User login
Necrobiotic Xanthogranuloma Without a Monoclonal Gammopathy
Necrobiotic xanthogranuloma (NXG) was first described in 1980 by Kossard and Winkelmann1 as a xanthomatosis associated with paraproteinemia. It is an indolent disorder characterized by indurated, yellow to violaceous red papules, plaques, or nodules often presenting with telangiectases and ulceration.2 The lesions have a predilection for the bilateral periorbital region in the majority of documented cases, consequently producing ocular findings such as periocular skin lesions, blepharoptosis, restricted ocular motility, and proptosis.3
Necrobiotic xanthogranuloma is a systemic disease that may involve extracutaneous sites such as the heart, respiratory tract, spleen, kidneys, ovaries, liver, skeletal muscle, and central nervous system.4-6 The most common sites include the respiratory tract and heart, with documented cases of pulmonary and myocardial giant cell granulomas.4 In a 2009 review, Spicknall and Mehregan7 reported an increased frequency of systemic involvement.
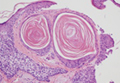
The distinctive histopathologic features of NXG consist of large bands of necrobiosis and a pattern of palisading histiocytic granulomas comprised of Touton giant cells, bizarre foreign body giant cells, foam cells, and cholesterol clefts.8 These histopathologic findings differentiate NXG from other clinical differential diagnoses such as necrobiosis lipoidica.
Necrobiotic xanthogranuloma is associated with paraproteinemia in 80% of documented cases, most commonly as an IgG monoclonal gammopathy.2 The etiology of this indolent disorder remains unclear despite proposed theories of its pathogenesis. Consequently, treatment proves difficult with no recommended first-line therapy and a tendency for recurrent cutaneous lesions. We report an unusual case of periorbital NXG without development of a monoclonal gammopathy.
Case Report
A 76-year-old man presented with a long-standing history (30 years) of bilateral periorbital NXG. Approximately 30 years prior to the current presentation, the patient presented to a dermatologist with dry eyes and periorbital cutaneous lesions that were originally diagnosed as xanthelasma. He later developed edema of the right periorbital region that progressed to involve the left periorbital region. He underwent surgical excision of the lesions 10 years prior to the current presentation, which showed the lesions were infiltrating into the muscle. At that time, a diagnosis of NXG was made. The department of plastic surgery at an outside institution evaluated the patient and identified no further treatment options; however, annual computed tomography scans were performed to detect disease progression.
The patient presented for increasing periorbital manifestations of NXG. He denied any other remarkable medical history or any family history of NXG, malignancy, or hematologic disorders. His surgical history was exclusive to the excisional surgery of the periorbital lesions. At the time of presentation he was not taking medications and had no known drug allergies. He denied tobacco use but occasionally consumed alcohol.
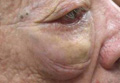
On systemic inquiry the patient’s only concerns were ocular in nature and included dry, sensitive, and painful eyes. Dermatologic examination revealed substantial periorbital edema with multiple yellow indurated plaques (Figure). There were no additional findings on physical examination.
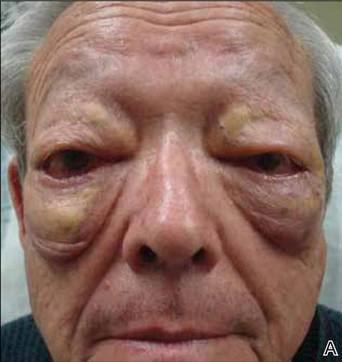 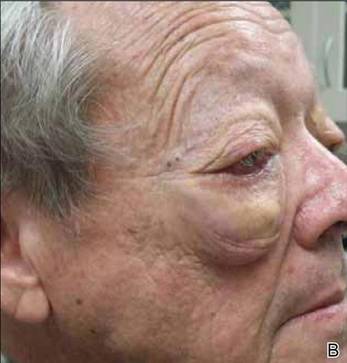 Bilateral periorbital edema with yellow indurated plaques (A). A lateral view showed substantial right periorbital edema with multiple yellow plaques (B). |
Extensive hematologic and oncologic investigations revealed the absence of a monoclonal gammopathy. Serum protein electrophoresis was negative for paraproteinemia and quantitative serum immunoglobulin testing was normal. A complete blood cell count, lipid panel, CD4 count, CD8 count, C3, C4, and computed tomography scan did not reveal any abnormalities. A complete metabolic panel identified elevated serum glucose levels (162 mg/dL [reference range, 74–118 mg/dL]), low serum albumin levels (3.3 g/dL [reference range, 3.5–4.8 g/dL]), and low serum calcium levels (8.8 mg/dL [reference range, 8.9–10.3 mg/dL]). IgG subclass (SC) proteins were mildly increasedwith an IgG SC1 of 950 mg/dL (reference range, 382–929 mg/dL), IgG SC3 of 211 mg/dL (reference range, 22–178 mg/dL), and IgG SC4 of 292 mg/dL (reference range, 4–86 mg/dL), and the plasma IgG was in the upper limit of the reference range with a value of 1591 mg/dL (reference range, 791–1643 mg/dL).
After the hematologic and oncologic workup was completed, intravenous immunoglobulin and acitretin were recommended to the patient as viable treatment options to reduce the cutaneous sequelae of NXG. A 6-month regimen of acitretin markedly improved cutaneous edema and plaque size. However, these sequelae returned to baseline just months after acitretin was discontinued.
Comment
Necrobiotic xanthogranuloma is a distinct granulomatous disorder with no predilection for sex and the average age of onset is 54 years.2 Consistent with prior reports, our patient presented with bilateral periorbital lesions and ophthalmic concerns of dryness, burning, and sensitivity. Reddy et al9 demonstrated that aggressive forms of periorbital NXG may involve ocular tissues and result in vision loss and corneal perforation. On follow-up, our patient underwent annual computed tomography scans to rule out further progression.
Eighty percent of patients diagnosed with NXG have an associated monoclonal gammopathy and 10% develop multiple myeloma.2 Our case presents an unusual variant of NXG due to the absence of a monoclonal gammopathy. Chang et al10 described a similar case of NXG without a monoclonal gammopathy and hypothesized that periorbital involvement, malignancy, and systemic involvement are the main contributing factors to the morbidity of NXG.
Our patient had mildly elevated IgG SC1, IgG SC3, and IgG SC4 levels. The most substantially elevated subclass was IgG SC4. Elevations of IgG SC4 often are associated with disorders that are allergic or autoimmune in nature, such as pemphigus vulgaris, autoimmune pancreatitis, and inflammatory pseudotumor.11 Our patient denied prior history and lacked manifestations of allergic or autoimmune disorders. A similar case was reported in a 67-year-old man with periorbital NXG and elevated IgG SC4 levels. Singh et al12 postulated that systemic elevation of IgG SC4 can be associated with NXG of the orbit.
Due to the rarity and uncertain etiology of NXG, there are no definitive first-line therapies.There have been encouraging results with intravenous immunoglobulin,13 autologous stem cell transplantation,14 lenalidomide,15 melphalan with prednisolone,16 and chlorambucil with low-dose corticosteroids.11 In 2007, Ho et al17 identified that CD20 and CD25 were both strongly expressed in tissue specimens of NXG, indicating the possible effectiveness of rituximab and denileukin diftitox, which target CD20 and CD25, respectively. It is unclear how these data pertain to patients without paraproteinemia because the treatment often is directed at the monoclonal gammopathy. These uncertainties are concerning because of the undesirable and often toxic side effects associated with these therapies. Psoralen plus UVA therapy was described as an alternative to cytotoxic drugs and immunosuppressive agents in 1 patient without paraproteinemia.18
Bullock et al19 proposed that NXG arises from a foreign body giant cell reaction resulting from circulating immune complexes precipitating in periorbital tissues. Although the relationship between the cutaneous manifestations and a monoclonal gammopathy remains poorly understood, cases of NXG without paraproteinemia challenge this theory. Our case supports the theory that there is no correlation between the histopathologic findings of NXG and the extent of the monoclonal gammopathy.
Conclusion
The etiology and pathogenesis of NXG remain elusive. We strive to attain a more sophisticated understanding of NXG to identify efficacious treatment options. Our case highlights the ambiguous association between the cutaneous lesions of NXG and a monoclonal gammopathy.
1. Kossard S, Winkelmann RK. Necrobiotic xanthogranuloma with paraproteinemia. J Am Acad Dermatol. 1980;3:257-270.
2. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma [published correction in Arch Dermatol. 1992;128:632]. Arch Dermatol. 1992;128:94-100.
3. Ugurlu S, Bartley GB, Gibson LE. Necrobiotic xanthogranuloma: long-term outcome of ocular and systemic involvement. Am J Ophthalmol. 2000;129:651-657.
4. Winkelmann RK, Litzow MR, Umbert IJ, et al. Giant cell granulomatous pulmonary and myocardial lesions in necrobiotic xanthogranuloma with paraproteinemia. Mayo Clin Proc. 1997;72:1028-1033.
5. Shah KC, Poonnoose SI, George R, et al. Necrobiotic xanthogranuloma with cutaneous and cerebral manifestations. case report and review of literature. J Neurosurg. 2004;100:1111-1114.
6. Umbert I, Winkelmann RK. Necrobiotic xanthogranuloma with cardiac involvement. Br J Dermatol. 1995;133:438-443.
7. Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
8. Finan MC, Winkelmann RK. Histopathology of necrobiotic xanthogranuloma with paraproteinemia. J Cutan Pathol. 1987;14:92-99.
9. Reddy VC, Salomão DR, Garrity JA, et al. Periorbital and ocular necrobiotic xanthogranuloma leading to perforation. Arch Ophthalmol. 2010;128:1493-1494.
10. Chang SE, Lee WS, Lee MW, et al. A case of necrobiotic xanthogranuloma without paraproteinemia presenting as a solitary tumor on the thigh. Int J Dermatol. 2003;42:470-472.
11. Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284.
12. Singh K, Rajan KD, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocul Immunol Inflamm. 2010;18:373-378.
13. Hallermann C, Tittelbach J, Norgauer J, et al. Successful treatment of necrobiotic xanthogranuloma with intravenous immunoglobulin. Arch Dermatol. 2009;146:957-960.
14. Goede JS, Misselwitz B, Taverna C, et al. Necrobiotic xanthogranuloma successfully treated with autologous stem cell transplantation. Ann Hematol. 2007;86:303-306.
15. Silapunt S, Chon SY. Generalized necrobiotic xanthogranuloma successfully treated with lenalidomide. J Drugs Dermatol. 2010;9:273-276.
16. Saeki H, Tomita M, Kai H, et al. Necrobiotic xanthogranuloma with paraproteinemia successfully treated with melphalan, prednisolone and skin graft. J Dermatol. 2007;34:795-797.
17. Ho VH, Chevez-Barrios P, Jorgensen JL, et al. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007;70:105-109.
18. Al-Niaimi FA, Dawn G, Cox NH. Necrobiotic xanthogranuloma without paraproteinemia: marked improvement with psoralen ultraviolet A treatment. Clin Exp Dermatol. 2010;35:275-277.
19. Bullock JD, Bartley GB, Campbell RJ, et al. Necrobiotic xanthogranuloma with paraproteinemia: case report and a pathogenetic theory. Trans Am Ophthalmol Soc. 1986;84:342-352.
Necrobiotic xanthogranuloma (NXG) was first described in 1980 by Kossard and Winkelmann1 as a xanthomatosis associated with paraproteinemia. It is an indolent disorder characterized by indurated, yellow to violaceous red papules, plaques, or nodules often presenting with telangiectases and ulceration.2 The lesions have a predilection for the bilateral periorbital region in the majority of documented cases, consequently producing ocular findings such as periocular skin lesions, blepharoptosis, restricted ocular motility, and proptosis.3
Necrobiotic xanthogranuloma is a systemic disease that may involve extracutaneous sites such as the heart, respiratory tract, spleen, kidneys, ovaries, liver, skeletal muscle, and central nervous system.4-6 The most common sites include the respiratory tract and heart, with documented cases of pulmonary and myocardial giant cell granulomas.4 In a 2009 review, Spicknall and Mehregan7 reported an increased frequency of systemic involvement.
The distinctive histopathologic features of NXG consist of large bands of necrobiosis and a pattern of palisading histiocytic granulomas comprised of Touton giant cells, bizarre foreign body giant cells, foam cells, and cholesterol clefts.8 These histopathologic findings differentiate NXG from other clinical differential diagnoses such as necrobiosis lipoidica.
Necrobiotic xanthogranuloma is associated with paraproteinemia in 80% of documented cases, most commonly as an IgG monoclonal gammopathy.2 The etiology of this indolent disorder remains unclear despite proposed theories of its pathogenesis. Consequently, treatment proves difficult with no recommended first-line therapy and a tendency for recurrent cutaneous lesions. We report an unusual case of periorbital NXG without development of a monoclonal gammopathy.
Case Report
A 76-year-old man presented with a long-standing history (30 years) of bilateral periorbital NXG. Approximately 30 years prior to the current presentation, the patient presented to a dermatologist with dry eyes and periorbital cutaneous lesions that were originally diagnosed as xanthelasma. He later developed edema of the right periorbital region that progressed to involve the left periorbital region. He underwent surgical excision of the lesions 10 years prior to the current presentation, which showed the lesions were infiltrating into the muscle. At that time, a diagnosis of NXG was made. The department of plastic surgery at an outside institution evaluated the patient and identified no further treatment options; however, annual computed tomography scans were performed to detect disease progression.
The patient presented for increasing periorbital manifestations of NXG. He denied any other remarkable medical history or any family history of NXG, malignancy, or hematologic disorders. His surgical history was exclusive to the excisional surgery of the periorbital lesions. At the time of presentation he was not taking medications and had no known drug allergies. He denied tobacco use but occasionally consumed alcohol.
On systemic inquiry the patient’s only concerns were ocular in nature and included dry, sensitive, and painful eyes. Dermatologic examination revealed substantial periorbital edema with multiple yellow indurated plaques (Figure). There were no additional findings on physical examination.
  Bilateral periorbital edema with yellow indurated plaques (A). A lateral view showed substantial right periorbital edema with multiple yellow plaques (B). |
Extensive hematologic and oncologic investigations revealed the absence of a monoclonal gammopathy. Serum protein electrophoresis was negative for paraproteinemia and quantitative serum immunoglobulin testing was normal. A complete blood cell count, lipid panel, CD4 count, CD8 count, C3, C4, and computed tomography scan did not reveal any abnormalities. A complete metabolic panel identified elevated serum glucose levels (162 mg/dL [reference range, 74–118 mg/dL]), low serum albumin levels (3.3 g/dL [reference range, 3.5–4.8 g/dL]), and low serum calcium levels (8.8 mg/dL [reference range, 8.9–10.3 mg/dL]). IgG subclass (SC) proteins were mildly increasedwith an IgG SC1 of 950 mg/dL (reference range, 382–929 mg/dL), IgG SC3 of 211 mg/dL (reference range, 22–178 mg/dL), and IgG SC4 of 292 mg/dL (reference range, 4–86 mg/dL), and the plasma IgG was in the upper limit of the reference range with a value of 1591 mg/dL (reference range, 791–1643 mg/dL).
After the hematologic and oncologic workup was completed, intravenous immunoglobulin and acitretin were recommended to the patient as viable treatment options to reduce the cutaneous sequelae of NXG. A 6-month regimen of acitretin markedly improved cutaneous edema and plaque size. However, these sequelae returned to baseline just months after acitretin was discontinued.
Comment
Necrobiotic xanthogranuloma is a distinct granulomatous disorder with no predilection for sex and the average age of onset is 54 years.2 Consistent with prior reports, our patient presented with bilateral periorbital lesions and ophthalmic concerns of dryness, burning, and sensitivity. Reddy et al9 demonstrated that aggressive forms of periorbital NXG may involve ocular tissues and result in vision loss and corneal perforation. On follow-up, our patient underwent annual computed tomography scans to rule out further progression.
Eighty percent of patients diagnosed with NXG have an associated monoclonal gammopathy and 10% develop multiple myeloma.2 Our case presents an unusual variant of NXG due to the absence of a monoclonal gammopathy. Chang et al10 described a similar case of NXG without a monoclonal gammopathy and hypothesized that periorbital involvement, malignancy, and systemic involvement are the main contributing factors to the morbidity of NXG.
Our patient had mildly elevated IgG SC1, IgG SC3, and IgG SC4 levels. The most substantially elevated subclass was IgG SC4. Elevations of IgG SC4 often are associated with disorders that are allergic or autoimmune in nature, such as pemphigus vulgaris, autoimmune pancreatitis, and inflammatory pseudotumor.11 Our patient denied prior history and lacked manifestations of allergic or autoimmune disorders. A similar case was reported in a 67-year-old man with periorbital NXG and elevated IgG SC4 levels. Singh et al12 postulated that systemic elevation of IgG SC4 can be associated with NXG of the orbit.
Due to the rarity and uncertain etiology of NXG, there are no definitive first-line therapies.There have been encouraging results with intravenous immunoglobulin,13 autologous stem cell transplantation,14 lenalidomide,15 melphalan with prednisolone,16 and chlorambucil with low-dose corticosteroids.11 In 2007, Ho et al17 identified that CD20 and CD25 were both strongly expressed in tissue specimens of NXG, indicating the possible effectiveness of rituximab and denileukin diftitox, which target CD20 and CD25, respectively. It is unclear how these data pertain to patients without paraproteinemia because the treatment often is directed at the monoclonal gammopathy. These uncertainties are concerning because of the undesirable and often toxic side effects associated with these therapies. Psoralen plus UVA therapy was described as an alternative to cytotoxic drugs and immunosuppressive agents in 1 patient without paraproteinemia.18
Bullock et al19 proposed that NXG arises from a foreign body giant cell reaction resulting from circulating immune complexes precipitating in periorbital tissues. Although the relationship between the cutaneous manifestations and a monoclonal gammopathy remains poorly understood, cases of NXG without paraproteinemia challenge this theory. Our case supports the theory that there is no correlation between the histopathologic findings of NXG and the extent of the monoclonal gammopathy.
Conclusion
The etiology and pathogenesis of NXG remain elusive. We strive to attain a more sophisticated understanding of NXG to identify efficacious treatment options. Our case highlights the ambiguous association between the cutaneous lesions of NXG and a monoclonal gammopathy.
Necrobiotic xanthogranuloma (NXG) was first described in 1980 by Kossard and Winkelmann1 as a xanthomatosis associated with paraproteinemia. It is an indolent disorder characterized by indurated, yellow to violaceous red papules, plaques, or nodules often presenting with telangiectases and ulceration.2 The lesions have a predilection for the bilateral periorbital region in the majority of documented cases, consequently producing ocular findings such as periocular skin lesions, blepharoptosis, restricted ocular motility, and proptosis.3
Necrobiotic xanthogranuloma is a systemic disease that may involve extracutaneous sites such as the heart, respiratory tract, spleen, kidneys, ovaries, liver, skeletal muscle, and central nervous system.4-6 The most common sites include the respiratory tract and heart, with documented cases of pulmonary and myocardial giant cell granulomas.4 In a 2009 review, Spicknall and Mehregan7 reported an increased frequency of systemic involvement.
The distinctive histopathologic features of NXG consist of large bands of necrobiosis and a pattern of palisading histiocytic granulomas comprised of Touton giant cells, bizarre foreign body giant cells, foam cells, and cholesterol clefts.8 These histopathologic findings differentiate NXG from other clinical differential diagnoses such as necrobiosis lipoidica.
Necrobiotic xanthogranuloma is associated with paraproteinemia in 80% of documented cases, most commonly as an IgG monoclonal gammopathy.2 The etiology of this indolent disorder remains unclear despite proposed theories of its pathogenesis. Consequently, treatment proves difficult with no recommended first-line therapy and a tendency for recurrent cutaneous lesions. We report an unusual case of periorbital NXG without development of a monoclonal gammopathy.
Case Report
A 76-year-old man presented with a long-standing history (30 years) of bilateral periorbital NXG. Approximately 30 years prior to the current presentation, the patient presented to a dermatologist with dry eyes and periorbital cutaneous lesions that were originally diagnosed as xanthelasma. He later developed edema of the right periorbital region that progressed to involve the left periorbital region. He underwent surgical excision of the lesions 10 years prior to the current presentation, which showed the lesions were infiltrating into the muscle. At that time, a diagnosis of NXG was made. The department of plastic surgery at an outside institution evaluated the patient and identified no further treatment options; however, annual computed tomography scans were performed to detect disease progression.
The patient presented for increasing periorbital manifestations of NXG. He denied any other remarkable medical history or any family history of NXG, malignancy, or hematologic disorders. His surgical history was exclusive to the excisional surgery of the periorbital lesions. At the time of presentation he was not taking medications and had no known drug allergies. He denied tobacco use but occasionally consumed alcohol.
On systemic inquiry the patient’s only concerns were ocular in nature and included dry, sensitive, and painful eyes. Dermatologic examination revealed substantial periorbital edema with multiple yellow indurated plaques (Figure). There were no additional findings on physical examination.
  Bilateral periorbital edema with yellow indurated plaques (A). A lateral view showed substantial right periorbital edema with multiple yellow plaques (B). |
Extensive hematologic and oncologic investigations revealed the absence of a monoclonal gammopathy. Serum protein electrophoresis was negative for paraproteinemia and quantitative serum immunoglobulin testing was normal. A complete blood cell count, lipid panel, CD4 count, CD8 count, C3, C4, and computed tomography scan did not reveal any abnormalities. A complete metabolic panel identified elevated serum glucose levels (162 mg/dL [reference range, 74–118 mg/dL]), low serum albumin levels (3.3 g/dL [reference range, 3.5–4.8 g/dL]), and low serum calcium levels (8.8 mg/dL [reference range, 8.9–10.3 mg/dL]). IgG subclass (SC) proteins were mildly increasedwith an IgG SC1 of 950 mg/dL (reference range, 382–929 mg/dL), IgG SC3 of 211 mg/dL (reference range, 22–178 mg/dL), and IgG SC4 of 292 mg/dL (reference range, 4–86 mg/dL), and the plasma IgG was in the upper limit of the reference range with a value of 1591 mg/dL (reference range, 791–1643 mg/dL).
After the hematologic and oncologic workup was completed, intravenous immunoglobulin and acitretin were recommended to the patient as viable treatment options to reduce the cutaneous sequelae of NXG. A 6-month regimen of acitretin markedly improved cutaneous edema and plaque size. However, these sequelae returned to baseline just months after acitretin was discontinued.
Comment
Necrobiotic xanthogranuloma is a distinct granulomatous disorder with no predilection for sex and the average age of onset is 54 years.2 Consistent with prior reports, our patient presented with bilateral periorbital lesions and ophthalmic concerns of dryness, burning, and sensitivity. Reddy et al9 demonstrated that aggressive forms of periorbital NXG may involve ocular tissues and result in vision loss and corneal perforation. On follow-up, our patient underwent annual computed tomography scans to rule out further progression.
Eighty percent of patients diagnosed with NXG have an associated monoclonal gammopathy and 10% develop multiple myeloma.2 Our case presents an unusual variant of NXG due to the absence of a monoclonal gammopathy. Chang et al10 described a similar case of NXG without a monoclonal gammopathy and hypothesized that periorbital involvement, malignancy, and systemic involvement are the main contributing factors to the morbidity of NXG.
Our patient had mildly elevated IgG SC1, IgG SC3, and IgG SC4 levels. The most substantially elevated subclass was IgG SC4. Elevations of IgG SC4 often are associated with disorders that are allergic or autoimmune in nature, such as pemphigus vulgaris, autoimmune pancreatitis, and inflammatory pseudotumor.11 Our patient denied prior history and lacked manifestations of allergic or autoimmune disorders. A similar case was reported in a 67-year-old man with periorbital NXG and elevated IgG SC4 levels. Singh et al12 postulated that systemic elevation of IgG SC4 can be associated with NXG of the orbit.
Due to the rarity and uncertain etiology of NXG, there are no definitive first-line therapies.There have been encouraging results with intravenous immunoglobulin,13 autologous stem cell transplantation,14 lenalidomide,15 melphalan with prednisolone,16 and chlorambucil with low-dose corticosteroids.11 In 2007, Ho et al17 identified that CD20 and CD25 were both strongly expressed in tissue specimens of NXG, indicating the possible effectiveness of rituximab and denileukin diftitox, which target CD20 and CD25, respectively. It is unclear how these data pertain to patients without paraproteinemia because the treatment often is directed at the monoclonal gammopathy. These uncertainties are concerning because of the undesirable and often toxic side effects associated with these therapies. Psoralen plus UVA therapy was described as an alternative to cytotoxic drugs and immunosuppressive agents in 1 patient without paraproteinemia.18
Bullock et al19 proposed that NXG arises from a foreign body giant cell reaction resulting from circulating immune complexes precipitating in periorbital tissues. Although the relationship between the cutaneous manifestations and a monoclonal gammopathy remains poorly understood, cases of NXG without paraproteinemia challenge this theory. Our case supports the theory that there is no correlation between the histopathologic findings of NXG and the extent of the monoclonal gammopathy.
Conclusion
The etiology and pathogenesis of NXG remain elusive. We strive to attain a more sophisticated understanding of NXG to identify efficacious treatment options. Our case highlights the ambiguous association between the cutaneous lesions of NXG and a monoclonal gammopathy.
1. Kossard S, Winkelmann RK. Necrobiotic xanthogranuloma with paraproteinemia. J Am Acad Dermatol. 1980;3:257-270.
2. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma [published correction in Arch Dermatol. 1992;128:632]. Arch Dermatol. 1992;128:94-100.
3. Ugurlu S, Bartley GB, Gibson LE. Necrobiotic xanthogranuloma: long-term outcome of ocular and systemic involvement. Am J Ophthalmol. 2000;129:651-657.
4. Winkelmann RK, Litzow MR, Umbert IJ, et al. Giant cell granulomatous pulmonary and myocardial lesions in necrobiotic xanthogranuloma with paraproteinemia. Mayo Clin Proc. 1997;72:1028-1033.
5. Shah KC, Poonnoose SI, George R, et al. Necrobiotic xanthogranuloma with cutaneous and cerebral manifestations. case report and review of literature. J Neurosurg. 2004;100:1111-1114.
6. Umbert I, Winkelmann RK. Necrobiotic xanthogranuloma with cardiac involvement. Br J Dermatol. 1995;133:438-443.
7. Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
8. Finan MC, Winkelmann RK. Histopathology of necrobiotic xanthogranuloma with paraproteinemia. J Cutan Pathol. 1987;14:92-99.
9. Reddy VC, Salomão DR, Garrity JA, et al. Periorbital and ocular necrobiotic xanthogranuloma leading to perforation. Arch Ophthalmol. 2010;128:1493-1494.
10. Chang SE, Lee WS, Lee MW, et al. A case of necrobiotic xanthogranuloma without paraproteinemia presenting as a solitary tumor on the thigh. Int J Dermatol. 2003;42:470-472.
11. Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284.
12. Singh K, Rajan KD, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocul Immunol Inflamm. 2010;18:373-378.
13. Hallermann C, Tittelbach J, Norgauer J, et al. Successful treatment of necrobiotic xanthogranuloma with intravenous immunoglobulin. Arch Dermatol. 2009;146:957-960.
14. Goede JS, Misselwitz B, Taverna C, et al. Necrobiotic xanthogranuloma successfully treated with autologous stem cell transplantation. Ann Hematol. 2007;86:303-306.
15. Silapunt S, Chon SY. Generalized necrobiotic xanthogranuloma successfully treated with lenalidomide. J Drugs Dermatol. 2010;9:273-276.
16. Saeki H, Tomita M, Kai H, et al. Necrobiotic xanthogranuloma with paraproteinemia successfully treated with melphalan, prednisolone and skin graft. J Dermatol. 2007;34:795-797.
17. Ho VH, Chevez-Barrios P, Jorgensen JL, et al. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007;70:105-109.
18. Al-Niaimi FA, Dawn G, Cox NH. Necrobiotic xanthogranuloma without paraproteinemia: marked improvement with psoralen ultraviolet A treatment. Clin Exp Dermatol. 2010;35:275-277.
19. Bullock JD, Bartley GB, Campbell RJ, et al. Necrobiotic xanthogranuloma with paraproteinemia: case report and a pathogenetic theory. Trans Am Ophthalmol Soc. 1986;84:342-352.
1. Kossard S, Winkelmann RK. Necrobiotic xanthogranuloma with paraproteinemia. J Am Acad Dermatol. 1980;3:257-270.
2. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma [published correction in Arch Dermatol. 1992;128:632]. Arch Dermatol. 1992;128:94-100.
3. Ugurlu S, Bartley GB, Gibson LE. Necrobiotic xanthogranuloma: long-term outcome of ocular and systemic involvement. Am J Ophthalmol. 2000;129:651-657.
4. Winkelmann RK, Litzow MR, Umbert IJ, et al. Giant cell granulomatous pulmonary and myocardial lesions in necrobiotic xanthogranuloma with paraproteinemia. Mayo Clin Proc. 1997;72:1028-1033.
5. Shah KC, Poonnoose SI, George R, et al. Necrobiotic xanthogranuloma with cutaneous and cerebral manifestations. case report and review of literature. J Neurosurg. 2004;100:1111-1114.
6. Umbert I, Winkelmann RK. Necrobiotic xanthogranuloma with cardiac involvement. Br J Dermatol. 1995;133:438-443.
7. Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
8. Finan MC, Winkelmann RK. Histopathology of necrobiotic xanthogranuloma with paraproteinemia. J Cutan Pathol. 1987;14:92-99.
9. Reddy VC, Salomão DR, Garrity JA, et al. Periorbital and ocular necrobiotic xanthogranuloma leading to perforation. Arch Ophthalmol. 2010;128:1493-1494.
10. Chang SE, Lee WS, Lee MW, et al. A case of necrobiotic xanthogranuloma without paraproteinemia presenting as a solitary tumor on the thigh. Int J Dermatol. 2003;42:470-472.
11. Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284.
12. Singh K, Rajan KD, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocul Immunol Inflamm. 2010;18:373-378.
13. Hallermann C, Tittelbach J, Norgauer J, et al. Successful treatment of necrobiotic xanthogranuloma with intravenous immunoglobulin. Arch Dermatol. 2009;146:957-960.
14. Goede JS, Misselwitz B, Taverna C, et al. Necrobiotic xanthogranuloma successfully treated with autologous stem cell transplantation. Ann Hematol. 2007;86:303-306.
15. Silapunt S, Chon SY. Generalized necrobiotic xanthogranuloma successfully treated with lenalidomide. J Drugs Dermatol. 2010;9:273-276.
16. Saeki H, Tomita M, Kai H, et al. Necrobiotic xanthogranuloma with paraproteinemia successfully treated with melphalan, prednisolone and skin graft. J Dermatol. 2007;34:795-797.
17. Ho VH, Chevez-Barrios P, Jorgensen JL, et al. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007;70:105-109.
18. Al-Niaimi FA, Dawn G, Cox NH. Necrobiotic xanthogranuloma without paraproteinemia: marked improvement with psoralen ultraviolet A treatment. Clin Exp Dermatol. 2010;35:275-277.
19. Bullock JD, Bartley GB, Campbell RJ, et al. Necrobiotic xanthogranuloma with paraproteinemia: case report and a pathogenetic theory. Trans Am Ophthalmol Soc. 1986;84:342-352.
Practice Points
- Necrobiotic xanthogranuloma is a rare histiocytic disease that is strongly associated with monoclonal gammopathy.
- Due to the rarity and uncertain etiology, there are no definitive first-line therapies.