg/dL, and creatinine is 1.5 μmol/L.
Scenario B: Consider the same patient undergoing elective cholecystectomy instead of a femoropopliteal bypass.
Scenario C: Consider the same patient scheduled for a cystoscopy instead of the other procedures. He had one episode of gross hematuria 1 week ago that resolved. Work-up by his urologist included a urinalysis and culture that were normal, cytology that was negative for malignancy, and a sonogram and computed tomography scan that were both negative. He has had no further bleeding and is not anemic. The urologist wants to do the cystoscopy for the sake of completeness.
Question 3.1: What would be your preoperative course of action in the above scenarios?
A. Order a dobutamine stress echocardiogram
B. Order nuclear imaging with dipyridamole or adenosine
C. Order coronary angiography
D. Order a resting two-dimensional echocardiogram
E. Continue his current medications and send to surgery with no further testing
Dr. Cohn: This is a man with an active cardiac condition and class III angina, which is considered severe angina in the ACC/AHA 2007 guidelines on perioperative cardiac evaluation and care.10 The guidelines’ recommendation is to delay surgery for further evaluation and treatment. He is already on maximal medical therapy, which has failed to control his symptoms. He has poor exercise capacity. The only difference among the case scenarios is a variation in surgical risk.
This patient has independent indications for coronary angiography regardless of whether or not he’s undergoing surgery. He deserves evaluation for possible revascularization to improve his quality of life and symptoms.
I would send the patient to the catheterization lab in every one of these instances, with the possible exception of the cystoscopy scenario, where one could argue that revascularization with stenting would require antiplatelet therapy that might increase the bleeding risk, and also that the antiplatelet therapy would have to be interrupted for the cystoscopy, potentially increasing thrombotic risk.
Dr. Sweitzer: I disagree. The ACC/AHA 2007 guidelines do not recommend going directly to catheterization but rather recommend delaying surgery for further evaluation and treatment.10 We must ask whether this patient is truly receiving optimal medical management. After all, he is not on an ACE inhibitor or an ARB.
We must also consider whether the surgery is truly elective. In the first scenario, if he has peripheral vascular disease, he is likely to develop gangrene and have a further decrease in exercise capacity, which reduces his functional ability and increases his risk of comorbid conditions. He is at significant risk of developing worsening renal insufficiency or renal failure if he undergoes angiography. Coronary revascularization will delay treatment of his peripheral vascular disease. The Coronary Artery Revascularization Prophylaxis (CARP) trial showed no benefit of coronary revascularization relative to medical management in patients undergoing vascular surgery,11 as is planned for this patient. I believe one must balance two competing risks and have an in-depth discussion with the patient.
In the second scenario, not treating gallstones or preventing cholelithiasis poses more risk to the health of this diabetic patient than does elective surgery if he needs a cholecystectomy. Emergency surgery, especially for acute cholecystitis, also significantly increases the risk of a cardiac event.
In the third scenario, the cystoscopy may uncover bladder cancer, which may be adversely affected by a delay of surgery. Regardless, the patient had gross hematuria and would be at risk for further bleeding should he undergo stenting with the requisite antiplatelet therapy.
Catheterization is not normally recommended unless CABG or stenting is being considered, yet I have seen no data that either of these procedures prolongs life except in very limited circumstances such as left main disease treated with bypass grafting. Though it is true that CABG reduces the incidence and severity of angina, it does not modify the physiologic cause of angina but rather may result in symptom improvement by damaging somatic nerve fibers to the heart. Putting a stent in this patient would be like applying a bandage: his symptoms will likely recur if he does not receive optimal medical management.
In a 2007 science advisory, several major medical societies cautioned against percutaneous coronary intervention (PCI) with drug-eluting stent placement in patients expected to undergo noncardiac surgery that would require interruption of antiplatelet therapy in the following 12 months (and against PCI with bare metal stent placement in patients undergoing such surgery in the following 4 to 6 weeks).12 Therefore, I would not recommend catheterization for a patient whose noncardiac disease is likely to require surgery in the very near future, as is the case in each of the surgical scenarios above. One could consider noninvasive stress testing, which would be a safer approach and would almost certainly identify either significant stenosis of the left main coronary artery or three-vessel disease, which would be the only possible reasons to recommend CABG. I don’t believe there is any role for PCI for this patient.
Dr. Cohn: I argue for symptom relief even if it doesn’t prolong life. This patient cannot walk across the room without having symptoms despite taking multiple medications. I think he deserves a chance at revascularization if the angiogram shows he has a stenosis amenable to it, but I agree that a drug-eluting stent should not be placed if we know that he will undergo surgery within a few months.
CASE 4: VENTRAL HERNIA REPAIR IN A MIDDLE-AGED WOMAN
A 60-year-old woman is scheduled for ventral hernia repair. Her medical history is unremarkable, with the exception of hypertension. She denies any bleeding problems and had no complications after a laparoscopic cholecystectomy 10 years ago. She has no family history of bleeding disorders.
Question 4.1: Would you order a prothrombin time (PT)/partial thromboplastin time (PTT)?
A. Yes
B. No
Dr. Cohn: I would not.
Dr. Sweitzer: I agree.
Question 4.2: Although not requested, a PT/PTT was ordered anyway. The PT is normal (12.2 sec/12 sec) and the PTT is abnormal (40 sec/25 sec). What is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Factor VII deficiency
C. Factor IX deficiency
D. Factor XI deficiency
E. Factor XII deficiency
Dr. Cohn: The most likely cause is a sample with insufficient blood in the tube. The test wasn’t indicated in the first place, but now it must be done again.
Question 4.3: The PTT is repeated and remains abnormal: 42 sec/25 sec. Mixing studies correct the abnormality to 29 sec/25 sec. Based on this information, what is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Lupus anticoagulant
C. Prekallikrein factor deficiency
D. Factor XII deficiency
Dr. Cohn: This is not a case of lupus anticoagulant because the abnormal PTT was corrected by the mixing study. Causes of a prolonged PTT include deficiencies of factors XII, XI, and IX, so factor XII deficiency is the most likely explanation, though a deficiency higher up the coagulation cascade (ie, prekallikrein factor deficiency) is possible. In the absence of any personal or family bleeding history, it is unlikely to be a deficiency of factors VII or IX (the hemophiliac) or of factor XI, so a deficiency of factor XII or one of the prekallikrein factors is more likely.
Dr. Sweitzer: A mixing study is indeed the appropriate first step. It is ordered from the lab and involves mixing the patient’s blood with normal plasma and incubating the mixture. If the mixture corrects the PTT result, as was the case with this patient, it indicates a coagulation factor deficiency in the patient’s blood; if it doesn’t correct, that should prompt evaluation for lupus anticoagulant or the presence of some other protein or hormone that’s prolonging the PTT.
Question 4.4: How would you manage this patient perioperatively?
A. Fresh frozen plasma
B. Platelet transfusion
C. Cryoprecipitate
D. Factor VII
E. No treatment necessary
Dr. Cohn: No treatment is necessary. Factor XII deficiency does not cause bleeding, regardless of the PTT. Factor XI deficiency is associated with bleeding, but usually there is a family history or a personal history of bleeding with surgery.
Screening coagulation studies are not usually indicated in a patient without a personal or family history of bleeding, liver disease, alcohol or drug use, or current anticoagulant therapy. Such studies are usually normal in such patients, and when they are not, it’s usually because of a lab error or a disease (hypercoagulable state) or factor deficiency that does not cause bleeding
Dr. Sweitzer: However, if the PTT is prolonged, the cause should be identified, because if the patient is sent to the operating room without an explanation for the prolongation, the perioperative team might think the patient has a bleeding problem and use fresh frozen plasma too readily. Fresh frozen plasma is not appropriate for everyone and may actually make a potentially hypercoagulable state worse.
DISCUSSION
Question from the audience: It was said that use of ACE inhibitors and ARBs should be avoided around the time of surgery. I’ve done an extensive literature search and found minimal to no evidence to support this practice. To the contrary, I found fairly good evidence to indicate that heart failure can be exacerbated significantly and acutely, as early as within 24 hours, when patients are taken off their ACE inhibitor or ARB. I would like your viewpoint on this basic pathology in perioperative medicine.
Dr. Cohn: The literature on the use of ACE inhibitors or ARBs prior to noncardiac surgery consists of five studies with fewer than 500 patients in total, as recently reviewed by Rosenman et al.13 Although there was no excess of death or MI associated with taking these medications on the morning of surgery, they did increase the need for fluid and pressors.
Dr. Sweitzer: Patients with hypertension have bigger variations of blood pressure, both hypo- and hypertension, in the perioperative period. For this reason, it was standard of care 30 years ago to stop all antihypertensive drugs, including beta-blockers, preoperatively. We soon found that although this practice prevented many episodes of hypotension, it increased the occurrence of perioperative hypertension and the likelihood of cardiac events. It then became standard of care to always continue antihypertensive drugs on the morning of surgery. In the late 1980s and early 1990s, several studies showed that ACE inhibitors and ARBs were associated with a more profound drop in blood pressure upon induction of general anesthesia compared with other antihypertensives.
The usual ways we treat drops in blood pressure—with phenylephrine and ephedrine—are not very effective in treating hypotension associated with general anesthesia in patients taking ACE inhibitors or ARBs. Vasopressin is effective in treating refractory hypotension during surgery, but anesthesiologists don’t use it often. Reducing the doses of induction agents is another means of attenuating the hypotension induced by ACE inhibitors and ARBs.
We should not routinely stop ACE inhibitors and ARBs on the day of surgery, particularly in patients being treated for heart failure, angina, or a prior MI. My bias is to selectively hold ACE inhibitors and ARBs on the morning of surgery in patients who are undergoing a significant operation with a high likelihood of hypotension, have well-controlled preoperative blood pressure, are taking multiple antihypertensive agents, and do not have heart failure. Otherwise, patients should continue their ACE inhibitors and ARBs on the morning of surgery, and the anesthesiologist should be prepared for significant hypotension upon induction of anesthesia, alter anesthesia induction doses accordingly, have vasopressin handy, and avoid the temptation to treat hypotension with fluids or repeated doses of phenylephrine and ephedrine. The previous comment about concerns with ACE inhibitors and ARBs was in the context of initiating new therapies in the immediate preoperative period.
Question from the audience: Urinalysis is ordered for many patients undergoing orthopedic surgery, and invariably some bacteriuria is found. Can you comment on the value of urinalysis and subsequent treatment of abnormal results?
Dr. Cohn: I believe you should never order a urinalysis in an asymptomatic patient, with the exception of patients undergoing procedures that involve genitourinary or gynecologic instrumentation. Ordering a urinalysis before joint replacement has been promoted in the orthopedic literature on the theoretical grounds that bacteria might somehow seed and colonize the joint. Orthopedic surgeons like to do it, but I disregard their requests for it.
Dr. Sweitzer: One study showed that we’d need to spend $1.5 million on screening urinalysis for asymptomatic patients scheduled for joint replacement surgery in order to prevent one joint infection.14
Dr. Cohn: Also, patients are going to get their one dose of cephalosporin before surgery anyway, and that will probably knock out any bacteria that would be found on urinalysis.
Question from the audience: Can you clarify how the 2007 ACC/AHA perioperative guidelines define an active cardiac condition? The patient in your third case report had class III angina, or angina with less than usual activities, but nothing was presented to suggest that his symptoms were unstable. I would suggest that despite his class III symptoms, his angina was stable, and I would have continued down the algorithm rather than defining his cardiac condition as active and considering an intervention.
Dr. Cohn: An active cardiac condition is defined by the ACC as unstable coronary syndromes, which include acute (within the prior 7 days) or recent (within the prior 30 days) MI, unstable angina, and severe (class III or IV) angina.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):454S–545S.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):299S–339S.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Regional Anesthesia & Pain Medicine. 2003; 28:172–197. Available at: http://www.asra.com/consensus-statements/2.html. Accessed May 11, 2009.
- Salpeter S, Gryeber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Syst Rev 2006; (1):CD002967.
- Duncan AI, Koch CG, Xu M, et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg 2007; 104:42–50.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Poldermans D. Fluvastatin XL use is associated with improved cardiac outcome after major vascular surgery: results from a randomized placebo controlled trial. Presented at: European Society of Cardiology Congress 2008; September 1, 2008; Munich, Germany.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation 2007; 115:813–818.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-agiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Lawrence VA, Gafni A, Gross M. The unproven utility of the preoperative urinalysis: economic evaluation. J Clin Epidemiol 1989; 42:1185–1192.
CASE 1: RADICAL PROSTATECTOMY IN A MAN WITH ACUTE DEEP VEIN THROMBOSIS
A 69-year-old man is seen in the preoperative clinic 1 week before a scheduled radical prostatectomy. He has been diagnosed with femoral deep vein thrombosis (DVT) following a complaint of calf soreness.
Question 1.1: How would you treat him for his DVT?
A. Intravenous (IV) unfractionated heparin (UFH)
B. Low-molecular-weight heparin (LMWH)
C. Inferior vena cava (IVC) filter
D. Combination of pharmacologic therapy and then an IVC filter
Dr. Steven L. Cohn: The latest edition of the American College of Chest Physicians (ACCP) evidence-based guidelines on antithrombotic therapy recommends the use of therapeutic-dose subcutaneous LMWH over IV UFH for initial treatment of acute DVT in the outpatient or inpatient setting.1 Additionally, indications for an IVC filter include the prevention of pulmonary embolism (PE) in a patient with DVT who requires full-dose anticoagulation but cannot receive it, as would be the case here if the patient proceeds with surgery as scheduled. So if surgery will be postponed, the best option is LMWH; if surgery will not be postponed, the best answer is a combination of pharmacologic therapy with low-dose LMWH and an IVC filter, preferably a retrievable one.2
Question 1.2: You recommend postponing surgery, but the patient is worried about metastatic disease. For how long should surgery be postponed?
A. 2 weeks
B. 1 month
C. 2 months
D. 3 months
E. 6 months
Dr. Cohn: In the absence of anticoagulation therapy, the risk of venous thromboembolism (VTE) is approximately 40% (~1% per day) during the first month following an acute VTE and then declines markedly, to approximately 10%, during the second and third months following the acute event.3 Therefore, I would suggest that the patient wait at least 1 month after an acute DVT before undergoing surgery.
Dr. BobbieJean Sweitzer: This patient is in a hypercoagulable state, and the surgery itself will induce excess hypercoagulability. With a femoral DVT already present, his risk of VTE or PE is likely to be greater than 1% per day during the first month. If he does develop a PE, it may potentially be fatal.
Question 1.3: According to the patient, the surgeon and the internist discussed options, but the surgeon “doesn’t believe in filters” and the patient doesn’t want to postpone the procedure, despite your recommendation. Two weeks later he shows up for surgery having stopped his LMWH 3 days before. What would you do?
A. Cancel the surgery and restart full-dose LMWH
B. Proceed with prophylactic-dose LMWH
C. Proceed after giving a full therapeutic dose
D. Insert a filter and give DVT prophylaxis
Dr. Cohn: A bridging protocol should have been discussed with the surgeon and anesthesiologist before the procedure. Therapeutic levels of LMWH persist as long as 18 hours after discontinuation; therefore, the ACCP recommends interrupting LMWH 24 hours before surgery.4
Dr. Sweitzer: The lack of a bridging protocol in this case created a problem. The patient was afraid to continue anticoagulation after hearing the internist and surgeon disagree about the plan, and thus stopped it entirely, and he did not want to delay surgery because he was fearful of metastasis. The surgeon was adamant that IVC filters don’t work. The internist was concerned that the patient was at high risk for a PE. Even though the documented risk of postponing radical prostatectomy for a short time is inconsequential, I was convinced that the patient would not believe this if metastasis were to develop in the future.
Question 1.4: How would you have managed his anticoagulation perioperatively?
A. Stop LMWH 12 hours before surgery and restart at full dose 12 to 24 hours after surgery
B. Stop LMWH 24 hours before surgery and restart at full dose 24 hours after surgery
C. Stop LMWH 24 hours before surgery and restart prophylactic dosing 12 to 24 hours after surgery, and then full-dose LMWH in 48 to 72 hours
D. Stop LMWH 24 hours before surgery and restart at full dose 72 hours after surgery
Dr. Cohn: The correct timing for stopping LMWH is 24 hours before surgery. As for how to resume anticoagulation in patients at high risk for VTE or those undergoing major surgery, the latest ACCP guidelines recommend the following4:
- Reinitiation of anticoagulation 12 to 24 hours postoperatively, assuming adequate hemostasis in patients not at high risk for bleeding
- Use of a prophylactic dose or no anticoagulation for up to 72 hours if the patient is at high risk for bleeding.
These recommendations are a departure from previous practice, in which we routinely restarted anticoagulation 6 to 12 hours postoperatively.
Dr. Sweitzer: According to guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA),5 if twice-daily LMWH is stopped 24 hours ahead of time (as long as patients have normal renal function), it is safe to perform epidural or spinal anesthesia, if either is an option. If full-dose UFH is used, the partial thromboplastin time (PTT) is monitored and central neuraxial blockade may be done if the PTT is in the normal range, which typically is 2 to 6 hours after UFH is stopped.
Additionally, the platelet count should be checked every 3 days postoperatively while the patient is on UFH or LMWH. It may be just as important to monitor the platelet count preoperatively if the patient has been on UFH or LMWH for an extended duration, especially if a central neuraxial anesthetic technique is planned.
Dr. Cohn: The reason for monitoring the platelet count is the potential for heparin-induced thrombocytopenia in patients on UFH. I recently encountered a patient who developed postoperative heparin-induced thrombocytopenia with thrombosis while on LMWH, which is relatively uncommon compared with UFH.
Case resolution
After much discussion of the risk of a significant PE with the patient, family, urologist, and vascular surgeon, it is decided that a temporary IVC filter will be placed in the operating room immediately after induction of general anesthesia and before the prostatectomy. The operation is delayed about 1 hour to allow this option. The patient is successfully treated and has the IVC filter removed 1 month postoperatively.
CASE 2: RADICAL CYSTECTOMY IN ELDERLY MAN WITH CARDIAC RISK FACTORS
A 78-year-old obese Russian-speaking man is seen in the preoperative clinic prior to a scheduled radical cystectomy for highly invasive bladder cancer. He is a poor historian and argues with the several family members accompanying him, but it is determined that his medical history includes hypertension, diabetes mellitus, a myocardial infarction (MI) 5 years previously (in Russia), and stable angina that is determined to be class II.
He had no previous work-up and no electrocardiogram (ECG). His medications are aspirin, metoprolol, and metformin. His blood pressure is 190/100 mm Hg, heart rate 90 beats per minute, and body mass index 32. On examination, there is no murmur, S3 gallop, or rales. His blood glucose is 220 mg/dL, and his creatinine is slightly elevated (1.4 mg/dL). ECG verifies a prior MI.
Question 2.1: Which of the following additional tests should be ordered preoperatively?
A. Hemoglobin (Hb) A1c
B. Lipid profile
C. Both
D. Neither
Dr. Sweitzer: Because the surgery is not elective, no immediate benefit would be achieved by ordering either an HbA1c or a lipid profile. However, if you view the preoperative evaluation as an opportunity to manage risk factors over the long term, then it may be a good idea to order the lipid profile because this patient has rarely engaged the health care system. Likewise, the HbA1c can be ordered to set in place his long-term management. Sometimes we focus on the preoperative visit only in the context of the surgery, but if a test or intervention is appropriate and needed for long-term management, then it is appropriate to do now.
Dr. Cohn: There is no evidence to support using the preoperative HbA1c to alter management decisions. I would not postpone surgery based on the HbA1c value, as I would if his glucose level were 600 mg/dL. Most of the studies that have assessed postoperative complications based on preoperative HbA1c did not control for postoperative glucose levels. The incidence of complications varies based on the type of complication and the type of surgery.
Similarly, I would not use lipid values to guide management of this patient. Studies suggest that perioperative statin therapy may reduce postoperative morbidity and mortality in patients undergoing vascular surgery (see article by Poldermans on page S79 of this supplement), but our patient already has indications for a statin—a remote MI and diabetes—independent of what his lipid values are.
Question 2.2: How would you manage his elevated blood pressure (190/100 mm Hg)?
A. Discontinue metoprolol and start a different antihypertensive drug
B. Increase the metoprolol dose
C. Continue metoprolol and add a second drug
D. Observe him on his current regimen
Dr. Cohn: I would increase the dose of metoprolol and consider adding another drug, in view of his heart rate (90 beats per minute) and his cardiac status. Beta-blocker therapy should not be discontinued because doing so in the perioperative period is associated with an increased risk of adverse events such as cardiac death and MI.
Dr. Sweitzer: I would push up the metoprolol a bit to reduce the heart rate, knowing that beta-blockers are probably not the most efficacious antihypertensive agents. I would caution against starting an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) because he is scheduled to undergo a fairly significant procedure with expected blood loss and fluid shifts, and either of those agents in combination with a beta-blocker would be challenging to manage on the day of surgery.
Question 2.3: How would you manage his metformin perioperatively?
A. Discontinue it 48 hours preoperatively
B. Discontinue it 24 hours preoperatively
C. Withhold it on the morning of surgery
D. Continue it on the morning of surgery
Dr. Sweitzer: We routinely advise patients to hold all their oral diabetes medications the morning of surgery, primarily because many anesthesiologists are uncertain about the differing risks of hypoglycemia associated with the various oral agents.
Most of us will never see a patient who has lactic acidosis from metformin use. A systematic literature review and analysis found no increase in the risk of lactic acidosis with metformin compared with other oral hypoglycemics,6 so fear of lactic acidosis is not a valid reason to discontinue metformin. In fact, I think it is inappropriate to ever postpone or cancel surgery simply because the patient inadvertently took metformin on the morning of surgery. Some may argue that patients with renal insufficiency are at higher risk of lactic acidosis from metformin use on the morning of surgery, but keep in mind that renal insufficiency is a relative contraindication to metformin use in the first place. Unless the patient is scheduled for a bilateral nephrectomy, his or her renal function is not going to be acutely reduced enough to enable a morning dose of metformin to cause lactic acidosis.
Dr. Cohn: Additionally, in a recent study of patients undergoing coronary artery bypass graft surgery (CABG), there was no increased risk of in-hospital morbidity or mortality in patients who received metformin on the morning of surgery,7 although I typically stop it 24 hours before major surgery.
Question 2.4: With respect to statin therapy, which course would you choose preoperatively?
A. Start a statin at a low dose
B. Start a statin at an intermediate dose
C. Start a statin at a high dose
D. Do not start a statin
Dr. Cohn: The answer to this question is not clear cut. The reason not to start a prophylactic statin would be the lack of evidence of benefit in patients undergoing noncardiac, nonvascular surgery, although there is evidence of potential benefit in patients undergoing vascular surgery.* The arguments in favor of starting a statin are that this patient has independent indications for a statin and the planned surgery is a high-risk procedure.
(* Editor’s note: In the time since this summit, results of the DECREASE-IV trial were published [Dunkelgrun et al, Ann Surg 2009; 249:921–926], showing a statisically nonsignificant trend toward improved outcomes at 30 days with fluvastatin in intermediate-risk patients undergoing noncardiovascular surgery.)
In cohort studies, perioperative death rates have been lower in statin recipients than in those not taking a statin.8 In the Dutch Echographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III), which randomized noncardiac vascular surgery patients to perioperative fluvastatin or placebo, rates of MI and the composite end point of nonfatal MI or cardiovascular death were significantly lower in the statin group than in the placebo group.9
Question 2.5: Which of the following cardiac tests would you order preoperatively?
A. Exercise ECG
B. Dobutamine stress echocardiogram
C. Dipyridamole nuclear imaging
D. Coronary angiography
E. No further cardiac testing
Dr. Cohn: I wouldn’t do any cardiac testing since this patient needs surgery for his malignancy and the results of any testing would be highly unlikely to change management, in terms of canceling the surgery. This approach is consistent with the 2007 guidelines on perioperative cardiovascular evaluation for noncardiac surgery issued by the American College of Cardiology (ACC) and the American Heart Association (AHA).10
Dr. Sweitzer: I would differ on this question. This patient has not been evaluated adequately for his coronary artery disease. He has poor functional capacity that complicates assessment of his symptoms. He also has diabetes, so he is more likely to have silent myocardial ischemia. At age 78, he is understandably concerned about his survival: radical cystectomy is a major operation associated with significant blood loss, fluid shifts, and a long-term recuperative state. In this case, a cardiac evaluation may change management, not in terms of considering coronary revascularization before the surgery, but in terms of affecting the assessment of his chance of surviving this major operation, his life span following the operation, and his quality of life. For example, a highly positive dobutamine stress echocardiogram or certain wall motion abnormalities would suggest that he might not be protected even by optimal perioperative medical management.
Question 2.6: Which of the following would you do preoperatively to assess pulmonary risk?
A. Obtain pulmonary function tests
B. Order a sleep study
C. Both
D. Neither
Dr. Sweitzer: There is no evidence supporting routine pulmonary function tests for patients undergoing procedures other than lung resection. If obstructive sleep apnea were suspected, I would order a sleep study only if I had access to one quickly to avoid delaying the surgery. Cancer surgery should never be delayed to get a sleep study. However, if this patient were seen in the primary care clinic, I would order a sleep study and, if indicated, put him on continuous positive airway pressure (CPAP). Whether or not preoperative CPAP makes a difference hasn’t been shown. No randomized controlled trials have been conducted, but there are some suggestions that the risks of ischemia and atrial arrhythmias in patients with known coronary artery disease can be reduced with CPAP. It is not always easy to initiate CPAP postoperatively because the number of CPAP machines is limited and titration by a respiratory technician is required, which is typically done in a sleep lab.
How the case was actually managed
Neither an HbA1c measurement nor a lipid profile was ordered preoperatively, for lack of supportive evidence. The patient was continued on his beta-blocker and the dosage was increased sufficiently to control his blood pressure and heart rate. Metformin was continued, and statin therapy was begun preoperatively in light of the patient’s independent indications for it and the high-risk nature of the procedure. Stress testing was not ordered, in light of the lack of indication, given the patient’s stable angina. The patient refused a sleep study. The operation was lengthy and involved significant blood loss. The patient had a complicated postoperative course and ultimately died from multiorgan failure.
CASE 3: OPERATIONS OF VARIABLE RISK IN ELDERLY MAN WITH ACTIVE CARDIAC CONDITION
Scenario A: A 75-year-old man with diabetes, class III angina, and Q waves in inferior leads on his ECG is scheduled for elective femoropopliteal bypass surgery. His medications include isosorbide mononitrate (120 mg), amlodipine (10 mg), metoprolol controlled release (100 mg), atorvastatin (80 mg), insulin, and aspirin (81 mg). His heart rate is 64 beats per minute, blood pressure is controlled at 120/80 mm Hg, low-density lipoprotein cholesterol is 80 mg/dL, and creatinine is 1.5 μmol/L.
Scenario B: Consider the same patient undergoing elective cholecystectomy instead of a femoropopliteal bypass.
Scenario C: Consider the same patient scheduled for a cystoscopy instead of the other procedures. He had one episode of gross hematuria 1 week ago that resolved. Work-up by his urologist included a urinalysis and culture that were normal, cytology that was negative for malignancy, and a sonogram and computed tomography scan that were both negative. He has had no further bleeding and is not anemic. The urologist wants to do the cystoscopy for the sake of completeness.
Question 3.1: What would be your preoperative course of action in the above scenarios?
A. Order a dobutamine stress echocardiogram
B. Order nuclear imaging with dipyridamole or adenosine
C. Order coronary angiography
D. Order a resting two-dimensional echocardiogram
E. Continue his current medications and send to surgery with no further testing
Dr. Cohn: This is a man with an active cardiac condition and class III angina, which is considered severe angina in the ACC/AHA 2007 guidelines on perioperative cardiac evaluation and care.10 The guidelines’ recommendation is to delay surgery for further evaluation and treatment. He is already on maximal medical therapy, which has failed to control his symptoms. He has poor exercise capacity. The only difference among the case scenarios is a variation in surgical risk.
This patient has independent indications for coronary angiography regardless of whether or not he’s undergoing surgery. He deserves evaluation for possible revascularization to improve his quality of life and symptoms.
I would send the patient to the catheterization lab in every one of these instances, with the possible exception of the cystoscopy scenario, where one could argue that revascularization with stenting would require antiplatelet therapy that might increase the bleeding risk, and also that the antiplatelet therapy would have to be interrupted for the cystoscopy, potentially increasing thrombotic risk.
Dr. Sweitzer: I disagree. The ACC/AHA 2007 guidelines do not recommend going directly to catheterization but rather recommend delaying surgery for further evaluation and treatment.10 We must ask whether this patient is truly receiving optimal medical management. After all, he is not on an ACE inhibitor or an ARB.
We must also consider whether the surgery is truly elective. In the first scenario, if he has peripheral vascular disease, he is likely to develop gangrene and have a further decrease in exercise capacity, which reduces his functional ability and increases his risk of comorbid conditions. He is at significant risk of developing worsening renal insufficiency or renal failure if he undergoes angiography. Coronary revascularization will delay treatment of his peripheral vascular disease. The Coronary Artery Revascularization Prophylaxis (CARP) trial showed no benefit of coronary revascularization relative to medical management in patients undergoing vascular surgery,11 as is planned for this patient. I believe one must balance two competing risks and have an in-depth discussion with the patient.
In the second scenario, not treating gallstones or preventing cholelithiasis poses more risk to the health of this diabetic patient than does elective surgery if he needs a cholecystectomy. Emergency surgery, especially for acute cholecystitis, also significantly increases the risk of a cardiac event.
In the third scenario, the cystoscopy may uncover bladder cancer, which may be adversely affected by a delay of surgery. Regardless, the patient had gross hematuria and would be at risk for further bleeding should he undergo stenting with the requisite antiplatelet therapy.
Catheterization is not normally recommended unless CABG or stenting is being considered, yet I have seen no data that either of these procedures prolongs life except in very limited circumstances such as left main disease treated with bypass grafting. Though it is true that CABG reduces the incidence and severity of angina, it does not modify the physiologic cause of angina but rather may result in symptom improvement by damaging somatic nerve fibers to the heart. Putting a stent in this patient would be like applying a bandage: his symptoms will likely recur if he does not receive optimal medical management.
In a 2007 science advisory, several major medical societies cautioned against percutaneous coronary intervention (PCI) with drug-eluting stent placement in patients expected to undergo noncardiac surgery that would require interruption of antiplatelet therapy in the following 12 months (and against PCI with bare metal stent placement in patients undergoing such surgery in the following 4 to 6 weeks).12 Therefore, I would not recommend catheterization for a patient whose noncardiac disease is likely to require surgery in the very near future, as is the case in each of the surgical scenarios above. One could consider noninvasive stress testing, which would be a safer approach and would almost certainly identify either significant stenosis of the left main coronary artery or three-vessel disease, which would be the only possible reasons to recommend CABG. I don’t believe there is any role for PCI for this patient.
Dr. Cohn: I argue for symptom relief even if it doesn’t prolong life. This patient cannot walk across the room without having symptoms despite taking multiple medications. I think he deserves a chance at revascularization if the angiogram shows he has a stenosis amenable to it, but I agree that a drug-eluting stent should not be placed if we know that he will undergo surgery within a few months.
CASE 4: VENTRAL HERNIA REPAIR IN A MIDDLE-AGED WOMAN
A 60-year-old woman is scheduled for ventral hernia repair. Her medical history is unremarkable, with the exception of hypertension. She denies any bleeding problems and had no complications after a laparoscopic cholecystectomy 10 years ago. She has no family history of bleeding disorders.
Question 4.1: Would you order a prothrombin time (PT)/partial thromboplastin time (PTT)?
A. Yes
B. No
Dr. Cohn: I would not.
Dr. Sweitzer: I agree.
Question 4.2: Although not requested, a PT/PTT was ordered anyway. The PT is normal (12.2 sec/12 sec) and the PTT is abnormal (40 sec/25 sec). What is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Factor VII deficiency
C. Factor IX deficiency
D. Factor XI deficiency
E. Factor XII deficiency
Dr. Cohn: The most likely cause is a sample with insufficient blood in the tube. The test wasn’t indicated in the first place, but now it must be done again.
Question 4.3: The PTT is repeated and remains abnormal: 42 sec/25 sec. Mixing studies correct the abnormality to 29 sec/25 sec. Based on this information, what is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Lupus anticoagulant
C. Prekallikrein factor deficiency
D. Factor XII deficiency
Dr. Cohn: This is not a case of lupus anticoagulant because the abnormal PTT was corrected by the mixing study. Causes of a prolonged PTT include deficiencies of factors XII, XI, and IX, so factor XII deficiency is the most likely explanation, though a deficiency higher up the coagulation cascade (ie, prekallikrein factor deficiency) is possible. In the absence of any personal or family bleeding history, it is unlikely to be a deficiency of factors VII or IX (the hemophiliac) or of factor XI, so a deficiency of factor XII or one of the prekallikrein factors is more likely.
Dr. Sweitzer: A mixing study is indeed the appropriate first step. It is ordered from the lab and involves mixing the patient’s blood with normal plasma and incubating the mixture. If the mixture corrects the PTT result, as was the case with this patient, it indicates a coagulation factor deficiency in the patient’s blood; if it doesn’t correct, that should prompt evaluation for lupus anticoagulant or the presence of some other protein or hormone that’s prolonging the PTT.
Question 4.4: How would you manage this patient perioperatively?
A. Fresh frozen plasma
B. Platelet transfusion
C. Cryoprecipitate
D. Factor VII
E. No treatment necessary
Dr. Cohn: No treatment is necessary. Factor XII deficiency does not cause bleeding, regardless of the PTT. Factor XI deficiency is associated with bleeding, but usually there is a family history or a personal history of bleeding with surgery.
Screening coagulation studies are not usually indicated in a patient without a personal or family history of bleeding, liver disease, alcohol or drug use, or current anticoagulant therapy. Such studies are usually normal in such patients, and when they are not, it’s usually because of a lab error or a disease (hypercoagulable state) or factor deficiency that does not cause bleeding
Dr. Sweitzer: However, if the PTT is prolonged, the cause should be identified, because if the patient is sent to the operating room without an explanation for the prolongation, the perioperative team might think the patient has a bleeding problem and use fresh frozen plasma too readily. Fresh frozen plasma is not appropriate for everyone and may actually make a potentially hypercoagulable state worse.
DISCUSSION
Question from the audience: It was said that use of ACE inhibitors and ARBs should be avoided around the time of surgery. I’ve done an extensive literature search and found minimal to no evidence to support this practice. To the contrary, I found fairly good evidence to indicate that heart failure can be exacerbated significantly and acutely, as early as within 24 hours, when patients are taken off their ACE inhibitor or ARB. I would like your viewpoint on this basic pathology in perioperative medicine.
Dr. Cohn: The literature on the use of ACE inhibitors or ARBs prior to noncardiac surgery consists of five studies with fewer than 500 patients in total, as recently reviewed by Rosenman et al.13 Although there was no excess of death or MI associated with taking these medications on the morning of surgery, they did increase the need for fluid and pressors.
Dr. Sweitzer: Patients with hypertension have bigger variations of blood pressure, both hypo- and hypertension, in the perioperative period. For this reason, it was standard of care 30 years ago to stop all antihypertensive drugs, including beta-blockers, preoperatively. We soon found that although this practice prevented many episodes of hypotension, it increased the occurrence of perioperative hypertension and the likelihood of cardiac events. It then became standard of care to always continue antihypertensive drugs on the morning of surgery. In the late 1980s and early 1990s, several studies showed that ACE inhibitors and ARBs were associated with a more profound drop in blood pressure upon induction of general anesthesia compared with other antihypertensives.
The usual ways we treat drops in blood pressure—with phenylephrine and ephedrine—are not very effective in treating hypotension associated with general anesthesia in patients taking ACE inhibitors or ARBs. Vasopressin is effective in treating refractory hypotension during surgery, but anesthesiologists don’t use it often. Reducing the doses of induction agents is another means of attenuating the hypotension induced by ACE inhibitors and ARBs.
We should not routinely stop ACE inhibitors and ARBs on the day of surgery, particularly in patients being treated for heart failure, angina, or a prior MI. My bias is to selectively hold ACE inhibitors and ARBs on the morning of surgery in patients who are undergoing a significant operation with a high likelihood of hypotension, have well-controlled preoperative blood pressure, are taking multiple antihypertensive agents, and do not have heart failure. Otherwise, patients should continue their ACE inhibitors and ARBs on the morning of surgery, and the anesthesiologist should be prepared for significant hypotension upon induction of anesthesia, alter anesthesia induction doses accordingly, have vasopressin handy, and avoid the temptation to treat hypotension with fluids or repeated doses of phenylephrine and ephedrine. The previous comment about concerns with ACE inhibitors and ARBs was in the context of initiating new therapies in the immediate preoperative period.
Question from the audience: Urinalysis is ordered for many patients undergoing orthopedic surgery, and invariably some bacteriuria is found. Can you comment on the value of urinalysis and subsequent treatment of abnormal results?
Dr. Cohn: I believe you should never order a urinalysis in an asymptomatic patient, with the exception of patients undergoing procedures that involve genitourinary or gynecologic instrumentation. Ordering a urinalysis before joint replacement has been promoted in the orthopedic literature on the theoretical grounds that bacteria might somehow seed and colonize the joint. Orthopedic surgeons like to do it, but I disregard their requests for it.
Dr. Sweitzer: One study showed that we’d need to spend $1.5 million on screening urinalysis for asymptomatic patients scheduled for joint replacement surgery in order to prevent one joint infection.14
Dr. Cohn: Also, patients are going to get their one dose of cephalosporin before surgery anyway, and that will probably knock out any bacteria that would be found on urinalysis.
Question from the audience: Can you clarify how the 2007 ACC/AHA perioperative guidelines define an active cardiac condition? The patient in your third case report had class III angina, or angina with less than usual activities, but nothing was presented to suggest that his symptoms were unstable. I would suggest that despite his class III symptoms, his angina was stable, and I would have continued down the algorithm rather than defining his cardiac condition as active and considering an intervention.
Dr. Cohn: An active cardiac condition is defined by the ACC as unstable coronary syndromes, which include acute (within the prior 7 days) or recent (within the prior 30 days) MI, unstable angina, and severe (class III or IV) angina.
CASE 1: RADICAL PROSTATECTOMY IN A MAN WITH ACUTE DEEP VEIN THROMBOSIS
A 69-year-old man is seen in the preoperative clinic 1 week before a scheduled radical prostatectomy. He has been diagnosed with femoral deep vein thrombosis (DVT) following a complaint of calf soreness.
Question 1.1: How would you treat him for his DVT?
A. Intravenous (IV) unfractionated heparin (UFH)
B. Low-molecular-weight heparin (LMWH)
C. Inferior vena cava (IVC) filter
D. Combination of pharmacologic therapy and then an IVC filter
Dr. Steven L. Cohn: The latest edition of the American College of Chest Physicians (ACCP) evidence-based guidelines on antithrombotic therapy recommends the use of therapeutic-dose subcutaneous LMWH over IV UFH for initial treatment of acute DVT in the outpatient or inpatient setting.1 Additionally, indications for an IVC filter include the prevention of pulmonary embolism (PE) in a patient with DVT who requires full-dose anticoagulation but cannot receive it, as would be the case here if the patient proceeds with surgery as scheduled. So if surgery will be postponed, the best option is LMWH; if surgery will not be postponed, the best answer is a combination of pharmacologic therapy with low-dose LMWH and an IVC filter, preferably a retrievable one.2
Question 1.2: You recommend postponing surgery, but the patient is worried about metastatic disease. For how long should surgery be postponed?
A. 2 weeks
B. 1 month
C. 2 months
D. 3 months
E. 6 months
Dr. Cohn: In the absence of anticoagulation therapy, the risk of venous thromboembolism (VTE) is approximately 40% (~1% per day) during the first month following an acute VTE and then declines markedly, to approximately 10%, during the second and third months following the acute event.3 Therefore, I would suggest that the patient wait at least 1 month after an acute DVT before undergoing surgery.
Dr. BobbieJean Sweitzer: This patient is in a hypercoagulable state, and the surgery itself will induce excess hypercoagulability. With a femoral DVT already present, his risk of VTE or PE is likely to be greater than 1% per day during the first month. If he does develop a PE, it may potentially be fatal.
Question 1.3: According to the patient, the surgeon and the internist discussed options, but the surgeon “doesn’t believe in filters” and the patient doesn’t want to postpone the procedure, despite your recommendation. Two weeks later he shows up for surgery having stopped his LMWH 3 days before. What would you do?
A. Cancel the surgery and restart full-dose LMWH
B. Proceed with prophylactic-dose LMWH
C. Proceed after giving a full therapeutic dose
D. Insert a filter and give DVT prophylaxis
Dr. Cohn: A bridging protocol should have been discussed with the surgeon and anesthesiologist before the procedure. Therapeutic levels of LMWH persist as long as 18 hours after discontinuation; therefore, the ACCP recommends interrupting LMWH 24 hours before surgery.4
Dr. Sweitzer: The lack of a bridging protocol in this case created a problem. The patient was afraid to continue anticoagulation after hearing the internist and surgeon disagree about the plan, and thus stopped it entirely, and he did not want to delay surgery because he was fearful of metastasis. The surgeon was adamant that IVC filters don’t work. The internist was concerned that the patient was at high risk for a PE. Even though the documented risk of postponing radical prostatectomy for a short time is inconsequential, I was convinced that the patient would not believe this if metastasis were to develop in the future.
Question 1.4: How would you have managed his anticoagulation perioperatively?
A. Stop LMWH 12 hours before surgery and restart at full dose 12 to 24 hours after surgery
B. Stop LMWH 24 hours before surgery and restart at full dose 24 hours after surgery
C. Stop LMWH 24 hours before surgery and restart prophylactic dosing 12 to 24 hours after surgery, and then full-dose LMWH in 48 to 72 hours
D. Stop LMWH 24 hours before surgery and restart at full dose 72 hours after surgery
Dr. Cohn: The correct timing for stopping LMWH is 24 hours before surgery. As for how to resume anticoagulation in patients at high risk for VTE or those undergoing major surgery, the latest ACCP guidelines recommend the following4:
- Reinitiation of anticoagulation 12 to 24 hours postoperatively, assuming adequate hemostasis in patients not at high risk for bleeding
- Use of a prophylactic dose or no anticoagulation for up to 72 hours if the patient is at high risk for bleeding.
These recommendations are a departure from previous practice, in which we routinely restarted anticoagulation 6 to 12 hours postoperatively.
Dr. Sweitzer: According to guidelines from the American Society of Regional Anesthesia and Pain Medicine (ASRA),5 if twice-daily LMWH is stopped 24 hours ahead of time (as long as patients have normal renal function), it is safe to perform epidural or spinal anesthesia, if either is an option. If full-dose UFH is used, the partial thromboplastin time (PTT) is monitored and central neuraxial blockade may be done if the PTT is in the normal range, which typically is 2 to 6 hours after UFH is stopped.
Additionally, the platelet count should be checked every 3 days postoperatively while the patient is on UFH or LMWH. It may be just as important to monitor the platelet count preoperatively if the patient has been on UFH or LMWH for an extended duration, especially if a central neuraxial anesthetic technique is planned.
Dr. Cohn: The reason for monitoring the platelet count is the potential for heparin-induced thrombocytopenia in patients on UFH. I recently encountered a patient who developed postoperative heparin-induced thrombocytopenia with thrombosis while on LMWH, which is relatively uncommon compared with UFH.
Case resolution
After much discussion of the risk of a significant PE with the patient, family, urologist, and vascular surgeon, it is decided that a temporary IVC filter will be placed in the operating room immediately after induction of general anesthesia and before the prostatectomy. The operation is delayed about 1 hour to allow this option. The patient is successfully treated and has the IVC filter removed 1 month postoperatively.
CASE 2: RADICAL CYSTECTOMY IN ELDERLY MAN WITH CARDIAC RISK FACTORS
A 78-year-old obese Russian-speaking man is seen in the preoperative clinic prior to a scheduled radical cystectomy for highly invasive bladder cancer. He is a poor historian and argues with the several family members accompanying him, but it is determined that his medical history includes hypertension, diabetes mellitus, a myocardial infarction (MI) 5 years previously (in Russia), and stable angina that is determined to be class II.
He had no previous work-up and no electrocardiogram (ECG). His medications are aspirin, metoprolol, and metformin. His blood pressure is 190/100 mm Hg, heart rate 90 beats per minute, and body mass index 32. On examination, there is no murmur, S3 gallop, or rales. His blood glucose is 220 mg/dL, and his creatinine is slightly elevated (1.4 mg/dL). ECG verifies a prior MI.
Question 2.1: Which of the following additional tests should be ordered preoperatively?
A. Hemoglobin (Hb) A1c
B. Lipid profile
C. Both
D. Neither
Dr. Sweitzer: Because the surgery is not elective, no immediate benefit would be achieved by ordering either an HbA1c or a lipid profile. However, if you view the preoperative evaluation as an opportunity to manage risk factors over the long term, then it may be a good idea to order the lipid profile because this patient has rarely engaged the health care system. Likewise, the HbA1c can be ordered to set in place his long-term management. Sometimes we focus on the preoperative visit only in the context of the surgery, but if a test or intervention is appropriate and needed for long-term management, then it is appropriate to do now.
Dr. Cohn: There is no evidence to support using the preoperative HbA1c to alter management decisions. I would not postpone surgery based on the HbA1c value, as I would if his glucose level were 600 mg/dL. Most of the studies that have assessed postoperative complications based on preoperative HbA1c did not control for postoperative glucose levels. The incidence of complications varies based on the type of complication and the type of surgery.
Similarly, I would not use lipid values to guide management of this patient. Studies suggest that perioperative statin therapy may reduce postoperative morbidity and mortality in patients undergoing vascular surgery (see article by Poldermans on page S79 of this supplement), but our patient already has indications for a statin—a remote MI and diabetes—independent of what his lipid values are.
Question 2.2: How would you manage his elevated blood pressure (190/100 mm Hg)?
A. Discontinue metoprolol and start a different antihypertensive drug
B. Increase the metoprolol dose
C. Continue metoprolol and add a second drug
D. Observe him on his current regimen
Dr. Cohn: I would increase the dose of metoprolol and consider adding another drug, in view of his heart rate (90 beats per minute) and his cardiac status. Beta-blocker therapy should not be discontinued because doing so in the perioperative period is associated with an increased risk of adverse events such as cardiac death and MI.
Dr. Sweitzer: I would push up the metoprolol a bit to reduce the heart rate, knowing that beta-blockers are probably not the most efficacious antihypertensive agents. I would caution against starting an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) because he is scheduled to undergo a fairly significant procedure with expected blood loss and fluid shifts, and either of those agents in combination with a beta-blocker would be challenging to manage on the day of surgery.
Question 2.3: How would you manage his metformin perioperatively?
A. Discontinue it 48 hours preoperatively
B. Discontinue it 24 hours preoperatively
C. Withhold it on the morning of surgery
D. Continue it on the morning of surgery
Dr. Sweitzer: We routinely advise patients to hold all their oral diabetes medications the morning of surgery, primarily because many anesthesiologists are uncertain about the differing risks of hypoglycemia associated with the various oral agents.
Most of us will never see a patient who has lactic acidosis from metformin use. A systematic literature review and analysis found no increase in the risk of lactic acidosis with metformin compared with other oral hypoglycemics,6 so fear of lactic acidosis is not a valid reason to discontinue metformin. In fact, I think it is inappropriate to ever postpone or cancel surgery simply because the patient inadvertently took metformin on the morning of surgery. Some may argue that patients with renal insufficiency are at higher risk of lactic acidosis from metformin use on the morning of surgery, but keep in mind that renal insufficiency is a relative contraindication to metformin use in the first place. Unless the patient is scheduled for a bilateral nephrectomy, his or her renal function is not going to be acutely reduced enough to enable a morning dose of metformin to cause lactic acidosis.
Dr. Cohn: Additionally, in a recent study of patients undergoing coronary artery bypass graft surgery (CABG), there was no increased risk of in-hospital morbidity or mortality in patients who received metformin on the morning of surgery,7 although I typically stop it 24 hours before major surgery.
Question 2.4: With respect to statin therapy, which course would you choose preoperatively?
A. Start a statin at a low dose
B. Start a statin at an intermediate dose
C. Start a statin at a high dose
D. Do not start a statin
Dr. Cohn: The answer to this question is not clear cut. The reason not to start a prophylactic statin would be the lack of evidence of benefit in patients undergoing noncardiac, nonvascular surgery, although there is evidence of potential benefit in patients undergoing vascular surgery.* The arguments in favor of starting a statin are that this patient has independent indications for a statin and the planned surgery is a high-risk procedure.
(* Editor’s note: In the time since this summit, results of the DECREASE-IV trial were published [Dunkelgrun et al, Ann Surg 2009; 249:921–926], showing a statisically nonsignificant trend toward improved outcomes at 30 days with fluvastatin in intermediate-risk patients undergoing noncardiovascular surgery.)
In cohort studies, perioperative death rates have been lower in statin recipients than in those not taking a statin.8 In the Dutch Echographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III), which randomized noncardiac vascular surgery patients to perioperative fluvastatin or placebo, rates of MI and the composite end point of nonfatal MI or cardiovascular death were significantly lower in the statin group than in the placebo group.9
Question 2.5: Which of the following cardiac tests would you order preoperatively?
A. Exercise ECG
B. Dobutamine stress echocardiogram
C. Dipyridamole nuclear imaging
D. Coronary angiography
E. No further cardiac testing
Dr. Cohn: I wouldn’t do any cardiac testing since this patient needs surgery for his malignancy and the results of any testing would be highly unlikely to change management, in terms of canceling the surgery. This approach is consistent with the 2007 guidelines on perioperative cardiovascular evaluation for noncardiac surgery issued by the American College of Cardiology (ACC) and the American Heart Association (AHA).10
Dr. Sweitzer: I would differ on this question. This patient has not been evaluated adequately for his coronary artery disease. He has poor functional capacity that complicates assessment of his symptoms. He also has diabetes, so he is more likely to have silent myocardial ischemia. At age 78, he is understandably concerned about his survival: radical cystectomy is a major operation associated with significant blood loss, fluid shifts, and a long-term recuperative state. In this case, a cardiac evaluation may change management, not in terms of considering coronary revascularization before the surgery, but in terms of affecting the assessment of his chance of surviving this major operation, his life span following the operation, and his quality of life. For example, a highly positive dobutamine stress echocardiogram or certain wall motion abnormalities would suggest that he might not be protected even by optimal perioperative medical management.
Question 2.6: Which of the following would you do preoperatively to assess pulmonary risk?
A. Obtain pulmonary function tests
B. Order a sleep study
C. Both
D. Neither
Dr. Sweitzer: There is no evidence supporting routine pulmonary function tests for patients undergoing procedures other than lung resection. If obstructive sleep apnea were suspected, I would order a sleep study only if I had access to one quickly to avoid delaying the surgery. Cancer surgery should never be delayed to get a sleep study. However, if this patient were seen in the primary care clinic, I would order a sleep study and, if indicated, put him on continuous positive airway pressure (CPAP). Whether or not preoperative CPAP makes a difference hasn’t been shown. No randomized controlled trials have been conducted, but there are some suggestions that the risks of ischemia and atrial arrhythmias in patients with known coronary artery disease can be reduced with CPAP. It is not always easy to initiate CPAP postoperatively because the number of CPAP machines is limited and titration by a respiratory technician is required, which is typically done in a sleep lab.
How the case was actually managed
Neither an HbA1c measurement nor a lipid profile was ordered preoperatively, for lack of supportive evidence. The patient was continued on his beta-blocker and the dosage was increased sufficiently to control his blood pressure and heart rate. Metformin was continued, and statin therapy was begun preoperatively in light of the patient’s independent indications for it and the high-risk nature of the procedure. Stress testing was not ordered, in light of the lack of indication, given the patient’s stable angina. The patient refused a sleep study. The operation was lengthy and involved significant blood loss. The patient had a complicated postoperative course and ultimately died from multiorgan failure.
CASE 3: OPERATIONS OF VARIABLE RISK IN ELDERLY MAN WITH ACTIVE CARDIAC CONDITION
Scenario A: A 75-year-old man with diabetes, class III angina, and Q waves in inferior leads on his ECG is scheduled for elective femoropopliteal bypass surgery. His medications include isosorbide mononitrate (120 mg), amlodipine (10 mg), metoprolol controlled release (100 mg), atorvastatin (80 mg), insulin, and aspirin (81 mg). His heart rate is 64 beats per minute, blood pressure is controlled at 120/80 mm Hg, low-density lipoprotein cholesterol is 80 mg/dL, and creatinine is 1.5 μmol/L.
Scenario B: Consider the same patient undergoing elective cholecystectomy instead of a femoropopliteal bypass.
Scenario C: Consider the same patient scheduled for a cystoscopy instead of the other procedures. He had one episode of gross hematuria 1 week ago that resolved. Work-up by his urologist included a urinalysis and culture that were normal, cytology that was negative for malignancy, and a sonogram and computed tomography scan that were both negative. He has had no further bleeding and is not anemic. The urologist wants to do the cystoscopy for the sake of completeness.
Question 3.1: What would be your preoperative course of action in the above scenarios?
A. Order a dobutamine stress echocardiogram
B. Order nuclear imaging with dipyridamole or adenosine
C. Order coronary angiography
D. Order a resting two-dimensional echocardiogram
E. Continue his current medications and send to surgery with no further testing
Dr. Cohn: This is a man with an active cardiac condition and class III angina, which is considered severe angina in the ACC/AHA 2007 guidelines on perioperative cardiac evaluation and care.10 The guidelines’ recommendation is to delay surgery for further evaluation and treatment. He is already on maximal medical therapy, which has failed to control his symptoms. He has poor exercise capacity. The only difference among the case scenarios is a variation in surgical risk.
This patient has independent indications for coronary angiography regardless of whether or not he’s undergoing surgery. He deserves evaluation for possible revascularization to improve his quality of life and symptoms.
I would send the patient to the catheterization lab in every one of these instances, with the possible exception of the cystoscopy scenario, where one could argue that revascularization with stenting would require antiplatelet therapy that might increase the bleeding risk, and also that the antiplatelet therapy would have to be interrupted for the cystoscopy, potentially increasing thrombotic risk.
Dr. Sweitzer: I disagree. The ACC/AHA 2007 guidelines do not recommend going directly to catheterization but rather recommend delaying surgery for further evaluation and treatment.10 We must ask whether this patient is truly receiving optimal medical management. After all, he is not on an ACE inhibitor or an ARB.
We must also consider whether the surgery is truly elective. In the first scenario, if he has peripheral vascular disease, he is likely to develop gangrene and have a further decrease in exercise capacity, which reduces his functional ability and increases his risk of comorbid conditions. He is at significant risk of developing worsening renal insufficiency or renal failure if he undergoes angiography. Coronary revascularization will delay treatment of his peripheral vascular disease. The Coronary Artery Revascularization Prophylaxis (CARP) trial showed no benefit of coronary revascularization relative to medical management in patients undergoing vascular surgery,11 as is planned for this patient. I believe one must balance two competing risks and have an in-depth discussion with the patient.
In the second scenario, not treating gallstones or preventing cholelithiasis poses more risk to the health of this diabetic patient than does elective surgery if he needs a cholecystectomy. Emergency surgery, especially for acute cholecystitis, also significantly increases the risk of a cardiac event.
In the third scenario, the cystoscopy may uncover bladder cancer, which may be adversely affected by a delay of surgery. Regardless, the patient had gross hematuria and would be at risk for further bleeding should he undergo stenting with the requisite antiplatelet therapy.
Catheterization is not normally recommended unless CABG or stenting is being considered, yet I have seen no data that either of these procedures prolongs life except in very limited circumstances such as left main disease treated with bypass grafting. Though it is true that CABG reduces the incidence and severity of angina, it does not modify the physiologic cause of angina but rather may result in symptom improvement by damaging somatic nerve fibers to the heart. Putting a stent in this patient would be like applying a bandage: his symptoms will likely recur if he does not receive optimal medical management.
In a 2007 science advisory, several major medical societies cautioned against percutaneous coronary intervention (PCI) with drug-eluting stent placement in patients expected to undergo noncardiac surgery that would require interruption of antiplatelet therapy in the following 12 months (and against PCI with bare metal stent placement in patients undergoing such surgery in the following 4 to 6 weeks).12 Therefore, I would not recommend catheterization for a patient whose noncardiac disease is likely to require surgery in the very near future, as is the case in each of the surgical scenarios above. One could consider noninvasive stress testing, which would be a safer approach and would almost certainly identify either significant stenosis of the left main coronary artery or three-vessel disease, which would be the only possible reasons to recommend CABG. I don’t believe there is any role for PCI for this patient.
Dr. Cohn: I argue for symptom relief even if it doesn’t prolong life. This patient cannot walk across the room without having symptoms despite taking multiple medications. I think he deserves a chance at revascularization if the angiogram shows he has a stenosis amenable to it, but I agree that a drug-eluting stent should not be placed if we know that he will undergo surgery within a few months.
CASE 4: VENTRAL HERNIA REPAIR IN A MIDDLE-AGED WOMAN
A 60-year-old woman is scheduled for ventral hernia repair. Her medical history is unremarkable, with the exception of hypertension. She denies any bleeding problems and had no complications after a laparoscopic cholecystectomy 10 years ago. She has no family history of bleeding disorders.
Question 4.1: Would you order a prothrombin time (PT)/partial thromboplastin time (PTT)?
A. Yes
B. No
Dr. Cohn: I would not.
Dr. Sweitzer: I agree.
Question 4.2: Although not requested, a PT/PTT was ordered anyway. The PT is normal (12.2 sec/12 sec) and the PTT is abnormal (40 sec/25 sec). What is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Factor VII deficiency
C. Factor IX deficiency
D. Factor XI deficiency
E. Factor XII deficiency
Dr. Cohn: The most likely cause is a sample with insufficient blood in the tube. The test wasn’t indicated in the first place, but now it must be done again.
Question 4.3: The PTT is repeated and remains abnormal: 42 sec/25 sec. Mixing studies correct the abnormality to 29 sec/25 sec. Based on this information, what is the most likely cause of the PTT abnormality?
A. Laboratory error
B. Lupus anticoagulant
C. Prekallikrein factor deficiency
D. Factor XII deficiency
Dr. Cohn: This is not a case of lupus anticoagulant because the abnormal PTT was corrected by the mixing study. Causes of a prolonged PTT include deficiencies of factors XII, XI, and IX, so factor XII deficiency is the most likely explanation, though a deficiency higher up the coagulation cascade (ie, prekallikrein factor deficiency) is possible. In the absence of any personal or family bleeding history, it is unlikely to be a deficiency of factors VII or IX (the hemophiliac) or of factor XI, so a deficiency of factor XII or one of the prekallikrein factors is more likely.
Dr. Sweitzer: A mixing study is indeed the appropriate first step. It is ordered from the lab and involves mixing the patient’s blood with normal plasma and incubating the mixture. If the mixture corrects the PTT result, as was the case with this patient, it indicates a coagulation factor deficiency in the patient’s blood; if it doesn’t correct, that should prompt evaluation for lupus anticoagulant or the presence of some other protein or hormone that’s prolonging the PTT.
Question 4.4: How would you manage this patient perioperatively?
A. Fresh frozen plasma
B. Platelet transfusion
C. Cryoprecipitate
D. Factor VII
E. No treatment necessary
Dr. Cohn: No treatment is necessary. Factor XII deficiency does not cause bleeding, regardless of the PTT. Factor XI deficiency is associated with bleeding, but usually there is a family history or a personal history of bleeding with surgery.
Screening coagulation studies are not usually indicated in a patient without a personal or family history of bleeding, liver disease, alcohol or drug use, or current anticoagulant therapy. Such studies are usually normal in such patients, and when they are not, it’s usually because of a lab error or a disease (hypercoagulable state) or factor deficiency that does not cause bleeding
Dr. Sweitzer: However, if the PTT is prolonged, the cause should be identified, because if the patient is sent to the operating room without an explanation for the prolongation, the perioperative team might think the patient has a bleeding problem and use fresh frozen plasma too readily. Fresh frozen plasma is not appropriate for everyone and may actually make a potentially hypercoagulable state worse.
DISCUSSION
Question from the audience: It was said that use of ACE inhibitors and ARBs should be avoided around the time of surgery. I’ve done an extensive literature search and found minimal to no evidence to support this practice. To the contrary, I found fairly good evidence to indicate that heart failure can be exacerbated significantly and acutely, as early as within 24 hours, when patients are taken off their ACE inhibitor or ARB. I would like your viewpoint on this basic pathology in perioperative medicine.
Dr. Cohn: The literature on the use of ACE inhibitors or ARBs prior to noncardiac surgery consists of five studies with fewer than 500 patients in total, as recently reviewed by Rosenman et al.13 Although there was no excess of death or MI associated with taking these medications on the morning of surgery, they did increase the need for fluid and pressors.
Dr. Sweitzer: Patients with hypertension have bigger variations of blood pressure, both hypo- and hypertension, in the perioperative period. For this reason, it was standard of care 30 years ago to stop all antihypertensive drugs, including beta-blockers, preoperatively. We soon found that although this practice prevented many episodes of hypotension, it increased the occurrence of perioperative hypertension and the likelihood of cardiac events. It then became standard of care to always continue antihypertensive drugs on the morning of surgery. In the late 1980s and early 1990s, several studies showed that ACE inhibitors and ARBs were associated with a more profound drop in blood pressure upon induction of general anesthesia compared with other antihypertensives.
The usual ways we treat drops in blood pressure—with phenylephrine and ephedrine—are not very effective in treating hypotension associated with general anesthesia in patients taking ACE inhibitors or ARBs. Vasopressin is effective in treating refractory hypotension during surgery, but anesthesiologists don’t use it often. Reducing the doses of induction agents is another means of attenuating the hypotension induced by ACE inhibitors and ARBs.
We should not routinely stop ACE inhibitors and ARBs on the day of surgery, particularly in patients being treated for heart failure, angina, or a prior MI. My bias is to selectively hold ACE inhibitors and ARBs on the morning of surgery in patients who are undergoing a significant operation with a high likelihood of hypotension, have well-controlled preoperative blood pressure, are taking multiple antihypertensive agents, and do not have heart failure. Otherwise, patients should continue their ACE inhibitors and ARBs on the morning of surgery, and the anesthesiologist should be prepared for significant hypotension upon induction of anesthesia, alter anesthesia induction doses accordingly, have vasopressin handy, and avoid the temptation to treat hypotension with fluids or repeated doses of phenylephrine and ephedrine. The previous comment about concerns with ACE inhibitors and ARBs was in the context of initiating new therapies in the immediate preoperative period.
Question from the audience: Urinalysis is ordered for many patients undergoing orthopedic surgery, and invariably some bacteriuria is found. Can you comment on the value of urinalysis and subsequent treatment of abnormal results?
Dr. Cohn: I believe you should never order a urinalysis in an asymptomatic patient, with the exception of patients undergoing procedures that involve genitourinary or gynecologic instrumentation. Ordering a urinalysis before joint replacement has been promoted in the orthopedic literature on the theoretical grounds that bacteria might somehow seed and colonize the joint. Orthopedic surgeons like to do it, but I disregard their requests for it.
Dr. Sweitzer: One study showed that we’d need to spend $1.5 million on screening urinalysis for asymptomatic patients scheduled for joint replacement surgery in order to prevent one joint infection.14
Dr. Cohn: Also, patients are going to get their one dose of cephalosporin before surgery anyway, and that will probably knock out any bacteria that would be found on urinalysis.
Question from the audience: Can you clarify how the 2007 ACC/AHA perioperative guidelines define an active cardiac condition? The patient in your third case report had class III angina, or angina with less than usual activities, but nothing was presented to suggest that his symptoms were unstable. I would suggest that despite his class III symptoms, his angina was stable, and I would have continued down the algorithm rather than defining his cardiac condition as active and considering an intervention.
Dr. Cohn: An active cardiac condition is defined by the ACC as unstable coronary syndromes, which include acute (within the prior 7 days) or recent (within the prior 30 days) MI, unstable angina, and severe (class III or IV) angina.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):454S–545S.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):299S–339S.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Regional Anesthesia & Pain Medicine. 2003; 28:172–197. Available at: http://www.asra.com/consensus-statements/2.html. Accessed May 11, 2009.
- Salpeter S, Gryeber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Syst Rev 2006; (1):CD002967.
- Duncan AI, Koch CG, Xu M, et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg 2007; 104:42–50.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Poldermans D. Fluvastatin XL use is associated with improved cardiac outcome after major vascular surgery: results from a randomized placebo controlled trial. Presented at: European Society of Cardiology Congress 2008; September 1, 2008; Munich, Germany.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation 2007; 115:813–818.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-agiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Lawrence VA, Gafni A, Gross M. The unproven utility of the preoperative urinalysis: economic evaluation. J Clin Epidemiol 1989; 42:1185–1192.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):454S–545S.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133(6 suppl):299S–339S.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Regional Anesthesia & Pain Medicine. 2003; 28:172–197. Available at: http://www.asra.com/consensus-statements/2.html. Accessed May 11, 2009.
- Salpeter S, Gryeber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Syst Rev 2006; (1):CD002967.
- Duncan AI, Koch CG, Xu M, et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg 2007; 104:42–50.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Poldermans D. Fluvastatin XL use is associated with improved cardiac outcome after major vascular surgery: results from a randomized placebo controlled trial. Presented at: European Society of Cardiology Congress 2008; September 1, 2008; Munich, Germany.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation 2007; 115:813–818.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-agiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med 2008; 3:319–325.
- Lawrence VA, Gafni A, Gross M. The unproven utility of the preoperative urinalysis: economic evaluation. J Clin Epidemiol 1989; 42:1185–1192.
Improved Prophylaxis Following Education
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).
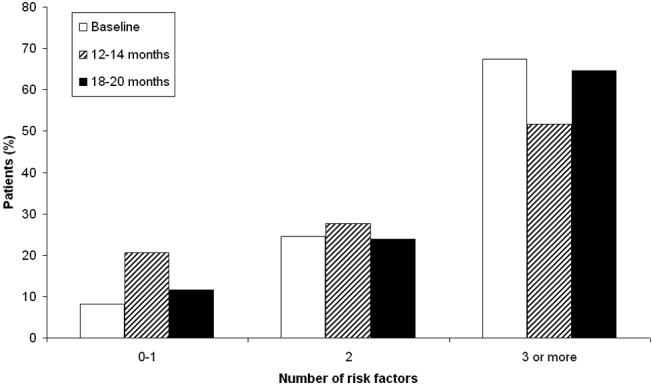
Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).
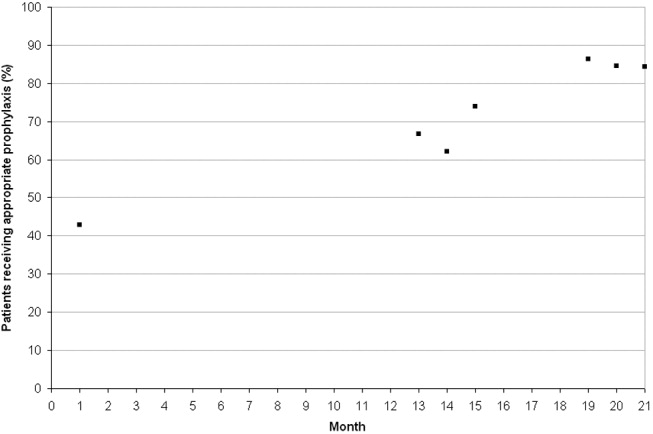
DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).

Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).

DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).

Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).

DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
Copyright © 2006 Society of Hospital Medicine