User login
Supplements Are Not a Synonym for Safe: Suspected Liver Injury From Ashwagandha
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
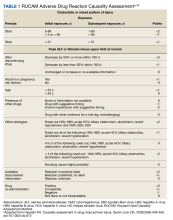
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Difluoroethane Inhalant Abuse, Skeletal Fluorosis, and Withdrawal
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
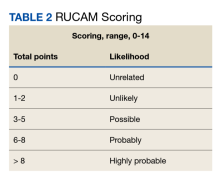
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
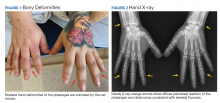
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Hospitalist‐Run Preoperative Clinic
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
Copyright © 2012 Society of Hospital Medicine