User login
Tranexamic Acid Reduces Perioperative Blood Loss and Hemarthrosis in Total Ankle Arthroplasty
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
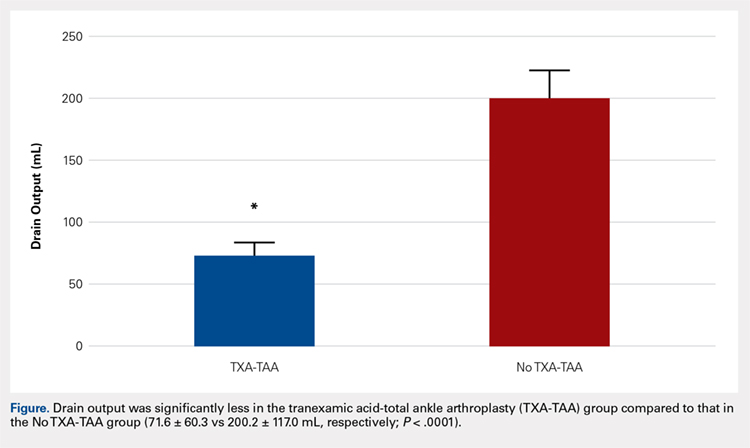
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).
RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
TAKE-HOME POINTS
- TXA is an inexpensive and effective hemostatic agent used during TAA.
- The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of bone during TAA are not as extensive when compared to TKA and THA, but the intra-articular volume is smaller and surrounding soft tissues may be less yielding when blood accumulation occurs.
- If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management during TAA.
- TXA decreases postoperative hemarthrosis and helps to reduce the risk of postoperative wound complications.
- The administration of TXA in the appropriate patient has the potential to decrease hospital cost by controlling postoperative pain and swelling allowing for earlier discharge.