User login
Does XR injectable naltrexone prevent relapse as effectively as daily sublingual buprenorphine-naloxone?
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE-BASED ANSWER:
Yes. Monthly extended-release injectable naltrexone (XR-NTX) treats opioid use disorder as effectively as daily sublingual buprenorphine-naloxone (BUP-NX) without causing any increase in serious adverse events or fatal overdoses. (strength of recommendation: A, 2 good-quality RCTs).
Which oral nonopioid agents are most effective for OA pain?
EVIDENCE SUMMARY
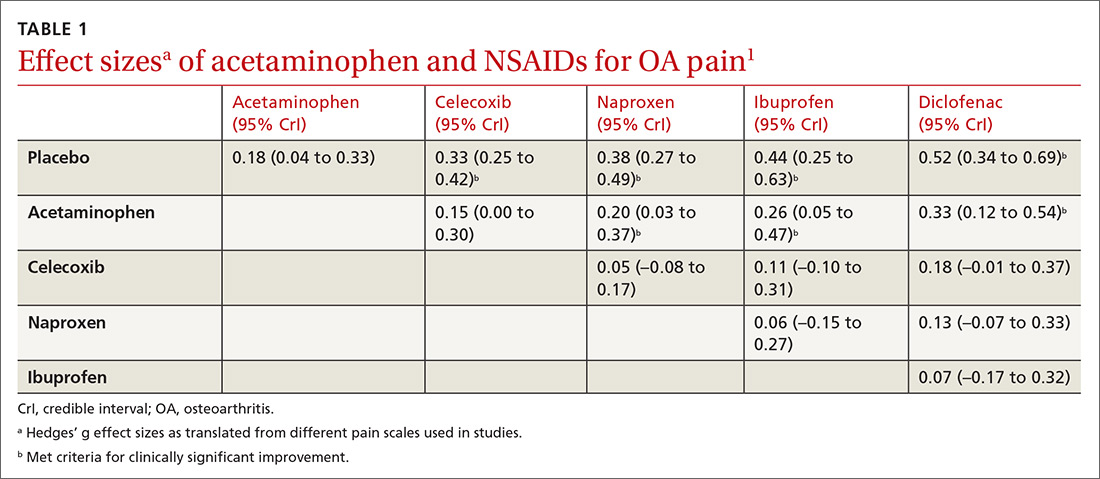
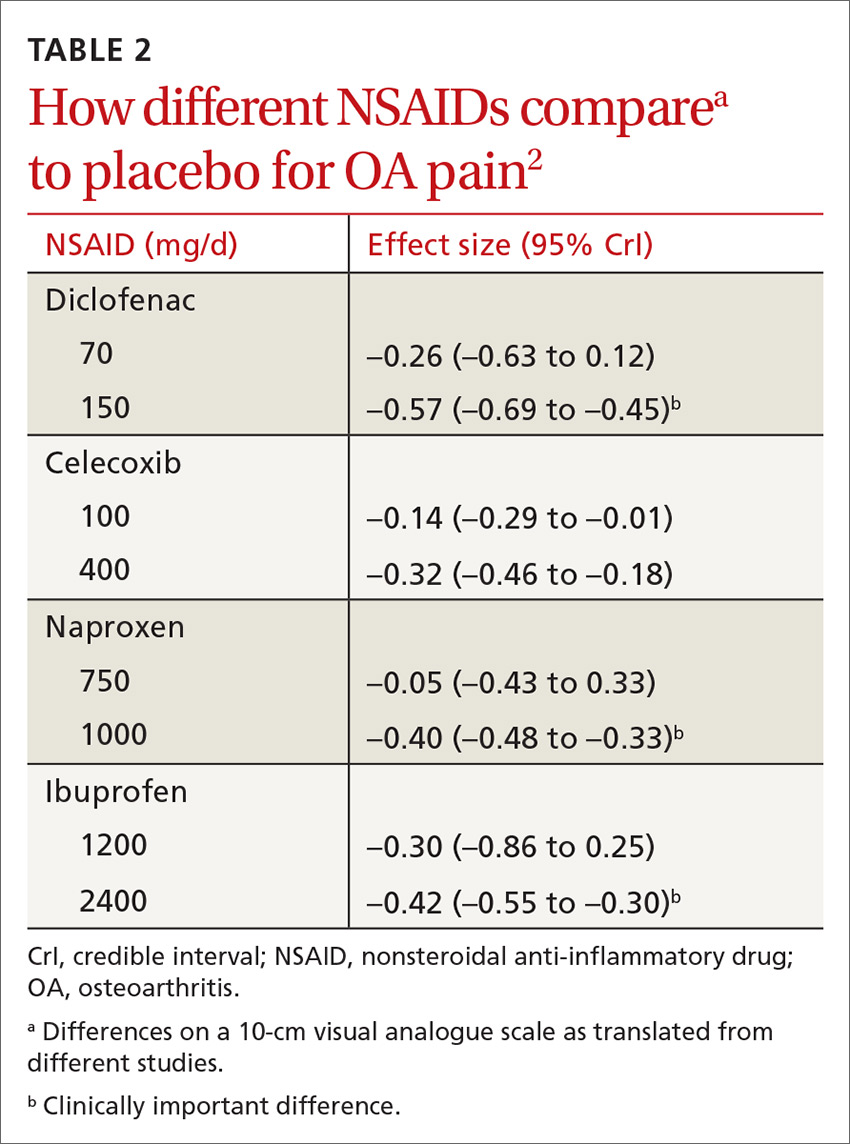
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2
Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1
Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
EVIDENCE SUMMARY
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2
Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1
Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
EVIDENCE SUMMARY
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2
Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1
Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
EVIDENCE-BASED ANSWER:
Nonsteroidal anti-inflammatory drugs (NSAIDs), when used at the maximum clinically effective dose, reduce osteoarthritis (OA) pain in large joints more effectively than either placebo or acetaminophen (strength of recommendation [SOR]: A, network meta-analysis of randomized controlled trials [RCTs]).
When ranked for efficacy, diclofenac 150 mg/d was the most effective (SOR: A, network meta-analysis of RCTs). The selective COX-2 inhibitors, such as celecoxib, are not more effective at reducing pain than the nonselective NSAIDs (SOR: A, meta-analysis of RCTs). Meloxicam is superior to placebo but marginally inferior to other NSAIDs (SOR: A, systematic review of RCTs).
Acetaminophen is no more effective than placebo (SOR: A, meta-analysis of RCTs).
How often does long-term PPI therapy cause clinically significant hypomagnesemia?
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
EVIDENCE-BASED ANSWER:
Rarely. Proton pump inhibitors (PPIs) may be associated with decreases in serum magnesium laboratory values to below 1.6 to 1.8 mg/dL, especially when used concurrently with diuretics and loop diuretics (strength of recommendation [SOR]: C, disease-oriented outcomes based on cohort, case-control, and cross-sectional studies). Clinically significant or symptomatic hypomagnesemia (below 1.2 mg/dL) appears to be quite rare, however.
What are the benefits and risks of daily low-dose aspirin for primary prevention of CV events?
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE BASED ANSWER:
One nonfatal myocardial infarction (MI) will be avoided for every 126 to 138 adults who take daily aspirin for 10 years (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of multiple randomized controlled trials [RCTs]).
Taking low-dose aspirin for primary prevention shows no clear mortality benefit. A benefit for primary prevention of stroke is less certain. Although no evidence establishes increased risk of hemorrhagic stroke from daily low-dose aspirin, one gastrointestinal hemorrhage will occur for every 72 to 357 adults who take aspirin for longer than 10 years (SOR: A, systematic reviews and meta-analyses of multiple RCTs and cohort studies).
What is the best beta-blocker for systolic heart failure?
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3
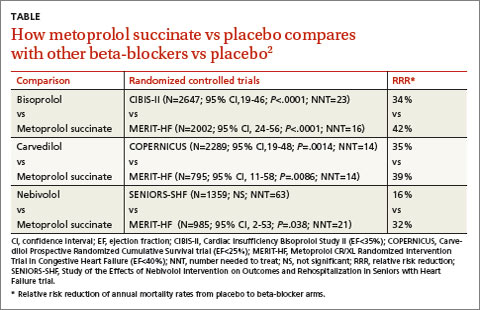
RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Evidence-based answers from the Family Physicians Inquiries Network
Does surgery relieve the pain of a herniated disc?
YES, in the short term. Patients with an acute episode of low back pain, radicular symptoms, and evidence of a herniated disc on imaging may experience short-term pain relief from discectomy if their symptoms haven’t improved after initial conservative therapy (strength of recommendation [SOR]: A, multiple randomized, controlled trials [RCTs]).
Although surgery may enhance pain relief initially, no evidence supports a long-term benefit for surgery over conservative management (SOR: A, multiple RCTs).
Evidence summary
Disc herniation is defined as any protrusion of the disc nucleus, cartilage, or other associated tissues from the normal disc space. Lumbar disc herniations (LDHs) are most likely to occur in the L4 to L5 and L5 to S1 levels, causing low back pain and sciatica. Many LDHs occur without symptoms, however, so it’s important to correlate level and side of herniation before assuming causality. Expert opinion recommends early surgical intervention for patients with cauda equina syndrome or progressive neurologic deficits.1
Surgery provides short-term gains
A search identified 4 RCTs that compared surgical intervention with conservative management. The first, published in 1983, evaluated 126 patients with radicular pain and confirmed LDH who did not improve after 2 weeks of conservative therapy. The study assigned patients to either open discectomy or back school.2 Patients rated their results as good, fair, poor, or bad; a good or fair rating was considered a positive outcome.
At 1 year, significantly more patients in the surgery group reported positive results (P<.001), based on working capacity, neurological deficits, pain, and lumbar spine mobility. At 4 years, no significant difference was found between the groups.
The study showed significant crossover, with 26% of conservatively managed patients receiving surgery within the first year. Evaluators weren’t blinded, and outcome measurements weren’t based on standardized evaluation tools.
Crossover complicates comparison of relative treatment effects
The Spine Patient Outcomes Research Trial (SPORT), published in 2006, compared 501 patients with confirmed LDH and persistent symptoms after 6 weeks.3 Patients were randomized to open discectomy or nonoperative “usual care.” Both groups showed improvement in pain scores and no significant differences in standardized pain scales at 3 months, 1 year, or 2 years.
Crossover for the study was high: 40% of the surgical group didn’t have surgery, and 45% of the nonoperative group underwent surgery. Although the pattern of care in the SPORT study resembles common clinical situations,4 the high degree of crossover makes it difficult to draw inferences about relative treatment effects.5
Greater patient satisfaction with surgery
Another RCT followed 56 patients with confirmed LDH and symptoms for 6 to 12 weeks.6 Patients were randomized to receive microdiscectomy within 2 weeks of randomization or nonoperative care. Outcomes were based on standardized pain scales for leg and back pain. The surgical group had significantly better leg pain relief (P<.01) at the 6-week evaluation. At 12 weeks, neither back pain nor leg pain differed between the groups.
Although pain didn’t differ significantly, patients in the surgical group were more satisfied with their care, and physicians were more likely to believe that surgery would improve outcomes. Crossover from the nonoperative group was high, with 39% of that group undergoing surgery.
Surgery improves leg pain, not disability, more than conservative therapy
Another RCT also directly compared microdiscectomy to conservative treatment in 283 patients with confirmed LDH and symptoms lasting 6 to 12 weeks.7 The surgical group underwent microdiscectomy within 2 weeks of randomization. Pain and disability measurements, based on standardized scales, showed significant improvement in leg pain (P<.001) for the surgical group, but no significant difference in disability.
Patient perception of recovery on a Likert-type scale showed a median recovery time of 4 weeks for the surgical group and 12 weeks for the conservative therapy group. No significant differences in perceived degree of recovery were noted between the groups at 1 year; 95% of participants had a satisfactory recovery.
Again, significant crossover occurred: 11% of patients allocated to surgery recovered before surgery, and 39% of the conservative therapy group experienced worsening symptoms or intractable pain that led them to undergo microdiscectomy.
Open discectomy, microdiscectomy produce similar results
A Cochrane review of interventions for LDH included only the Weber2 and SPORT3 RCTs. The review also included 3 RCTs that compared open discectomy and microdiscectomy. These studies found no difference in pain relief or complications between the 2 interventions.8
Recommendations
The Institute for Clinical Systems Improvement guidelines for adult low back pain list cauda equina, progressive neurologic deficits, or uncontrolled pain as reasons for direct referral to a spine specialist.1 Patients can be treated conservatively for 6 weeks without imaging, unless other symptoms or concerns are present.
The guidelines recommend that patients with chronic sciatica (lasting >6 weeks) receive further imaging or referral to a specialist if the patient is a potential candidate for surgery.
1. Institute for Clinical Systems Improvement. Health Care Guideline: Adult Low Back Pain. 13th ed. Bloomington, Minn: Institute for Clinical Systems Improvement; 2008. Available at: www.icsi.org/low_back_pain/adult_low_back_pain__8.html. Accessed December 11, 2009.
2. Weber H. Lumbar disc herniation. a controlled, prospective study with 10 years of observation. Spine. 1983;8:131-140.
3. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441-2450.
4. Angevine PD McCormick PC Inference and validity in the SPORT herniated lumbar disc randomized clinical trial. Spine J. 2007;7:387-391.
5. Mirza SK, Goodkin R. What patients know. Surg Neurol. 2008;70:5-7.
6. Osterman H, Seitsalo S, Karpinen J, et al. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine. 2006;21:2409-2414.
7. Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment in sciatica. N Engl J Med. 2007;356:2245-2256.
8. Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev. 2007;(2):CD001350.-
YES, in the short term. Patients with an acute episode of low back pain, radicular symptoms, and evidence of a herniated disc on imaging may experience short-term pain relief from discectomy if their symptoms haven’t improved after initial conservative therapy (strength of recommendation [SOR]: A, multiple randomized, controlled trials [RCTs]).
Although surgery may enhance pain relief initially, no evidence supports a long-term benefit for surgery over conservative management (SOR: A, multiple RCTs).
Evidence summary
Disc herniation is defined as any protrusion of the disc nucleus, cartilage, or other associated tissues from the normal disc space. Lumbar disc herniations (LDHs) are most likely to occur in the L4 to L5 and L5 to S1 levels, causing low back pain and sciatica. Many LDHs occur without symptoms, however, so it’s important to correlate level and side of herniation before assuming causality. Expert opinion recommends early surgical intervention for patients with cauda equina syndrome or progressive neurologic deficits.1
Surgery provides short-term gains
A search identified 4 RCTs that compared surgical intervention with conservative management. The first, published in 1983, evaluated 126 patients with radicular pain and confirmed LDH who did not improve after 2 weeks of conservative therapy. The study assigned patients to either open discectomy or back school.2 Patients rated their results as good, fair, poor, or bad; a good or fair rating was considered a positive outcome.
At 1 year, significantly more patients in the surgery group reported positive results (P<.001), based on working capacity, neurological deficits, pain, and lumbar spine mobility. At 4 years, no significant difference was found between the groups.
The study showed significant crossover, with 26% of conservatively managed patients receiving surgery within the first year. Evaluators weren’t blinded, and outcome measurements weren’t based on standardized evaluation tools.
Crossover complicates comparison of relative treatment effects
The Spine Patient Outcomes Research Trial (SPORT), published in 2006, compared 501 patients with confirmed LDH and persistent symptoms after 6 weeks.3 Patients were randomized to open discectomy or nonoperative “usual care.” Both groups showed improvement in pain scores and no significant differences in standardized pain scales at 3 months, 1 year, or 2 years.
Crossover for the study was high: 40% of the surgical group didn’t have surgery, and 45% of the nonoperative group underwent surgery. Although the pattern of care in the SPORT study resembles common clinical situations,4 the high degree of crossover makes it difficult to draw inferences about relative treatment effects.5
Greater patient satisfaction with surgery
Another RCT followed 56 patients with confirmed LDH and symptoms for 6 to 12 weeks.6 Patients were randomized to receive microdiscectomy within 2 weeks of randomization or nonoperative care. Outcomes were based on standardized pain scales for leg and back pain. The surgical group had significantly better leg pain relief (P<.01) at the 6-week evaluation. At 12 weeks, neither back pain nor leg pain differed between the groups.
Although pain didn’t differ significantly, patients in the surgical group were more satisfied with their care, and physicians were more likely to believe that surgery would improve outcomes. Crossover from the nonoperative group was high, with 39% of that group undergoing surgery.
Surgery improves leg pain, not disability, more than conservative therapy
Another RCT also directly compared microdiscectomy to conservative treatment in 283 patients with confirmed LDH and symptoms lasting 6 to 12 weeks.7 The surgical group underwent microdiscectomy within 2 weeks of randomization. Pain and disability measurements, based on standardized scales, showed significant improvement in leg pain (P<.001) for the surgical group, but no significant difference in disability.
Patient perception of recovery on a Likert-type scale showed a median recovery time of 4 weeks for the surgical group and 12 weeks for the conservative therapy group. No significant differences in perceived degree of recovery were noted between the groups at 1 year; 95% of participants had a satisfactory recovery.
Again, significant crossover occurred: 11% of patients allocated to surgery recovered before surgery, and 39% of the conservative therapy group experienced worsening symptoms or intractable pain that led them to undergo microdiscectomy.
Open discectomy, microdiscectomy produce similar results
A Cochrane review of interventions for LDH included only the Weber2 and SPORT3 RCTs. The review also included 3 RCTs that compared open discectomy and microdiscectomy. These studies found no difference in pain relief or complications between the 2 interventions.8
Recommendations
The Institute for Clinical Systems Improvement guidelines for adult low back pain list cauda equina, progressive neurologic deficits, or uncontrolled pain as reasons for direct referral to a spine specialist.1 Patients can be treated conservatively for 6 weeks without imaging, unless other symptoms or concerns are present.
The guidelines recommend that patients with chronic sciatica (lasting >6 weeks) receive further imaging or referral to a specialist if the patient is a potential candidate for surgery.
YES, in the short term. Patients with an acute episode of low back pain, radicular symptoms, and evidence of a herniated disc on imaging may experience short-term pain relief from discectomy if their symptoms haven’t improved after initial conservative therapy (strength of recommendation [SOR]: A, multiple randomized, controlled trials [RCTs]).
Although surgery may enhance pain relief initially, no evidence supports a long-term benefit for surgery over conservative management (SOR: A, multiple RCTs).
Evidence summary
Disc herniation is defined as any protrusion of the disc nucleus, cartilage, or other associated tissues from the normal disc space. Lumbar disc herniations (LDHs) are most likely to occur in the L4 to L5 and L5 to S1 levels, causing low back pain and sciatica. Many LDHs occur without symptoms, however, so it’s important to correlate level and side of herniation before assuming causality. Expert opinion recommends early surgical intervention for patients with cauda equina syndrome or progressive neurologic deficits.1
Surgery provides short-term gains
A search identified 4 RCTs that compared surgical intervention with conservative management. The first, published in 1983, evaluated 126 patients with radicular pain and confirmed LDH who did not improve after 2 weeks of conservative therapy. The study assigned patients to either open discectomy or back school.2 Patients rated their results as good, fair, poor, or bad; a good or fair rating was considered a positive outcome.
At 1 year, significantly more patients in the surgery group reported positive results (P<.001), based on working capacity, neurological deficits, pain, and lumbar spine mobility. At 4 years, no significant difference was found between the groups.
The study showed significant crossover, with 26% of conservatively managed patients receiving surgery within the first year. Evaluators weren’t blinded, and outcome measurements weren’t based on standardized evaluation tools.
Crossover complicates comparison of relative treatment effects
The Spine Patient Outcomes Research Trial (SPORT), published in 2006, compared 501 patients with confirmed LDH and persistent symptoms after 6 weeks.3 Patients were randomized to open discectomy or nonoperative “usual care.” Both groups showed improvement in pain scores and no significant differences in standardized pain scales at 3 months, 1 year, or 2 years.
Crossover for the study was high: 40% of the surgical group didn’t have surgery, and 45% of the nonoperative group underwent surgery. Although the pattern of care in the SPORT study resembles common clinical situations,4 the high degree of crossover makes it difficult to draw inferences about relative treatment effects.5
Greater patient satisfaction with surgery
Another RCT followed 56 patients with confirmed LDH and symptoms for 6 to 12 weeks.6 Patients were randomized to receive microdiscectomy within 2 weeks of randomization or nonoperative care. Outcomes were based on standardized pain scales for leg and back pain. The surgical group had significantly better leg pain relief (P<.01) at the 6-week evaluation. At 12 weeks, neither back pain nor leg pain differed between the groups.
Although pain didn’t differ significantly, patients in the surgical group were more satisfied with their care, and physicians were more likely to believe that surgery would improve outcomes. Crossover from the nonoperative group was high, with 39% of that group undergoing surgery.
Surgery improves leg pain, not disability, more than conservative therapy
Another RCT also directly compared microdiscectomy to conservative treatment in 283 patients with confirmed LDH and symptoms lasting 6 to 12 weeks.7 The surgical group underwent microdiscectomy within 2 weeks of randomization. Pain and disability measurements, based on standardized scales, showed significant improvement in leg pain (P<.001) for the surgical group, but no significant difference in disability.
Patient perception of recovery on a Likert-type scale showed a median recovery time of 4 weeks for the surgical group and 12 weeks for the conservative therapy group. No significant differences in perceived degree of recovery were noted between the groups at 1 year; 95% of participants had a satisfactory recovery.
Again, significant crossover occurred: 11% of patients allocated to surgery recovered before surgery, and 39% of the conservative therapy group experienced worsening symptoms or intractable pain that led them to undergo microdiscectomy.
Open discectomy, microdiscectomy produce similar results
A Cochrane review of interventions for LDH included only the Weber2 and SPORT3 RCTs. The review also included 3 RCTs that compared open discectomy and microdiscectomy. These studies found no difference in pain relief or complications between the 2 interventions.8
Recommendations
The Institute for Clinical Systems Improvement guidelines for adult low back pain list cauda equina, progressive neurologic deficits, or uncontrolled pain as reasons for direct referral to a spine specialist.1 Patients can be treated conservatively for 6 weeks without imaging, unless other symptoms or concerns are present.
The guidelines recommend that patients with chronic sciatica (lasting >6 weeks) receive further imaging or referral to a specialist if the patient is a potential candidate for surgery.
1. Institute for Clinical Systems Improvement. Health Care Guideline: Adult Low Back Pain. 13th ed. Bloomington, Minn: Institute for Clinical Systems Improvement; 2008. Available at: www.icsi.org/low_back_pain/adult_low_back_pain__8.html. Accessed December 11, 2009.
2. Weber H. Lumbar disc herniation. a controlled, prospective study with 10 years of observation. Spine. 1983;8:131-140.
3. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441-2450.
4. Angevine PD McCormick PC Inference and validity in the SPORT herniated lumbar disc randomized clinical trial. Spine J. 2007;7:387-391.
5. Mirza SK, Goodkin R. What patients know. Surg Neurol. 2008;70:5-7.
6. Osterman H, Seitsalo S, Karpinen J, et al. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine. 2006;21:2409-2414.
7. Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment in sciatica. N Engl J Med. 2007;356:2245-2256.
8. Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev. 2007;(2):CD001350.-
1. Institute for Clinical Systems Improvement. Health Care Guideline: Adult Low Back Pain. 13th ed. Bloomington, Minn: Institute for Clinical Systems Improvement; 2008. Available at: www.icsi.org/low_back_pain/adult_low_back_pain__8.html. Accessed December 11, 2009.
2. Weber H. Lumbar disc herniation. a controlled, prospective study with 10 years of observation. Spine. 1983;8:131-140.
3. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441-2450.
4. Angevine PD McCormick PC Inference and validity in the SPORT herniated lumbar disc randomized clinical trial. Spine J. 2007;7:387-391.
5. Mirza SK, Goodkin R. What patients know. Surg Neurol. 2008;70:5-7.
6. Osterman H, Seitsalo S, Karpinen J, et al. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine. 2006;21:2409-2414.
7. Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment in sciatica. N Engl J Med. 2007;356:2245-2256.
8. Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev. 2007;(2):CD001350.-
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best treatment for sebaceous cysts?
Punch biopsy excision appears to be superior to traditional wide elliptical excision for the treatment of sebaceous cysts when intervention is necessary (strength of recommendation [SOR]: B, based on 1 small randomized study). No rigorous methodological studies have compared punch biopsy excision of sebaceous cysts with the minimal excision technique.
Cyst qualities dictate technique
Gabrielle O’Sullivan, MD
University of Washington, Seattle
There are 3 main techniques for the removal of sebaceous cysts: traditional wide excision, minimal excision, and punch biopsy excision. For large cysts that have never become inflamed or ruptured, I favor the minimal excision technique because it’s likely that I’ll be able to remove the entire capsule with minimal scarring and faster healing times. Also, for cysts on the face, this method produces a better cosmetic result because of the significantly smaller scar.
However, for a cyst that has ruptured internally, has been expressed manually in the past, or recurs following minimal excision, I find traditional wide excision to be best. In these scenarios, it is extremely time-consuming and often impossible to remove the entire capsule using the minimal excision technique.
Evidence summary
Sebaceous cysts—more correctly referred to as epidermal inclusion cysts—are benign lesions of the skin. They rarely require intervention out of medical necessity, but are removed for cosmetic reasons. If the cysts become inflamed, secondary to internal discharge of the cysts’ contents, or grow so large that they interfere with the patient’s functioning, they may need to be removed.1
Traditional wide excision—involving dissection and removal of the cyst completely from the surrounding tissue through an elliptical incision—is considered the gold standard of treatment. This time-consuming endeavor frequently leads to significant scarring in comparison with minimal excision or punch biopsy, but has almost no recurrence when the cyst wall is entirely removed.2
Minimal excision and punch biopsy techniques are purported to produce minimal bleeding, have faster healing times, and produce less scarring.2 Though both techniques offer a shorter procedural time, they appear to have a slightly higher rates of recurrence.
The minimal incision technique involves kneading the lesion following injection of anesthetic and expressing the cyst contents through a 2- to 3-mm incision. Following expulsion of the cyst contents, the loosened capsule is delivered through the small opening. Closure with suture is optional.3
Punch biopsy excision is similar to the minimal excision technique except that the incision is made using a single-use disposable dermal punch following injection of lidocaine. Expulsion of the cyst contents, with cyst wall, via lateral pressure is performed and occasionally followed by closure with one suture.2
The majority of authors agree that inflamed cysts should be allowed to convalesce prior to attempted removal, though one group (Kitamura et al4) suggests primary resection, wound lavage, and primary suture without drainage for infected epidermal cysts. Rarely are these cysts truly infected. The inflammation is secondary to sebaceous cyst wall rupture with leakage of cyst contents, which elicits the inflammatory response.5
A small study points to cosmetic benefits of punch biopsy
To date, no randomized controlled trials have been published that compare the 3 most common techniques for treatment of sebaceous cysts. Only 1 small (n=60) randomized study compared traditional wide excision with punch biopsy.6 They found punch biopsy to be less time-consuming and to offer superior cosmetic results. However, cysts larger than 2 cm took longer with the punch biopsy technique.
Only a single dermatologist performed all of the surgeries, which could introduce bias. There was no mention of blinding of the researcher that subsequently measured the wounds. Of the 31 patients randomized to the punch biopsy technique, there was 1 recurrence in the 16 months of follow-up compared with none in the wide excision arm. This study excluded patients with infected, inflamed, or recurrent cysts.
Recommendations from others
UpToDate does not recommend excision of an inflamed cyst, suggesting that the inflamed cyst wall is more friable and, therefore, more difficult to remove completely.7 This may lead to a higher rate of recurrence.
Lookingbill and Marks in Principles of Dermatology8 suggest that, frequently, no therapy is indicated for these lesions. If removal is desired or indicated, every effort should be made to remove the entire cyst lining in order to prevent recurrence of the cyst. They recommend removal of the cyst via the traditional wide excision technique. If the cyst ruptures accidentally during the procedure they suggest removing the remaining contents and wall with a curette.
1. GP Notebook [online database]. Stratford-on-Avon, Warwickshire, UK: Oxbridge Solutions Limited; 2003. Sebaceous Cyst. Available at: www.gpnotebook.co.uk. Accessed on March 7, 2007.
2. Mehrabi D, Leonhardt JM, Brodell RT. Removal of keratinous and pilar cysts with the punch incision technique: analysis of surgical outcomes. Dermatol Surg 2002;28:673-677.
3. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician 2002;65:1409-1412, 14171418, 1420.
4. Kitamura K, Takahashi T, Yamaguchi T, Shimotsuma M, Majima T. Primary resection of infectious epidermal cyst. J Am Coll Surg 1994;179:607.-
5. Diven DG, Dozier SE, Meyer DJ, Smith EB. Bacteriology of inflamed and uninflamed epidermal inclusion cysts. Arch Dermatol 1998;134:49-51.
6. Lee HE, Yang CH, Chen CH, Hong HS, Kuan YZ. Comparison of the surgical outcomes of punch incision and elliptical excision in treating epidermal inclusion cysts: a prospective, randomized study. Dermatol Surg 2006;32:520-525.
7. Goldstein BG, Goldstein AO. Benign neoplasms of the skin. UpToDate [online database]. Updated November 21, 2005. Waltham, Mass: UpToDate.
8. Lookingbill DP, Marks JG. Principles of Dermatology. 3rd ed. Philadelphia, Pa: WB saunders Company; 2000.
Punch biopsy excision appears to be superior to traditional wide elliptical excision for the treatment of sebaceous cysts when intervention is necessary (strength of recommendation [SOR]: B, based on 1 small randomized study). No rigorous methodological studies have compared punch biopsy excision of sebaceous cysts with the minimal excision technique.
Cyst qualities dictate technique
Gabrielle O’Sullivan, MD
University of Washington, Seattle
There are 3 main techniques for the removal of sebaceous cysts: traditional wide excision, minimal excision, and punch biopsy excision. For large cysts that have never become inflamed or ruptured, I favor the minimal excision technique because it’s likely that I’ll be able to remove the entire capsule with minimal scarring and faster healing times. Also, for cysts on the face, this method produces a better cosmetic result because of the significantly smaller scar.
However, for a cyst that has ruptured internally, has been expressed manually in the past, or recurs following minimal excision, I find traditional wide excision to be best. In these scenarios, it is extremely time-consuming and often impossible to remove the entire capsule using the minimal excision technique.
Evidence summary
Sebaceous cysts—more correctly referred to as epidermal inclusion cysts—are benign lesions of the skin. They rarely require intervention out of medical necessity, but are removed for cosmetic reasons. If the cysts become inflamed, secondary to internal discharge of the cysts’ contents, or grow so large that they interfere with the patient’s functioning, they may need to be removed.1
Traditional wide excision—involving dissection and removal of the cyst completely from the surrounding tissue through an elliptical incision—is considered the gold standard of treatment. This time-consuming endeavor frequently leads to significant scarring in comparison with minimal excision or punch biopsy, but has almost no recurrence when the cyst wall is entirely removed.2
Minimal excision and punch biopsy techniques are purported to produce minimal bleeding, have faster healing times, and produce less scarring.2 Though both techniques offer a shorter procedural time, they appear to have a slightly higher rates of recurrence.
The minimal incision technique involves kneading the lesion following injection of anesthetic and expressing the cyst contents through a 2- to 3-mm incision. Following expulsion of the cyst contents, the loosened capsule is delivered through the small opening. Closure with suture is optional.3
Punch biopsy excision is similar to the minimal excision technique except that the incision is made using a single-use disposable dermal punch following injection of lidocaine. Expulsion of the cyst contents, with cyst wall, via lateral pressure is performed and occasionally followed by closure with one suture.2
The majority of authors agree that inflamed cysts should be allowed to convalesce prior to attempted removal, though one group (Kitamura et al4) suggests primary resection, wound lavage, and primary suture without drainage for infected epidermal cysts. Rarely are these cysts truly infected. The inflammation is secondary to sebaceous cyst wall rupture with leakage of cyst contents, which elicits the inflammatory response.5
A small study points to cosmetic benefits of punch biopsy
To date, no randomized controlled trials have been published that compare the 3 most common techniques for treatment of sebaceous cysts. Only 1 small (n=60) randomized study compared traditional wide excision with punch biopsy.6 They found punch biopsy to be less time-consuming and to offer superior cosmetic results. However, cysts larger than 2 cm took longer with the punch biopsy technique.
Only a single dermatologist performed all of the surgeries, which could introduce bias. There was no mention of blinding of the researcher that subsequently measured the wounds. Of the 31 patients randomized to the punch biopsy technique, there was 1 recurrence in the 16 months of follow-up compared with none in the wide excision arm. This study excluded patients with infected, inflamed, or recurrent cysts.
Recommendations from others
UpToDate does not recommend excision of an inflamed cyst, suggesting that the inflamed cyst wall is more friable and, therefore, more difficult to remove completely.7 This may lead to a higher rate of recurrence.
Lookingbill and Marks in Principles of Dermatology8 suggest that, frequently, no therapy is indicated for these lesions. If removal is desired or indicated, every effort should be made to remove the entire cyst lining in order to prevent recurrence of the cyst. They recommend removal of the cyst via the traditional wide excision technique. If the cyst ruptures accidentally during the procedure they suggest removing the remaining contents and wall with a curette.
Punch biopsy excision appears to be superior to traditional wide elliptical excision for the treatment of sebaceous cysts when intervention is necessary (strength of recommendation [SOR]: B, based on 1 small randomized study). No rigorous methodological studies have compared punch biopsy excision of sebaceous cysts with the minimal excision technique.
Cyst qualities dictate technique
Gabrielle O’Sullivan, MD
University of Washington, Seattle
There are 3 main techniques for the removal of sebaceous cysts: traditional wide excision, minimal excision, and punch biopsy excision. For large cysts that have never become inflamed or ruptured, I favor the minimal excision technique because it’s likely that I’ll be able to remove the entire capsule with minimal scarring and faster healing times. Also, for cysts on the face, this method produces a better cosmetic result because of the significantly smaller scar.
However, for a cyst that has ruptured internally, has been expressed manually in the past, or recurs following minimal excision, I find traditional wide excision to be best. In these scenarios, it is extremely time-consuming and often impossible to remove the entire capsule using the minimal excision technique.
Evidence summary
Sebaceous cysts—more correctly referred to as epidermal inclusion cysts—are benign lesions of the skin. They rarely require intervention out of medical necessity, but are removed for cosmetic reasons. If the cysts become inflamed, secondary to internal discharge of the cysts’ contents, or grow so large that they interfere with the patient’s functioning, they may need to be removed.1
Traditional wide excision—involving dissection and removal of the cyst completely from the surrounding tissue through an elliptical incision—is considered the gold standard of treatment. This time-consuming endeavor frequently leads to significant scarring in comparison with minimal excision or punch biopsy, but has almost no recurrence when the cyst wall is entirely removed.2
Minimal excision and punch biopsy techniques are purported to produce minimal bleeding, have faster healing times, and produce less scarring.2 Though both techniques offer a shorter procedural time, they appear to have a slightly higher rates of recurrence.
The minimal incision technique involves kneading the lesion following injection of anesthetic and expressing the cyst contents through a 2- to 3-mm incision. Following expulsion of the cyst contents, the loosened capsule is delivered through the small opening. Closure with suture is optional.3
Punch biopsy excision is similar to the minimal excision technique except that the incision is made using a single-use disposable dermal punch following injection of lidocaine. Expulsion of the cyst contents, with cyst wall, via lateral pressure is performed and occasionally followed by closure with one suture.2
The majority of authors agree that inflamed cysts should be allowed to convalesce prior to attempted removal, though one group (Kitamura et al4) suggests primary resection, wound lavage, and primary suture without drainage for infected epidermal cysts. Rarely are these cysts truly infected. The inflammation is secondary to sebaceous cyst wall rupture with leakage of cyst contents, which elicits the inflammatory response.5
A small study points to cosmetic benefits of punch biopsy
To date, no randomized controlled trials have been published that compare the 3 most common techniques for treatment of sebaceous cysts. Only 1 small (n=60) randomized study compared traditional wide excision with punch biopsy.6 They found punch biopsy to be less time-consuming and to offer superior cosmetic results. However, cysts larger than 2 cm took longer with the punch biopsy technique.
Only a single dermatologist performed all of the surgeries, which could introduce bias. There was no mention of blinding of the researcher that subsequently measured the wounds. Of the 31 patients randomized to the punch biopsy technique, there was 1 recurrence in the 16 months of follow-up compared with none in the wide excision arm. This study excluded patients with infected, inflamed, or recurrent cysts.
Recommendations from others
UpToDate does not recommend excision of an inflamed cyst, suggesting that the inflamed cyst wall is more friable and, therefore, more difficult to remove completely.7 This may lead to a higher rate of recurrence.
Lookingbill and Marks in Principles of Dermatology8 suggest that, frequently, no therapy is indicated for these lesions. If removal is desired or indicated, every effort should be made to remove the entire cyst lining in order to prevent recurrence of the cyst. They recommend removal of the cyst via the traditional wide excision technique. If the cyst ruptures accidentally during the procedure they suggest removing the remaining contents and wall with a curette.
1. GP Notebook [online database]. Stratford-on-Avon, Warwickshire, UK: Oxbridge Solutions Limited; 2003. Sebaceous Cyst. Available at: www.gpnotebook.co.uk. Accessed on March 7, 2007.
2. Mehrabi D, Leonhardt JM, Brodell RT. Removal of keratinous and pilar cysts with the punch incision technique: analysis of surgical outcomes. Dermatol Surg 2002;28:673-677.
3. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician 2002;65:1409-1412, 14171418, 1420.
4. Kitamura K, Takahashi T, Yamaguchi T, Shimotsuma M, Majima T. Primary resection of infectious epidermal cyst. J Am Coll Surg 1994;179:607.-
5. Diven DG, Dozier SE, Meyer DJ, Smith EB. Bacteriology of inflamed and uninflamed epidermal inclusion cysts. Arch Dermatol 1998;134:49-51.
6. Lee HE, Yang CH, Chen CH, Hong HS, Kuan YZ. Comparison of the surgical outcomes of punch incision and elliptical excision in treating epidermal inclusion cysts: a prospective, randomized study. Dermatol Surg 2006;32:520-525.
7. Goldstein BG, Goldstein AO. Benign neoplasms of the skin. UpToDate [online database]. Updated November 21, 2005. Waltham, Mass: UpToDate.
8. Lookingbill DP, Marks JG. Principles of Dermatology. 3rd ed. Philadelphia, Pa: WB saunders Company; 2000.
1. GP Notebook [online database]. Stratford-on-Avon, Warwickshire, UK: Oxbridge Solutions Limited; 2003. Sebaceous Cyst. Available at: www.gpnotebook.co.uk. Accessed on March 7, 2007.
2. Mehrabi D, Leonhardt JM, Brodell RT. Removal of keratinous and pilar cysts with the punch incision technique: analysis of surgical outcomes. Dermatol Surg 2002;28:673-677.
3. Zuber TJ. Minimal excision technique for epidermoid (sebaceous) cysts. Am Fam Physician 2002;65:1409-1412, 14171418, 1420.
4. Kitamura K, Takahashi T, Yamaguchi T, Shimotsuma M, Majima T. Primary resection of infectious epidermal cyst. J Am Coll Surg 1994;179:607.-
5. Diven DG, Dozier SE, Meyer DJ, Smith EB. Bacteriology of inflamed and uninflamed epidermal inclusion cysts. Arch Dermatol 1998;134:49-51.
6. Lee HE, Yang CH, Chen CH, Hong HS, Kuan YZ. Comparison of the surgical outcomes of punch incision and elliptical excision in treating epidermal inclusion cysts: a prospective, randomized study. Dermatol Surg 2006;32:520-525.
7. Goldstein BG, Goldstein AO. Benign neoplasms of the skin. UpToDate [online database]. Updated November 21, 2005. Waltham, Mass: UpToDate.
8. Lookingbill DP, Marks JG. Principles of Dermatology. 3rd ed. Philadelphia, Pa: WB saunders Company; 2000.
Evidence-based answers from the Family Physicians Inquiries Network
Do preparticipation clinical exams reduce morbidity and mortality for athletes?
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
Though clinical preparticipation exams (PPE) are recommended by experts and required in most states, we found no medium- or better-quality evidence that demonstrates they reduce mortality or morbidity. PPEs detect only a very small percentage of cardiac abnormalities among athletes who subsequently die suddenly (strength of recommendation [SOR]: C, case series study). PPEs are also unable to accurately identify athletes with exercise-induced bronchospasm (SOR: C, small cross-sectional study) and are poorly predictive of which athletes are at increased risk of orthopedic injuries (SOR: C, cross-sectional study).
Evidence summary
A systematic review of the literature on PPE identified 310 studies of athletes age <36 years. The authors searched multiple electronic databases and reviewed the bibliographies of retrieved articles but did not perform hand searches of journals or contact authors directly. The review did not find any prospective cohort or randomized trials addressing the effectiveness of clinical PPE. The 5 studies that assessed the format of the PPE concluded that it is not adequately standardized, does not consistently address the American Heart Association (AHA) recommendations for cardiovascular screening and exam, and is administered by a variety of health care professionals, some without proper training.1
Sudden cardiac death is defined as a nontraumatic, nonviolent, unexpected event resulting from sudden cardiac arrest within 6 hours of a previously witnessed state of normal health.2 Such events occur in about 1 in 200,000 high school athletes per academic year (about 16 deaths in the US annually). Detection of cardiovascular abnormalities that may cause morbidity or mortality is difficult. A case series reviewed 158 sudden deaths that occurred in trained athletes in the US from 1985 to 1995. The athletes were identified from news accounts, the National Center for Catastrophic Sports Injury Registry, and informal communications and reports. The authors interviewed families, witnesses, and coaches, and they analyzed postmortem information. Of the 115 athletes who had a standard preparticipation medical evaluation, only 4 (3%) were suspected of having cardiovascular disease. The cardiovascular abnormality responsible for sudden death was prospectively identified in only 1 athlete.3
PPE does not accurately identify student athletes with exercise-induced bronchospasm (EIB). Of the studies on EIB, the best was a prospective cross-sectional study of 352 adolescents from 3 suburban Washington state schools. The students completed a 14-item EIB questionnaire, had a physical exam, and underwent a 7-minute exercise challenge spirometry. Complete data were available for 256 of the students. EIB was diagnosed by spirometry in 9.4% of the athletes. No student had EIB detected solely by physical exam. Using a cutoff of 2 positive questions, the questionnaire had a sensitivity of 71% and a specificity of 47%, with a negative and positive predictive value of 6% and 12%, respectively. This study concluded that EIB occurs frequently in adolescent athletes, but screening by physical exam and medical history does not accurately detect it.4
PPEs are not able to predict which student athletes will experience an orthopedic injury, and no controlled studies have been done to determine whether PPE prevents or reduces the severity of orthopedic injuries. A study surveyed 1204 student athletes (aged 13–20 years) from Richmond County, Georgia, who had a standardized PPE before participating in sports. The questionnaire was administered via mail or telephone and inquired about injuries sustained after the PPE. The response rate to the survey was 56%. The study found that a history of knee or ankle injury and abnormal findings on exam in male athletes slightly increased the likelihood of repeated injury of the same joint. However, the sensitivities of history or physical exam for ankle or knee injuries were all <25%.5
Recommendations from others
The AHA recommends a national standard for PPE and that screening should be mandatory for all high school and college athletes before participation in organized sports, with screening repeated every 2 years, and an interim history obtained during the intervening years. Specific items are given in the TABLE.6
In 2004, the American Academy of Family Physicians, along with the American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine, published recommendations for PPEs. They suggested a detailed history (consisting of a 16-point questionnaire incorporating AHA recommendations for cardiovascular screening), limited medical exam, and a detailed musculoskeletal exam evaluating strength, flexibility, and stability of major joints.7
TABLE
AHA recommendations for preparticipation exams
| CARDIOVASCULAR SCREENING QUESTIONS |
|
| CARDIOVASCULAR SCREENING EXAM |
|
| CARDIAC FINDINGS REQUIRING FURTHER EVALUATION |
|
The PPE provides us an opportunity to address preventive health issues
Beth Anne Fox, MD, MPH
East Tennessee State University, Kingsport Family Medicine Residency, Kingsport, Tennessee
Most physicians involved in screening athletes recognize the limitations of PPEs in detecting those at risk for sports-related morbidity and mortality. The history is the most important part of the examination for identifying athletes who might be at risk and should be thorough. Prepared PPE forms such as those endorsed by the AAFP and ACSM can assist in obtaining this history. Because this may be the only occasion for the athlete to see a physician, the examination is best performed by a primary care provider who can use the opportunity to address preventive health issues such as tobacco, alcohol, and drug use, depression and suicidality, sexuality, nutrition, and accident prevention. This kind of counseling is difficult to do in a group format.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
1. Wingfield K, Matheson GO, Meeuwisse WH. Preparticipation evaluation: an evidence-based review. Clin J Sport Med 2004;14:109-122.
2. Lyznicki JM, Nielsen NH, Schneider JF. Cardiovascular screening of athletes. Am Fam Physician 2000;62:765-774.Erratum in: Am Fam Physician, 2001; 63:2332.
3. Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199-204.
4. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr 2002;141:343-348.
5. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85.-
6. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-856.
7. Smith DM. American Academy of Family Physicians, Preparticipation Physical Evaluation Task Force. Preparticipation Physical Evaluation. 3rd ed. Minneapolis: McGraw-Hill Healthcare; 2004.
Evidence-based answers from the Family Physicians Inquiries Network