User login
Pediatric Conditions Requiring Minimal Intervention or Observation After Interfacility Transfer
Regionalization of pediatric acute care is increasing across the United States, with rates of interfacility transfer for general medical conditions in children similar to those of high-risk conditions in adults.1 The inability for children to receive definitive care (ie, care provided to conclusively manage a patient’s condition without requiring an interfacility transfer) within their local community has implications on public health as well as family function and financial burden.1,2 Previous studies demonstrated that 30% to 80% of interfacility transfers are potentially unnecessary,3-6 as indicated by a high proportion of short lengths of stay after transfer.
To highlight conditions that referring hospitals may prioritize for pediatric capacity building, we aimed to identify the most common medical diagnoses among pediatric transfer patients that did not require advanced evaluation or intervention and that had high rates of discharge within 1 day of interfacility transfer.
METHODS
We conducted a retrospective, cross-sectional, descriptive study using the Pediatric Health Information System (PHIS) database, which contains administrative data from 48 geographically diverse US children’s hospitals.
We included children <18 years old who were transferred to a participating PHIS hospital in 2019, including emergency department (ED), observation, and inpatient encounters. We identified patients through the source-of-admission code labeled as “transfer.”
For each diagnosis, we determined the number of transfers and frequency of rapid discharge, defined as either discharge from the ED without admission or admission and discharge within 1 day from a general inpatient unit. As discharge times are not reliably available in PHIS, all patients discharged on the day of transfer or the following calendar day were identified as rapid discharge. Medical complexity was determined through applying the Pediatric Medical Complexity Algorithm (PMCA).8
For descriptive statistics, we calculated means for normally distributed variables, medians for continuous variables with nonnormal distributions, and percentages for binary variables. Comparisons were made using t-tests and chi-square tests.
This study was approved by the Seattle Children’s Institutional Review Board.
RESULTS
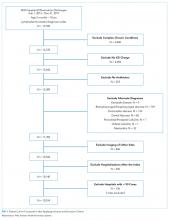
We identified 286,905 transfers into participating PHIS hospitals in 2019. Of these, 89,519 (31.2%) were excluded (Appendix Table 2), leaving 197,386 (68.6%) transfers. Patients discharged within 1 day were more likely to have public or unknown insurance (65.1% vs 61.5%, P < 0.01), to have no co-occurring chronic conditions (60.2% vs 28.5%, P < 0.01), and to reside within the Northeast (35.0% vs 11.0%, P < 0.01) (Appendix Table 3).
The most common medical diagnoses among these transfers included acute bronchiolitis (4.3% of all interfacility transfers, n = 8,425), chemotherapy (4.0%, n = 7,819), and asthma (3.3%, n = 6,430) (Appendix Table 4); 45.9% of bronchiolitis, 15.0% of chemotherapy, and 67.4% of asthma transfers were rapidly discharged.
The Table shows the medical conditions among transfers that most frequently experienced rapid discharge (primary surgical diagnoses are presented in Appendix Table 5). 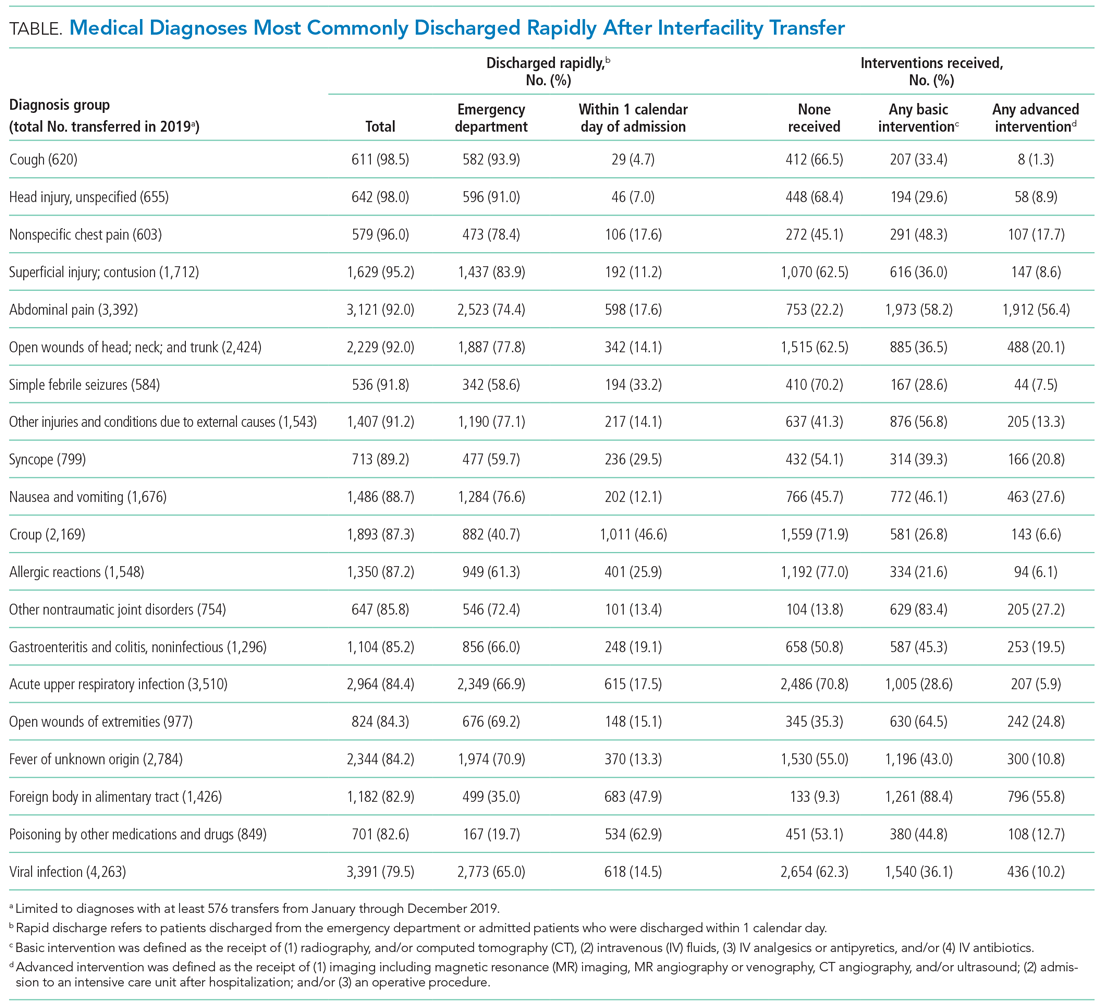
DISCUSSION
We have identified medical conditions that not only had high rates of rapid discharge after transfer, but also received minimal intervention from the accepting institution. Although bronchiolitis and chemotherapy were the most common conditions for which patients were transferred, the range of severity varied widely, with more than 50% of bronchiolitis and 85% of chemotherapy transfers requiring hospitalization for longer than 1 day
Identifying conditions as potential targets to reduce the number of interfacility transfers requires balancing a hospital’s capacity (or lack thereof) for pediatric admissions, perceived risk of decompensation, referring provider discomfort, and parental preference.9-11
The rapid upscale of telehealth may provide a unique opportunity to support the provision of pediatric care within local communities.12,13
Building infrastructure to prevent interfacility transfers may improve healthcare access for children in rural areas proportionately more than children in urban areas. Children in rural communities experience significantly higher rates of interfacility transfers than children in urban areas.14 This increases financial burden and causes additional distress and inconvenience for families.15 With constraints in staffing capacity, equipment, and finances, identifying a subset of medical conditions is a critical initial step to inform the design of targeted interventions to support pediatric healthcare delivery in local communities and avoid costly transfers, although it is not the wholesale solution. Additional utilization of tools such as informed shared decision-making resources and implementation of pediatric-specific protocols likely represent additional necessary steps.
Our study has several limitations. Because we used administrative data, there is a risk of misclassifying diagnoses. We attempted to mitigate this by using a standard ICD-10-based, pediatric-specific grouper.
CONCLUSION
Our exploration of pediatric interfacility transfers that experienced rapid discharge with minimal intervention provides a building block to support the provision of definitive pediatric care in non-pediatric hospitals and represents a step towards addressing limited access to care in general hospitals.
1. França UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096
2. Mumford V, Baysari MT, Kalinin D, et al. Measuring the financial and productivity burden of paediatric hospitalisation on the wider family network. J Paediatr Child Health. 2018;54(9):987-996. https://doi.org/10.1111/jpc.13923
3. Richard KR, Glisson KL, Shah N, et al. Predictors of potentially unnecessary transfers to pediatric emergency departments. Hosp Pediatr. 2020;10(5):424-429. https://doi.org/10.1542/hpeds.2019-0307
4. Gattu RK, Teshome G, Cai L, Wright C, Lichenstein R. Interhospital pediatric patient transfers-factors influencing rapid disposition after transfer. Pediatr Emerg Care. 2014;30(1):26-30. https://doi.org/10.1097/PEC.0000000000000061
5. Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130(1):83-92. https://doi.org/10.1542/peds.2011-1819
6. Rosenthal JL, Lieng MK, Marcin JP, Romano PS. Profiling pediatric potentially avoidable transfers using procedure and diagnosis codes. Pediatr Emerg Care. 2019 Mar 19;10.1097/PEC.0000000000001777. https://doi.org/10.1097/PEC.0000000000001777
7. Pediatric clinical classification system (PECCS) codes. Children’s Hospital Association. December 11, 2020. Accessed June 3, 2021. https://www.childrenshospitals.org/Research-and-Data/Pediatric-Data-and-Trends/2020/Pediatric-Clinical-Classification-System-PECCS
8. Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and validation of the pediatric medical complexity algorithm (PMCA) version 3.0. Acad Pediatr. 2018;18(5):577-580. https://doi.org/10.1016/j.acap.2018.02.010
9. Rosenthal JL, Okumura MJ, Hernandez L, Li ST, Rehm RS. Interfacility transfers to general pediatric floors: a qualitative study exploring the role of communication. Acad Pediatr. 2016;16(7):692-699. https://doi.org/10.1016/j.acap.2016.04.003
10. Rosenthal JL, Li ST, Hernandez L, Alvarez M, Rehm RS, Okumura MJ. Familial caregiver and physician perceptions of the family-physician interactions during interfacility transfers. Hosp Pediatr. 2017;7(6):344-351. https://doi.org/10.1542/hpeds.2017-0017
11. Peebles ER, Miller MR, Lynch TP, Tijssen JA. Factors associated with discharge home after transfer to a pediatric emergency department. Pediatr Emerg Care. 2018;34(9):650-655. https://doi.org/10.1097/PEC.0000000000001098
12. Labarbera JM, Ellenby MS, Bouressa P, Burrell J, Flori HR, Marcin JP. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303
13. Haynes SC, Dharmar M, Hill BC, et al. The impact of telemedicine on transfer rates of newborns at rural community hospitals. Acad Pediatr. 2020;20(5):636-641. https://doi.org/10.1016/j.acap.2020.02.013
14. Michelson KA, Hudgins JD, Lyons TW, Monuteaux MC, Bachur RG, Finkelstein JA. Trends in capability of hospitals to provide definitive acute care for children: 2008 to 2016. Pediatrics. 2020;145(1). https://doi.org/10.1542/peds.2019-2203
15. Mohr NM, Harland KK, Shane DM, Miller SL, Torner JC. Potentially avoidable pediatric interfacility transfer is a costly burden for rural families: a cohort study. Acad Emerg Med. 2016;23(8):885-894. https://doi.org/10.1111/acem.12972
Regionalization of pediatric acute care is increasing across the United States, with rates of interfacility transfer for general medical conditions in children similar to those of high-risk conditions in adults.1 The inability for children to receive definitive care (ie, care provided to conclusively manage a patient’s condition without requiring an interfacility transfer) within their local community has implications on public health as well as family function and financial burden.1,2 Previous studies demonstrated that 30% to 80% of interfacility transfers are potentially unnecessary,3-6 as indicated by a high proportion of short lengths of stay after transfer.
To highlight conditions that referring hospitals may prioritize for pediatric capacity building, we aimed to identify the most common medical diagnoses among pediatric transfer patients that did not require advanced evaluation or intervention and that had high rates of discharge within 1 day of interfacility transfer.
METHODS
We conducted a retrospective, cross-sectional, descriptive study using the Pediatric Health Information System (PHIS) database, which contains administrative data from 48 geographically diverse US children’s hospitals.
We included children <18 years old who were transferred to a participating PHIS hospital in 2019, including emergency department (ED), observation, and inpatient encounters. We identified patients through the source-of-admission code labeled as “transfer.”
For each diagnosis, we determined the number of transfers and frequency of rapid discharge, defined as either discharge from the ED without admission or admission and discharge within 1 day from a general inpatient unit. As discharge times are not reliably available in PHIS, all patients discharged on the day of transfer or the following calendar day were identified as rapid discharge. Medical complexity was determined through applying the Pediatric Medical Complexity Algorithm (PMCA).8
For descriptive statistics, we calculated means for normally distributed variables, medians for continuous variables with nonnormal distributions, and percentages for binary variables. Comparisons were made using t-tests and chi-square tests.
This study was approved by the Seattle Children’s Institutional Review Board.
RESULTS
We identified 286,905 transfers into participating PHIS hospitals in 2019. Of these, 89,519 (31.2%) were excluded (Appendix Table 2), leaving 197,386 (68.6%) transfers. Patients discharged within 1 day were more likely to have public or unknown insurance (65.1% vs 61.5%, P < 0.01), to have no co-occurring chronic conditions (60.2% vs 28.5%, P < 0.01), and to reside within the Northeast (35.0% vs 11.0%, P < 0.01) (Appendix Table 3).
The most common medical diagnoses among these transfers included acute bronchiolitis (4.3% of all interfacility transfers, n = 8,425), chemotherapy (4.0%, n = 7,819), and asthma (3.3%, n = 6,430) (Appendix Table 4); 45.9% of bronchiolitis, 15.0% of chemotherapy, and 67.4% of asthma transfers were rapidly discharged.
The Table shows the medical conditions among transfers that most frequently experienced rapid discharge (primary surgical diagnoses are presented in Appendix Table 5). 
DISCUSSION
We have identified medical conditions that not only had high rates of rapid discharge after transfer, but also received minimal intervention from the accepting institution. Although bronchiolitis and chemotherapy were the most common conditions for which patients were transferred, the range of severity varied widely, with more than 50% of bronchiolitis and 85% of chemotherapy transfers requiring hospitalization for longer than 1 day
Identifying conditions as potential targets to reduce the number of interfacility transfers requires balancing a hospital’s capacity (or lack thereof) for pediatric admissions, perceived risk of decompensation, referring provider discomfort, and parental preference.9-11
The rapid upscale of telehealth may provide a unique opportunity to support the provision of pediatric care within local communities.12,13
Building infrastructure to prevent interfacility transfers may improve healthcare access for children in rural areas proportionately more than children in urban areas. Children in rural communities experience significantly higher rates of interfacility transfers than children in urban areas.14 This increases financial burden and causes additional distress and inconvenience for families.15 With constraints in staffing capacity, equipment, and finances, identifying a subset of medical conditions is a critical initial step to inform the design of targeted interventions to support pediatric healthcare delivery in local communities and avoid costly transfers, although it is not the wholesale solution. Additional utilization of tools such as informed shared decision-making resources and implementation of pediatric-specific protocols likely represent additional necessary steps.
Our study has several limitations. Because we used administrative data, there is a risk of misclassifying diagnoses. We attempted to mitigate this by using a standard ICD-10-based, pediatric-specific grouper.
CONCLUSION
Our exploration of pediatric interfacility transfers that experienced rapid discharge with minimal intervention provides a building block to support the provision of definitive pediatric care in non-pediatric hospitals and represents a step towards addressing limited access to care in general hospitals.
Regionalization of pediatric acute care is increasing across the United States, with rates of interfacility transfer for general medical conditions in children similar to those of high-risk conditions in adults.1 The inability for children to receive definitive care (ie, care provided to conclusively manage a patient’s condition without requiring an interfacility transfer) within their local community has implications on public health as well as family function and financial burden.1,2 Previous studies demonstrated that 30% to 80% of interfacility transfers are potentially unnecessary,3-6 as indicated by a high proportion of short lengths of stay after transfer.
To highlight conditions that referring hospitals may prioritize for pediatric capacity building, we aimed to identify the most common medical diagnoses among pediatric transfer patients that did not require advanced evaluation or intervention and that had high rates of discharge within 1 day of interfacility transfer.
METHODS
We conducted a retrospective, cross-sectional, descriptive study using the Pediatric Health Information System (PHIS) database, which contains administrative data from 48 geographically diverse US children’s hospitals.
We included children <18 years old who were transferred to a participating PHIS hospital in 2019, including emergency department (ED), observation, and inpatient encounters. We identified patients through the source-of-admission code labeled as “transfer.”
For each diagnosis, we determined the number of transfers and frequency of rapid discharge, defined as either discharge from the ED without admission or admission and discharge within 1 day from a general inpatient unit. As discharge times are not reliably available in PHIS, all patients discharged on the day of transfer or the following calendar day were identified as rapid discharge. Medical complexity was determined through applying the Pediatric Medical Complexity Algorithm (PMCA).8
For descriptive statistics, we calculated means for normally distributed variables, medians for continuous variables with nonnormal distributions, and percentages for binary variables. Comparisons were made using t-tests and chi-square tests.
This study was approved by the Seattle Children’s Institutional Review Board.
RESULTS
We identified 286,905 transfers into participating PHIS hospitals in 2019. Of these, 89,519 (31.2%) were excluded (Appendix Table 2), leaving 197,386 (68.6%) transfers. Patients discharged within 1 day were more likely to have public or unknown insurance (65.1% vs 61.5%, P < 0.01), to have no co-occurring chronic conditions (60.2% vs 28.5%, P < 0.01), and to reside within the Northeast (35.0% vs 11.0%, P < 0.01) (Appendix Table 3).
The most common medical diagnoses among these transfers included acute bronchiolitis (4.3% of all interfacility transfers, n = 8,425), chemotherapy (4.0%, n = 7,819), and asthma (3.3%, n = 6,430) (Appendix Table 4); 45.9% of bronchiolitis, 15.0% of chemotherapy, and 67.4% of asthma transfers were rapidly discharged.
The Table shows the medical conditions among transfers that most frequently experienced rapid discharge (primary surgical diagnoses are presented in Appendix Table 5). 
DISCUSSION
We have identified medical conditions that not only had high rates of rapid discharge after transfer, but also received minimal intervention from the accepting institution. Although bronchiolitis and chemotherapy were the most common conditions for which patients were transferred, the range of severity varied widely, with more than 50% of bronchiolitis and 85% of chemotherapy transfers requiring hospitalization for longer than 1 day
Identifying conditions as potential targets to reduce the number of interfacility transfers requires balancing a hospital’s capacity (or lack thereof) for pediatric admissions, perceived risk of decompensation, referring provider discomfort, and parental preference.9-11
The rapid upscale of telehealth may provide a unique opportunity to support the provision of pediatric care within local communities.12,13
Building infrastructure to prevent interfacility transfers may improve healthcare access for children in rural areas proportionately more than children in urban areas. Children in rural communities experience significantly higher rates of interfacility transfers than children in urban areas.14 This increases financial burden and causes additional distress and inconvenience for families.15 With constraints in staffing capacity, equipment, and finances, identifying a subset of medical conditions is a critical initial step to inform the design of targeted interventions to support pediatric healthcare delivery in local communities and avoid costly transfers, although it is not the wholesale solution. Additional utilization of tools such as informed shared decision-making resources and implementation of pediatric-specific protocols likely represent additional necessary steps.
Our study has several limitations. Because we used administrative data, there is a risk of misclassifying diagnoses. We attempted to mitigate this by using a standard ICD-10-based, pediatric-specific grouper.
CONCLUSION
Our exploration of pediatric interfacility transfers that experienced rapid discharge with minimal intervention provides a building block to support the provision of definitive pediatric care in non-pediatric hospitals and represents a step towards addressing limited access to care in general hospitals.
1. França UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096
2. Mumford V, Baysari MT, Kalinin D, et al. Measuring the financial and productivity burden of paediatric hospitalisation on the wider family network. J Paediatr Child Health. 2018;54(9):987-996. https://doi.org/10.1111/jpc.13923
3. Richard KR, Glisson KL, Shah N, et al. Predictors of potentially unnecessary transfers to pediatric emergency departments. Hosp Pediatr. 2020;10(5):424-429. https://doi.org/10.1542/hpeds.2019-0307
4. Gattu RK, Teshome G, Cai L, Wright C, Lichenstein R. Interhospital pediatric patient transfers-factors influencing rapid disposition after transfer. Pediatr Emerg Care. 2014;30(1):26-30. https://doi.org/10.1097/PEC.0000000000000061
5. Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130(1):83-92. https://doi.org/10.1542/peds.2011-1819
6. Rosenthal JL, Lieng MK, Marcin JP, Romano PS. Profiling pediatric potentially avoidable transfers using procedure and diagnosis codes. Pediatr Emerg Care. 2019 Mar 19;10.1097/PEC.0000000000001777. https://doi.org/10.1097/PEC.0000000000001777
7. Pediatric clinical classification system (PECCS) codes. Children’s Hospital Association. December 11, 2020. Accessed June 3, 2021. https://www.childrenshospitals.org/Research-and-Data/Pediatric-Data-and-Trends/2020/Pediatric-Clinical-Classification-System-PECCS
8. Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and validation of the pediatric medical complexity algorithm (PMCA) version 3.0. Acad Pediatr. 2018;18(5):577-580. https://doi.org/10.1016/j.acap.2018.02.010
9. Rosenthal JL, Okumura MJ, Hernandez L, Li ST, Rehm RS. Interfacility transfers to general pediatric floors: a qualitative study exploring the role of communication. Acad Pediatr. 2016;16(7):692-699. https://doi.org/10.1016/j.acap.2016.04.003
10. Rosenthal JL, Li ST, Hernandez L, Alvarez M, Rehm RS, Okumura MJ. Familial caregiver and physician perceptions of the family-physician interactions during interfacility transfers. Hosp Pediatr. 2017;7(6):344-351. https://doi.org/10.1542/hpeds.2017-0017
11. Peebles ER, Miller MR, Lynch TP, Tijssen JA. Factors associated with discharge home after transfer to a pediatric emergency department. Pediatr Emerg Care. 2018;34(9):650-655. https://doi.org/10.1097/PEC.0000000000001098
12. Labarbera JM, Ellenby MS, Bouressa P, Burrell J, Flori HR, Marcin JP. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303
13. Haynes SC, Dharmar M, Hill BC, et al. The impact of telemedicine on transfer rates of newborns at rural community hospitals. Acad Pediatr. 2020;20(5):636-641. https://doi.org/10.1016/j.acap.2020.02.013
14. Michelson KA, Hudgins JD, Lyons TW, Monuteaux MC, Bachur RG, Finkelstein JA. Trends in capability of hospitals to provide definitive acute care for children: 2008 to 2016. Pediatrics. 2020;145(1). https://doi.org/10.1542/peds.2019-2203
15. Mohr NM, Harland KK, Shane DM, Miller SL, Torner JC. Potentially avoidable pediatric interfacility transfer is a costly burden for rural families: a cohort study. Acad Emerg Med. 2016;23(8):885-894. https://doi.org/10.1111/acem.12972
1. França UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096
2. Mumford V, Baysari MT, Kalinin D, et al. Measuring the financial and productivity burden of paediatric hospitalisation on the wider family network. J Paediatr Child Health. 2018;54(9):987-996. https://doi.org/10.1111/jpc.13923
3. Richard KR, Glisson KL, Shah N, et al. Predictors of potentially unnecessary transfers to pediatric emergency departments. Hosp Pediatr. 2020;10(5):424-429. https://doi.org/10.1542/hpeds.2019-0307
4. Gattu RK, Teshome G, Cai L, Wright C, Lichenstein R. Interhospital pediatric patient transfers-factors influencing rapid disposition after transfer. Pediatr Emerg Care. 2014;30(1):26-30. https://doi.org/10.1097/PEC.0000000000000061
5. Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130(1):83-92. https://doi.org/10.1542/peds.2011-1819
6. Rosenthal JL, Lieng MK, Marcin JP, Romano PS. Profiling pediatric potentially avoidable transfers using procedure and diagnosis codes. Pediatr Emerg Care. 2019 Mar 19;10.1097/PEC.0000000000001777. https://doi.org/10.1097/PEC.0000000000001777
7. Pediatric clinical classification system (PECCS) codes. Children’s Hospital Association. December 11, 2020. Accessed June 3, 2021. https://www.childrenshospitals.org/Research-and-Data/Pediatric-Data-and-Trends/2020/Pediatric-Clinical-Classification-System-PECCS
8. Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and validation of the pediatric medical complexity algorithm (PMCA) version 3.0. Acad Pediatr. 2018;18(5):577-580. https://doi.org/10.1016/j.acap.2018.02.010
9. Rosenthal JL, Okumura MJ, Hernandez L, Li ST, Rehm RS. Interfacility transfers to general pediatric floors: a qualitative study exploring the role of communication. Acad Pediatr. 2016;16(7):692-699. https://doi.org/10.1016/j.acap.2016.04.003
10. Rosenthal JL, Li ST, Hernandez L, Alvarez M, Rehm RS, Okumura MJ. Familial caregiver and physician perceptions of the family-physician interactions during interfacility transfers. Hosp Pediatr. 2017;7(6):344-351. https://doi.org/10.1542/hpeds.2017-0017
11. Peebles ER, Miller MR, Lynch TP, Tijssen JA. Factors associated with discharge home after transfer to a pediatric emergency department. Pediatr Emerg Care. 2018;34(9):650-655. https://doi.org/10.1097/PEC.0000000000001098
12. Labarbera JM, Ellenby MS, Bouressa P, Burrell J, Flori HR, Marcin JP. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303
13. Haynes SC, Dharmar M, Hill BC, et al. The impact of telemedicine on transfer rates of newborns at rural community hospitals. Acad Pediatr. 2020;20(5):636-641. https://doi.org/10.1016/j.acap.2020.02.013
14. Michelson KA, Hudgins JD, Lyons TW, Monuteaux MC, Bachur RG, Finkelstein JA. Trends in capability of hospitals to provide definitive acute care for children: 2008 to 2016. Pediatrics. 2020;145(1). https://doi.org/10.1542/peds.2019-2203
15. Mohr NM, Harland KK, Shane DM, Miller SL, Torner JC. Potentially avoidable pediatric interfacility transfer is a costly burden for rural families: a cohort study. Acad Emerg Med. 2016;23(8):885-894. https://doi.org/10.1111/acem.12972
© 2021 Society of Hospital Medicine
Decreasing Hospital Observation Time for Febrile Infants
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
© 2021 Society of Hospital Medicine
Imaging Strategies and Outcomes in Children Hospitalized with Cervical Lymphadenitis
Cervical lymphadenitis is a common superficial neck infection in childhood. While most children with cervical lymphadenitis recover with antibiotic therapy, a subset can develop an abscess that may require surgical drainage. Radiologic imaging, most commonly ultrasound or computed tomography (CT), is often performed to identify such an abscess.1-3 However, no national standards exist to guide clinician decision making around imaging in this population. In the absence of evidence-based guidelines, variability in frequency, timing, and modality of imaging likely exists in children hospitalized with cervical lymphadenitis.
As demonstrated for several other common pediatric conditions,4,5 variability in imaging practices may contribute to overutilization of resources in children with cervical lymphadenitis. In particular, routinely conducting imaging on presentation may constitute overuse, as children with cervical lymphadenitis who present with less than 72 hours of neck swelling rarely undergo surgical drainage within the first 24 hours of hospitalization.1,6,7 Imaging performed on presentation is often repeated later during hospitalization, particularly if the patient has not improved with antibiotic therapy. The net result may be unnecessary, redundant radiologic studies. Furthermore, serious complications such as bacteremia, extension of infection into the retropharyngeal space, or involvement of the airway or vasculature rarely occur in children with cervical lymphadenitis.6,8 In this context, deferring initial imaging in this population is unlikely to lead to adverse outcomes and may reduce radiation exposure.
The overall objectives of this study are to describe hospital-level variation in imaging practices for pediatric cervical lymphadenitis and to examine the association between early imaging and outcomes in this population.
METHODS
Study Design and Data Source
We conducted a multicenter, cross-sectional study using the Pediatric Health Information Systems (PHIS) database, which contains administrative and billing data from 49 geographically diverse children’s hospitals across the United States (US) affiliated with the Children’s Hospital Association (Lenexa, Kansas). PHIS includes data on patient demographics, discharge diagnoses, and procedures using the International Classification of Diseases, 9th (ICD-9) and 10th Revision (ICD-10) diagnosis codes, as well as daily billed resource utilization for laboratory tests, imaging studies, and medications. Encrypted medical record numbers permit longitudinal identification of children across multiple visits to the same hospital. Use of de-identified PHIS data was deemed to be nonhuman subjects research; our approach to validation of ICD codes using local electronic medical record review was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Study Population
Our study team developed an algorithm to identify children with cervical lymphadenitis and minimize misclassification using PHIS (Appendix A). All children with lymphadenitis-related ICD-9 and ICD-10 discharge diagnosis codes were eligible for inclusion.
This algorithm was subsequently applied to the PHIS database. Children ages two months to 18 years hospitalized at participating PHIS institutions between July 2013 and December 2017 with a diagnosis of cervical lymphadenitis as per th
Measures of Interest
To examine hospital-level variation in imaging practices, we measured the proportion of children at each hospital who underwent any neck imaging study, CT or ultrasound imaging, early imaging, and multiple imaging studies within a single hospitalization. Neck imaging was defined as the presence of a billing code for ultrasound, CT, or magnetic resonance imaging (MRI) study of the neck (Appendix B). Early imaging was defined as neck imaging conducted on day 0 of hospitalization (ie, calendar day of admission and ending at midnight). Multiple imaging studies were defined as the receipt of more than one imaging study, regardless of timing or modality. We also measured the proportion of children by hospital who received surgical drainage, defined by the presence of procedure codes for incision and drainage of abscess of the neck (Appendix B).
In examining patient-level association between early imaging and clinical outcomes, our primary outcome of interest was the receipt of multiple imaging studies. Secondary outcomes included rates of surgical drainage, length of stay (in hospital days), and rates of lymphadenitis-related hospital readmission within 30 days of index discharge.
Covariates
Baseline demographic characteristics included age, gender, race/ethnicity, and insurance type. We measured ED visits associated with lymphadenitis-related diagnosis codes in the 30 days prior to admission as a proxy measure for illness duration prior to presentation. To approximate illness severity, we included the following covariates: rates of intensive care unit admission on presentation, rates of receipt of intravenous (IV) analgesia (Appendix B) on hospital days prior to surgical drainage, and rates of receipt of broad-spectrum antibiotics on day 0 or 1 of hospitalization. Broad-spectrum antibiotics (Appendix B) were defined by an independent three-person review of available antibiotic codes (SD, SSS, and JT); differences were resolved by group consensus.
Analysis
Categorical variables were described using frequencies and percentages, while continuous data were described using median and interquartile range. We described hospital-level variation in imaging practices by calculating and comparing the proportion of children at each hospital who underwent any neck imaging study, CT imaging, ultrasound imaging, early imaging, multiple imaging studies, and surgical drainage.
Patient-level demographics and clinical characteristics were compared across groups using chi-square test. To examine the association between early imaging and outcomes, we used generalized linear or logistic mixed effects models to control for patient demographic characteristics and clinical markers of illness duration and severity, with a random effect for hospital to account for clustering. Patient demographics in the model defined a priori included age, race/ethnicity, and insurance type; clinical characteristics included prior ED visit for lymphadenitis, initial intensive care unit (ICU) admission, use of IV analgesia, and use of broad-spectrum antibiotics on day 0 or 1 of hospitalization. To assess the potential for misclassification related to the availability of calendar day but not time of imaging in PHIS, we conducted a secondary analysis to examine the patient-level association between early imaging and outcomes using an alternative definition for early imaging (defined as imaging conducted on day 0 or day 1 of hospitalization).
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, North Carolina); P < .05 was considered statistically significant.
RESULTS
We identified 19,785 PHIS hospitalizations with lymphadenitis-related discharge diagnosis codes between July 1, 2013 and December 31, 2017. Applying our algorithm and exclusion criteria, we assembled a cohort of 10,014 children hospitalized with cervical lymphadenitis (Figure 1). Two-thirds of the children in our cohort were <4 years old, 42% were non-Hispanic white, and 63% had a government payor (Table 1). Neck imaging (ultrasound, CT, or MRI) was conducted in 8,103 (81%) children. CT imaging was performed in 4,097 (41%) of children, and early imaging was conducted in 6,111 (61%) of children with cervical lymphadenitis.
We noted hospital-level variation in rates of any neck imaging (median: 82.1%, interquartile range [IQR]: 77.7%-85.5%, full range: 68.7%-93.1%), CT imaging (median: 42.3%, IQR: 26.7%-55.2%, full range: 12.0%-81.5%), early imaging (median: 64.4%, IQR: 59.8%-68.4%, full range: 13.8%-76.9%), and multiple imaging studies (median: 23.7%, IQR: 18.6%-28.9%, full range: 1.2%-40.7%; Figure 2). Rates of surgical drainage also varied by hospital (median: 35.1%, IQR: 31.3%-42.0%, full range: 17.1%-54.5%).
At the patient level, children who received early imaging were more likely to be <1 year old (21% vs 16%, P < .001), or Hispanic or Black when compared with children who did not receive early imaging (Table 1). Children who received early imaging were more likely to have had an ED visit for lymphadenitis in the preceding 30 days (8% vs 6%, P = .001). However, they were less likely to have received broad-spectrum antibiotics on admission (6% vs 8%, P < .001; Table 1). Of the 6,111 patients who received early imaging, 2,538 (41.5%) received CT imaging and 3,902 (63.9%) received ultrasound imaging on day 0. Of the 2,272 patients receiving multiple imaging studies, 116 (5.1%) received two or more CT scans.
In multivariable analysis at the patient level, early imaging was associated with higher adjusted odds of receiving multiple imaging studies (adjusted odds ratio [aOR] 3.0, 95% CI: 2.6-3.6). Similarly, early imaging was associated with higher adjusted odds of surgical drainage (aOR: 1.3, 95% CI: 1.1-1.4), increased 30-day readmission for lymphadenitis (aOR: 1.5, 95% CI: 1.2-1.9), and longer length of stay (adjusted rate ratio: 1.2, 95% CI: 1.1-1.2; Table 2). For the subset of patients who did not receive surgical drainage during the index admission, the adjusted odds ratio for the association between early imaging at index admission and 30-day readmission was 1.7 (95% CI: 1.3-2.1). About 63% of readmissions occurred within 7 days of index discharge; 89% occurred within 14 days (Appendix Figure).
In secondary analysis using an alternative definition for early imaging (ie, imaging conducted on day 0 or day 1 of hospitalization), the adjusted odds ratio for multiple imaging studies was 22.6 (95% CI: 15.8-32.4). The adjusted odds and rate ratios for the remaining outcomes were similar to our primary analysis.
DISCUSSION
In this large multicenter study of children with cervical lymphadenitis, we found variation in imaging practices across 44 US children’s hospitals. Children with cervical lymphadenitis who underwent early imaging were more likely to receive multiple imaging studies during a single hospitalization than those who did not receive early imaging. At the patient level, early imaging was also associated with higher rates of surgical drainage, more frequent 30-day readmission, and longer lengths of stay.
To our knowledge, imaging practices in the population of children hospitalized with cervical lymphadenitis have not been previously characterized in the US; one study from Atlanta, Georgia, describes imaging practices in all children evaluated in the ED.1 Single-center studies of children hospitalized with cervical lymphadenitis have been previously conducted in Canada6 and New Zealand,8 in which 42%-51% of children received imaging. In our study, most (81%) children hospitalized with lymphadenitis received some form of imaging, with 61% of all children receiving early imaging. Furthermore, 41% received CT imaging, as compared with 8%-10% of children in the aforementioned studies from Canada and New Zealand.6,8 This finding is consistent with a pattern of imaging overuse in the US, which has amongst the highest utilization rates globally for advanced imaging such as CT and MRI.10,11 Identifying opportunities to safely reduce routine imaging, particularly CT imaging, in this population could decrease unnecessary radiation exposure without compromising outcomes.
We also noted variability in imaging practices across PHIS hospitals. Some of this variability may be partially explained by differences in the patient population or illness severity across hospitals. However, given the absence of evidence-based best practices for children with cervical lymphadenitis, clinicians may rely on anecdotal experience or local practice culture to guide their decision making,12 leading to variability in frequency, timing, and modality of imaging.
At the patient level, we found that children who received early imaging were more likely to receive multiple imaging studies. This finding supports our hypothesis that clinicians often order a second imaging study when the initial imaging study does not clearly demonstrate an abscess, and the child subsequently fails to demonstrate clear improvement after 24-48 hours of antibiotics.
Furthermore, early imaging was associated with overall increased utilization in our cohort, including increased likelihood of surgical drainage, 30-day readmission for lymphadenitis, as well as longer lengths of stay. Confounding may be one explanation for this finding. For instance, clinicians may pursue early imaging in children who present with longer duration of symptoms or more severe illness on presentation, as these factors may be associated with abscess formation.1,6,7 These clinical covariates are not available in PHIS. Thus, we used prior ED visits for lymphadenitis to approximate illness duration, and initial admission to ICU, receipt of IV analgesia, and receipt of broad-spectrum antibiotics to approximate illness severity in an attempt to mitigate confounding.
On the other hand, it is also possible that a proportion of children with a small fluid collection on imaging may have improved with antibiotics alone. There is a growing body of evidence in children with other head and neck infections (eg, retropharyngeal abscess and orbital cellulitis with periosteal abscess)13-15 that suggests that children with small abscesses often improve with antibiotic therapy alone. In children with cervical lymphadenitis who have small or developing abscesses identified via routine imaging on presentation, clinicians may be driven to pursue a surgical intervention with uncertain benefit. Deferring routine imaging in this population may provide an opportunity to improve the value of care in children with lymphadenitis without adversely affecting outcomes.
This study has several limitations given our use of an administrative database. Children with lymphadenitis may have been misclassified as these patients were identified using discharge diagnosis codes
Furthermore, we were unable to measure the exact time of imaging study in PHIS; we used imaging conducted on hospital day 0 as a proxy measure for imaging conducted within the first 24 hours of presentation. With this definition, some children who had early imaging were likely misclassified as not having received early imaging. For example, a patient who arrived in the ED at 9
Additionally, there may be a subset of children who underwent imaging prior to presentation at the PHIS hospital ED for further workup and admission. Imaging conducted outside a PHIS hospital was not captured in this database. Similarly, children who had a readmission at a different hospital than their index admission would not be captured using PHIS. Finally, PHIS captures data from children’s hospitals; practices at these hospitals may not be generalizable to practices in the community hospital setting.
CONCLUSION
In conclusion, we found that imaging practices in children hospitalized with cervical lymphadenitis were widely variable across hospitals. Children receiving early imaging had more resource utilization and intervention when compared with children who did not receive early imaging. Our findings may represent a cascade effect, in which routinely conducted early imaging prompts clinicians to pursue more testing and interventions in this population. Future studies should obtain more detailed patient level covariates to further characterize clinical factors that may impact decisions around imaging and clinical outcomes for children with cervical lymphadenitis.
Acknowledgments
The authors would like to acknowledge the following investigators for their contributions to data interpretation and review of the final manuscript: Angela Choe MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Margaret Rush MD, Children’s National Medical Center, Washington, DC; Ryosuke Takei MD, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Wallis Molchen DO, Texas Children’s Hospital, Houston, Texas; Stephanie Royer Moss MD, Cleveland Clinic, Cleveland, Ohio; Rebecca Dang, MD, Lucile Packard Children’s Hospital Stanford, Palo Alto, California; Joy Solano MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas; Nathaniel P. Goodrich MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Ngozi Eboh MD, Texas Tech University Health Sciences Center, Dallas, Texas; Ashley Jenkins MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Rebecca Steuart MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sonya Tang Girdwood MD, PhD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Alissa McInerney MD, Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, New York; Sumeet Banker MD, MPH, New York Presbyterian Morgan Stanley Children’s Hospital, New York, New York; Corrie McDaniel DO, Seattle Children’s Hospital, Seattle, Washington; Christiane Lenzen MD, Rady Children’s Hospital, San Diego, California; Aleisha Nabower MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Waheeda Samady MD, Ann & Robert H. Lurie Children’s Hospital, Chicago, Illinois; Jennifer Chen MD, Rady Children’s Hospital, San Diego, California; Marquita Genies MD, MPH, John’s Hopkins Children’s Center, Baltimore, Maryland; Justin Lockwood MD, Children’s Hospital Colorado, Aurora, Colorado; David Synhorst MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas.
1. Sauer MW, Sharma S, Hirsh DA et al. Acute neck infections in children: who is likely to undergo surgical drainage? Am J Emerg Med. 2013;31(6):906-909. https://doi.org/10.1016/j.ajem.2013.02.043.
2. Sethia R, Mahida JB, Subbarayan RA, et al. Evaluation of an imaging protocol using ultrasound as the primary diagnostic modality in pediatric patients with superficial soft tissue infections of the face and neck. Int J Pediatr Otorhinolaryngol. 2017;96:89-93. https://doi.org/10.1016/j.ijporl.2017.02.027.
3. Neff L, Newland JG, Sykes KJ, Selvarangan R, Wei JL. Microbiology and antimicrobial treatment of pediatric cervical lymphadenitis requiring surgical intervention. Int J Pediatr Otorhinolaryngol. 2013;77(5):817-820. https://doi.org/10.1016/j.ijporl.2013.02.018.
4. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789-796. https://doi.org/10.1016/j.jpeds.2009.01.010.
6. Luu TM, Chevalier I, Gauthier M et al. Acute adenitis in children: clinical course and factors predictive of surgical drainage. J Paediatr Child Health. 2005;41(5-6):273-277. https://doi.org/10.1111/j.1440-1754.2005.00610.x.
7. Golriz F, Bisset GS, 3rd, D’Amico B, et al. A clinical decision rule for the use of ultrasound in children presenting with acute inflammatory neck masses. Pediatr Rad. 2017;47(4):422-428. https://doi.org/10.1007/s00247-016-3774-9.
8. Courtney MJ, Miteff A, Mahadevan M. Management of pediatric lateral neck infections: does the adage “… never let the sun go down on undrained pus …” hold true? Int J Pediatr Otorhinolaryngol. 2007;71(1):95-100. https://doi.org/10.1016/j.ijporl.2006.09.009.
9. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
10. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. https://doi.org/10.1001/jama.2018.1150.
11. Oren O, Kebebew E, Ioannidis JPA. Curbing unnecessary and wasted diagnostic imaging. JAMA. 2019;321(3):245-246. https://doi.org/10.1001/jama.2018.20295.
12. Palmer RH, Miller MR. Methodologic challenges in developing and implementing measures of quality for child health care. Ambul Pediatr Off J Ambul Pediatr Assoc. 2001;1(1):39-52. https://doi.org/10.1367/1539-4409(2001)001<0039:MCIDAI>2.0.CO;2.
13. Daya H, Lo S, Papsin BC, et al. Retropharyngeal and parapharyngeal infections in children: the Toronto experience. Int J Pediatr Otorhinolaryngol. 2005;69(1):81-86. https://doi.org/10.1016/j.ijporl.2004.08.010.
14. Wong SJ, Levi J. Management of pediatric orbital cellulitis: A systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006.
15. Wong DK, Brown C, Mills N, Spielmann P, Neeff M. To drain or not to drain-management of pediatric deep neck abscesses: a case-control study. Int J Pediatr Otorhinolaryngol. 2012;76(12):1810-1813. https://doi.org/10.1016/j.ijporl.2012.09.006.
Cervical lymphadenitis is a common superficial neck infection in childhood. While most children with cervical lymphadenitis recover with antibiotic therapy, a subset can develop an abscess that may require surgical drainage. Radiologic imaging, most commonly ultrasound or computed tomography (CT), is often performed to identify such an abscess.1-3 However, no national standards exist to guide clinician decision making around imaging in this population. In the absence of evidence-based guidelines, variability in frequency, timing, and modality of imaging likely exists in children hospitalized with cervical lymphadenitis.
As demonstrated for several other common pediatric conditions,4,5 variability in imaging practices may contribute to overutilization of resources in children with cervical lymphadenitis. In particular, routinely conducting imaging on presentation may constitute overuse, as children with cervical lymphadenitis who present with less than 72 hours of neck swelling rarely undergo surgical drainage within the first 24 hours of hospitalization.1,6,7 Imaging performed on presentation is often repeated later during hospitalization, particularly if the patient has not improved with antibiotic therapy. The net result may be unnecessary, redundant radiologic studies. Furthermore, serious complications such as bacteremia, extension of infection into the retropharyngeal space, or involvement of the airway or vasculature rarely occur in children with cervical lymphadenitis.6,8 In this context, deferring initial imaging in this population is unlikely to lead to adverse outcomes and may reduce radiation exposure.
The overall objectives of this study are to describe hospital-level variation in imaging practices for pediatric cervical lymphadenitis and to examine the association between early imaging and outcomes in this population.
METHODS
Study Design and Data Source
We conducted a multicenter, cross-sectional study using the Pediatric Health Information Systems (PHIS) database, which contains administrative and billing data from 49 geographically diverse children’s hospitals across the United States (US) affiliated with the Children’s Hospital Association (Lenexa, Kansas). PHIS includes data on patient demographics, discharge diagnoses, and procedures using the International Classification of Diseases, 9th (ICD-9) and 10th Revision (ICD-10) diagnosis codes, as well as daily billed resource utilization for laboratory tests, imaging studies, and medications. Encrypted medical record numbers permit longitudinal identification of children across multiple visits to the same hospital. Use of de-identified PHIS data was deemed to be nonhuman subjects research; our approach to validation of ICD codes using local electronic medical record review was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Study Population
Our study team developed an algorithm to identify children with cervical lymphadenitis and minimize misclassification using PHIS (Appendix A). All children with lymphadenitis-related ICD-9 and ICD-10 discharge diagnosis codes were eligible for inclusion.
This algorithm was subsequently applied to the PHIS database. Children ages two months to 18 years hospitalized at participating PHIS institutions between July 2013 and December 2017 with a diagnosis of cervical lymphadenitis as per th
Measures of Interest
To examine hospital-level variation in imaging practices, we measured the proportion of children at each hospital who underwent any neck imaging study, CT or ultrasound imaging, early imaging, and multiple imaging studies within a single hospitalization. Neck imaging was defined as the presence of a billing code for ultrasound, CT, or magnetic resonance imaging (MRI) study of the neck (Appendix B). Early imaging was defined as neck imaging conducted on day 0 of hospitalization (ie, calendar day of admission and ending at midnight). Multiple imaging studies were defined as the receipt of more than one imaging study, regardless of timing or modality. We also measured the proportion of children by hospital who received surgical drainage, defined by the presence of procedure codes for incision and drainage of abscess of the neck (Appendix B).
In examining patient-level association between early imaging and clinical outcomes, our primary outcome of interest was the receipt of multiple imaging studies. Secondary outcomes included rates of surgical drainage, length of stay (in hospital days), and rates of lymphadenitis-related hospital readmission within 30 days of index discharge.
Covariates
Baseline demographic characteristics included age, gender, race/ethnicity, and insurance type. We measured ED visits associated with lymphadenitis-related diagnosis codes in the 30 days prior to admission as a proxy measure for illness duration prior to presentation. To approximate illness severity, we included the following covariates: rates of intensive care unit admission on presentation, rates of receipt of intravenous (IV) analgesia (Appendix B) on hospital days prior to surgical drainage, and rates of receipt of broad-spectrum antibiotics on day 0 or 1 of hospitalization. Broad-spectrum antibiotics (Appendix B) were defined by an independent three-person review of available antibiotic codes (SD, SSS, and JT); differences were resolved by group consensus.
Analysis
Categorical variables were described using frequencies and percentages, while continuous data were described using median and interquartile range. We described hospital-level variation in imaging practices by calculating and comparing the proportion of children at each hospital who underwent any neck imaging study, CT imaging, ultrasound imaging, early imaging, multiple imaging studies, and surgical drainage.
Patient-level demographics and clinical characteristics were compared across groups using chi-square test. To examine the association between early imaging and outcomes, we used generalized linear or logistic mixed effects models to control for patient demographic characteristics and clinical markers of illness duration and severity, with a random effect for hospital to account for clustering. Patient demographics in the model defined a priori included age, race/ethnicity, and insurance type; clinical characteristics included prior ED visit for lymphadenitis, initial intensive care unit (ICU) admission, use of IV analgesia, and use of broad-spectrum antibiotics on day 0 or 1 of hospitalization. To assess the potential for misclassification related to the availability of calendar day but not time of imaging in PHIS, we conducted a secondary analysis to examine the patient-level association between early imaging and outcomes using an alternative definition for early imaging (defined as imaging conducted on day 0 or day 1 of hospitalization).
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, North Carolina); P < .05 was considered statistically significant.
RESULTS
We identified 19,785 PHIS hospitalizations with lymphadenitis-related discharge diagnosis codes between July 1, 2013 and December 31, 2017. Applying our algorithm and exclusion criteria, we assembled a cohort of 10,014 children hospitalized with cervical lymphadenitis (Figure 1). Two-thirds of the children in our cohort were <4 years old, 42% were non-Hispanic white, and 63% had a government payor (Table 1). Neck imaging (ultrasound, CT, or MRI) was conducted in 8,103 (81%) children. CT imaging was performed in 4,097 (41%) of children, and early imaging was conducted in 6,111 (61%) of children with cervical lymphadenitis.
We noted hospital-level variation in rates of any neck imaging (median: 82.1%, interquartile range [IQR]: 77.7%-85.5%, full range: 68.7%-93.1%), CT imaging (median: 42.3%, IQR: 26.7%-55.2%, full range: 12.0%-81.5%), early imaging (median: 64.4%, IQR: 59.8%-68.4%, full range: 13.8%-76.9%), and multiple imaging studies (median: 23.7%, IQR: 18.6%-28.9%, full range: 1.2%-40.7%; Figure 2). Rates of surgical drainage also varied by hospital (median: 35.1%, IQR: 31.3%-42.0%, full range: 17.1%-54.5%).
At the patient level, children who received early imaging were more likely to be <1 year old (21% vs 16%, P < .001), or Hispanic or Black when compared with children who did not receive early imaging (Table 1). Children who received early imaging were more likely to have had an ED visit for lymphadenitis in the preceding 30 days (8% vs 6%, P = .001). However, they were less likely to have received broad-spectrum antibiotics on admission (6% vs 8%, P < .001; Table 1). Of the 6,111 patients who received early imaging, 2,538 (41.5%) received CT imaging and 3,902 (63.9%) received ultrasound imaging on day 0. Of the 2,272 patients receiving multiple imaging studies, 116 (5.1%) received two or more CT scans.
In multivariable analysis at the patient level, early imaging was associated with higher adjusted odds of receiving multiple imaging studies (adjusted odds ratio [aOR] 3.0, 95% CI: 2.6-3.6). Similarly, early imaging was associated with higher adjusted odds of surgical drainage (aOR: 1.3, 95% CI: 1.1-1.4), increased 30-day readmission for lymphadenitis (aOR: 1.5, 95% CI: 1.2-1.9), and longer length of stay (adjusted rate ratio: 1.2, 95% CI: 1.1-1.2; Table 2). For the subset of patients who did not receive surgical drainage during the index admission, the adjusted odds ratio for the association between early imaging at index admission and 30-day readmission was 1.7 (95% CI: 1.3-2.1). About 63% of readmissions occurred within 7 days of index discharge; 89% occurred within 14 days (Appendix Figure).
In secondary analysis using an alternative definition for early imaging (ie, imaging conducted on day 0 or day 1 of hospitalization), the adjusted odds ratio for multiple imaging studies was 22.6 (95% CI: 15.8-32.4). The adjusted odds and rate ratios for the remaining outcomes were similar to our primary analysis.
DISCUSSION
In this large multicenter study of children with cervical lymphadenitis, we found variation in imaging practices across 44 US children’s hospitals. Children with cervical lymphadenitis who underwent early imaging were more likely to receive multiple imaging studies during a single hospitalization than those who did not receive early imaging. At the patient level, early imaging was also associated with higher rates of surgical drainage, more frequent 30-day readmission, and longer lengths of stay.
To our knowledge, imaging practices in the population of children hospitalized with cervical lymphadenitis have not been previously characterized in the US; one study from Atlanta, Georgia, describes imaging practices in all children evaluated in the ED.1 Single-center studies of children hospitalized with cervical lymphadenitis have been previously conducted in Canada6 and New Zealand,8 in which 42%-51% of children received imaging. In our study, most (81%) children hospitalized with lymphadenitis received some form of imaging, with 61% of all children receiving early imaging. Furthermore, 41% received CT imaging, as compared with 8%-10% of children in the aforementioned studies from Canada and New Zealand.6,8 This finding is consistent with a pattern of imaging overuse in the US, which has amongst the highest utilization rates globally for advanced imaging such as CT and MRI.10,11 Identifying opportunities to safely reduce routine imaging, particularly CT imaging, in this population could decrease unnecessary radiation exposure without compromising outcomes.
We also noted variability in imaging practices across PHIS hospitals. Some of this variability may be partially explained by differences in the patient population or illness severity across hospitals. However, given the absence of evidence-based best practices for children with cervical lymphadenitis, clinicians may rely on anecdotal experience or local practice culture to guide their decision making,12 leading to variability in frequency, timing, and modality of imaging.
At the patient level, we found that children who received early imaging were more likely to receive multiple imaging studies. This finding supports our hypothesis that clinicians often order a second imaging study when the initial imaging study does not clearly demonstrate an abscess, and the child subsequently fails to demonstrate clear improvement after 24-48 hours of antibiotics.
Furthermore, early imaging was associated with overall increased utilization in our cohort, including increased likelihood of surgical drainage, 30-day readmission for lymphadenitis, as well as longer lengths of stay. Confounding may be one explanation for this finding. For instance, clinicians may pursue early imaging in children who present with longer duration of symptoms or more severe illness on presentation, as these factors may be associated with abscess formation.1,6,7 These clinical covariates are not available in PHIS. Thus, we used prior ED visits for lymphadenitis to approximate illness duration, and initial admission to ICU, receipt of IV analgesia, and receipt of broad-spectrum antibiotics to approximate illness severity in an attempt to mitigate confounding.
On the other hand, it is also possible that a proportion of children with a small fluid collection on imaging may have improved with antibiotics alone. There is a growing body of evidence in children with other head and neck infections (eg, retropharyngeal abscess and orbital cellulitis with periosteal abscess)13-15 that suggests that children with small abscesses often improve with antibiotic therapy alone. In children with cervical lymphadenitis who have small or developing abscesses identified via routine imaging on presentation, clinicians may be driven to pursue a surgical intervention with uncertain benefit. Deferring routine imaging in this population may provide an opportunity to improve the value of care in children with lymphadenitis without adversely affecting outcomes.
This study has several limitations given our use of an administrative database. Children with lymphadenitis may have been misclassified as these patients were identified using discharge diagnosis codes
Furthermore, we were unable to measure the exact time of imaging study in PHIS; we used imaging conducted on hospital day 0 as a proxy measure for imaging conducted within the first 24 hours of presentation. With this definition, some children who had early imaging were likely misclassified as not having received early imaging. For example, a patient who arrived in the ED at 9
Additionally, there may be a subset of children who underwent imaging prior to presentation at the PHIS hospital ED for further workup and admission. Imaging conducted outside a PHIS hospital was not captured in this database. Similarly, children who had a readmission at a different hospital than their index admission would not be captured using PHIS. Finally, PHIS captures data from children’s hospitals; practices at these hospitals may not be generalizable to practices in the community hospital setting.
CONCLUSION
In conclusion, we found that imaging practices in children hospitalized with cervical lymphadenitis were widely variable across hospitals. Children receiving early imaging had more resource utilization and intervention when compared with children who did not receive early imaging. Our findings may represent a cascade effect, in which routinely conducted early imaging prompts clinicians to pursue more testing and interventions in this population. Future studies should obtain more detailed patient level covariates to further characterize clinical factors that may impact decisions around imaging and clinical outcomes for children with cervical lymphadenitis.
Acknowledgments
The authors would like to acknowledge the following investigators for their contributions to data interpretation and review of the final manuscript: Angela Choe MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Margaret Rush MD, Children’s National Medical Center, Washington, DC; Ryosuke Takei MD, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Wallis Molchen DO, Texas Children’s Hospital, Houston, Texas; Stephanie Royer Moss MD, Cleveland Clinic, Cleveland, Ohio; Rebecca Dang, MD, Lucile Packard Children’s Hospital Stanford, Palo Alto, California; Joy Solano MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas; Nathaniel P. Goodrich MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Ngozi Eboh MD, Texas Tech University Health Sciences Center, Dallas, Texas; Ashley Jenkins MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Rebecca Steuart MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sonya Tang Girdwood MD, PhD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Alissa McInerney MD, Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, New York; Sumeet Banker MD, MPH, New York Presbyterian Morgan Stanley Children’s Hospital, New York, New York; Corrie McDaniel DO, Seattle Children’s Hospital, Seattle, Washington; Christiane Lenzen MD, Rady Children’s Hospital, San Diego, California; Aleisha Nabower MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Waheeda Samady MD, Ann & Robert H. Lurie Children’s Hospital, Chicago, Illinois; Jennifer Chen MD, Rady Children’s Hospital, San Diego, California; Marquita Genies MD, MPH, John’s Hopkins Children’s Center, Baltimore, Maryland; Justin Lockwood MD, Children’s Hospital Colorado, Aurora, Colorado; David Synhorst MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas.
Cervical lymphadenitis is a common superficial neck infection in childhood. While most children with cervical lymphadenitis recover with antibiotic therapy, a subset can develop an abscess that may require surgical drainage. Radiologic imaging, most commonly ultrasound or computed tomography (CT), is often performed to identify such an abscess.1-3 However, no national standards exist to guide clinician decision making around imaging in this population. In the absence of evidence-based guidelines, variability in frequency, timing, and modality of imaging likely exists in children hospitalized with cervical lymphadenitis.
As demonstrated for several other common pediatric conditions,4,5 variability in imaging practices may contribute to overutilization of resources in children with cervical lymphadenitis. In particular, routinely conducting imaging on presentation may constitute overuse, as children with cervical lymphadenitis who present with less than 72 hours of neck swelling rarely undergo surgical drainage within the first 24 hours of hospitalization.1,6,7 Imaging performed on presentation is often repeated later during hospitalization, particularly if the patient has not improved with antibiotic therapy. The net result may be unnecessary, redundant radiologic studies. Furthermore, serious complications such as bacteremia, extension of infection into the retropharyngeal space, or involvement of the airway or vasculature rarely occur in children with cervical lymphadenitis.6,8 In this context, deferring initial imaging in this population is unlikely to lead to adverse outcomes and may reduce radiation exposure.
The overall objectives of this study are to describe hospital-level variation in imaging practices for pediatric cervical lymphadenitis and to examine the association between early imaging and outcomes in this population.
METHODS
Study Design and Data Source
We conducted a multicenter, cross-sectional study using the Pediatric Health Information Systems (PHIS) database, which contains administrative and billing data from 49 geographically diverse children’s hospitals across the United States (US) affiliated with the Children’s Hospital Association (Lenexa, Kansas). PHIS includes data on patient demographics, discharge diagnoses, and procedures using the International Classification of Diseases, 9th (ICD-9) and 10th Revision (ICD-10) diagnosis codes, as well as daily billed resource utilization for laboratory tests, imaging studies, and medications. Encrypted medical record numbers permit longitudinal identification of children across multiple visits to the same hospital. Use of de-identified PHIS data was deemed to be nonhuman subjects research; our approach to validation of ICD codes using local electronic medical record review was reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Study Population
Our study team developed an algorithm to identify children with cervical lymphadenitis and minimize misclassification using PHIS (Appendix A). All children with lymphadenitis-related ICD-9 and ICD-10 discharge diagnosis codes were eligible for inclusion.
This algorithm was subsequently applied to the PHIS database. Children ages two months to 18 years hospitalized at participating PHIS institutions between July 2013 and December 2017 with a diagnosis of cervical lymphadenitis as per th
Measures of Interest
To examine hospital-level variation in imaging practices, we measured the proportion of children at each hospital who underwent any neck imaging study, CT or ultrasound imaging, early imaging, and multiple imaging studies within a single hospitalization. Neck imaging was defined as the presence of a billing code for ultrasound, CT, or magnetic resonance imaging (MRI) study of the neck (Appendix B). Early imaging was defined as neck imaging conducted on day 0 of hospitalization (ie, calendar day of admission and ending at midnight). Multiple imaging studies were defined as the receipt of more than one imaging study, regardless of timing or modality. We also measured the proportion of children by hospital who received surgical drainage, defined by the presence of procedure codes for incision and drainage of abscess of the neck (Appendix B).
In examining patient-level association between early imaging and clinical outcomes, our primary outcome of interest was the receipt of multiple imaging studies. Secondary outcomes included rates of surgical drainage, length of stay (in hospital days), and rates of lymphadenitis-related hospital readmission within 30 days of index discharge.
Covariates
Baseline demographic characteristics included age, gender, race/ethnicity, and insurance type. We measured ED visits associated with lymphadenitis-related diagnosis codes in the 30 days prior to admission as a proxy measure for illness duration prior to presentation. To approximate illness severity, we included the following covariates: rates of intensive care unit admission on presentation, rates of receipt of intravenous (IV) analgesia (Appendix B) on hospital days prior to surgical drainage, and rates of receipt of broad-spectrum antibiotics on day 0 or 1 of hospitalization. Broad-spectrum antibiotics (Appendix B) were defined by an independent three-person review of available antibiotic codes (SD, SSS, and JT); differences were resolved by group consensus.
Analysis
Categorical variables were described using frequencies and percentages, while continuous data were described using median and interquartile range. We described hospital-level variation in imaging practices by calculating and comparing the proportion of children at each hospital who underwent any neck imaging study, CT imaging, ultrasound imaging, early imaging, multiple imaging studies, and surgical drainage.
Patient-level demographics and clinical characteristics were compared across groups using chi-square test. To examine the association between early imaging and outcomes, we used generalized linear or logistic mixed effects models to control for patient demographic characteristics and clinical markers of illness duration and severity, with a random effect for hospital to account for clustering. Patient demographics in the model defined a priori included age, race/ethnicity, and insurance type; clinical characteristics included prior ED visit for lymphadenitis, initial intensive care unit (ICU) admission, use of IV analgesia, and use of broad-spectrum antibiotics on day 0 or 1 of hospitalization. To assess the potential for misclassification related to the availability of calendar day but not time of imaging in PHIS, we conducted a secondary analysis to examine the patient-level association between early imaging and outcomes using an alternative definition for early imaging (defined as imaging conducted on day 0 or day 1 of hospitalization).
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, North Carolina); P < .05 was considered statistically significant.
RESULTS
We identified 19,785 PHIS hospitalizations with lymphadenitis-related discharge diagnosis codes between July 1, 2013 and December 31, 2017. Applying our algorithm and exclusion criteria, we assembled a cohort of 10,014 children hospitalized with cervical lymphadenitis (Figure 1). Two-thirds of the children in our cohort were <4 years old, 42% were non-Hispanic white, and 63% had a government payor (Table 1). Neck imaging (ultrasound, CT, or MRI) was conducted in 8,103 (81%) children. CT imaging was performed in 4,097 (41%) of children, and early imaging was conducted in 6,111 (61%) of children with cervical lymphadenitis.
We noted hospital-level variation in rates of any neck imaging (median: 82.1%, interquartile range [IQR]: 77.7%-85.5%, full range: 68.7%-93.1%), CT imaging (median: 42.3%, IQR: 26.7%-55.2%, full range: 12.0%-81.5%), early imaging (median: 64.4%, IQR: 59.8%-68.4%, full range: 13.8%-76.9%), and multiple imaging studies (median: 23.7%, IQR: 18.6%-28.9%, full range: 1.2%-40.7%; Figure 2). Rates of surgical drainage also varied by hospital (median: 35.1%, IQR: 31.3%-42.0%, full range: 17.1%-54.5%).
At the patient level, children who received early imaging were more likely to be <1 year old (21% vs 16%, P < .001), or Hispanic or Black when compared with children who did not receive early imaging (Table 1). Children who received early imaging were more likely to have had an ED visit for lymphadenitis in the preceding 30 days (8% vs 6%, P = .001). However, they were less likely to have received broad-spectrum antibiotics on admission (6% vs 8%, P < .001; Table 1). Of the 6,111 patients who received early imaging, 2,538 (41.5%) received CT imaging and 3,902 (63.9%) received ultrasound imaging on day 0. Of the 2,272 patients receiving multiple imaging studies, 116 (5.1%) received two or more CT scans.
In multivariable analysis at the patient level, early imaging was associated with higher adjusted odds of receiving multiple imaging studies (adjusted odds ratio [aOR] 3.0, 95% CI: 2.6-3.6). Similarly, early imaging was associated with higher adjusted odds of surgical drainage (aOR: 1.3, 95% CI: 1.1-1.4), increased 30-day readmission for lymphadenitis (aOR: 1.5, 95% CI: 1.2-1.9), and longer length of stay (adjusted rate ratio: 1.2, 95% CI: 1.1-1.2; Table 2). For the subset of patients who did not receive surgical drainage during the index admission, the adjusted odds ratio for the association between early imaging at index admission and 30-day readmission was 1.7 (95% CI: 1.3-2.1). About 63% of readmissions occurred within 7 days of index discharge; 89% occurred within 14 days (Appendix Figure).
In secondary analysis using an alternative definition for early imaging (ie, imaging conducted on day 0 or day 1 of hospitalization), the adjusted odds ratio for multiple imaging studies was 22.6 (95% CI: 15.8-32.4). The adjusted odds and rate ratios for the remaining outcomes were similar to our primary analysis.
DISCUSSION
In this large multicenter study of children with cervical lymphadenitis, we found variation in imaging practices across 44 US children’s hospitals. Children with cervical lymphadenitis who underwent early imaging were more likely to receive multiple imaging studies during a single hospitalization than those who did not receive early imaging. At the patient level, early imaging was also associated with higher rates of surgical drainage, more frequent 30-day readmission, and longer lengths of stay.
To our knowledge, imaging practices in the population of children hospitalized with cervical lymphadenitis have not been previously characterized in the US; one study from Atlanta, Georgia, describes imaging practices in all children evaluated in the ED.1 Single-center studies of children hospitalized with cervical lymphadenitis have been previously conducted in Canada6 and New Zealand,8 in which 42%-51% of children received imaging. In our study, most (81%) children hospitalized with lymphadenitis received some form of imaging, with 61% of all children receiving early imaging. Furthermore, 41% received CT imaging, as compared with 8%-10% of children in the aforementioned studies from Canada and New Zealand.6,8 This finding is consistent with a pattern of imaging overuse in the US, which has amongst the highest utilization rates globally for advanced imaging such as CT and MRI.10,11 Identifying opportunities to safely reduce routine imaging, particularly CT imaging, in this population could decrease unnecessary radiation exposure without compromising outcomes.
We also noted variability in imaging practices across PHIS hospitals. Some of this variability may be partially explained by differences in the patient population or illness severity across hospitals. However, given the absence of evidence-based best practices for children with cervical lymphadenitis, clinicians may rely on anecdotal experience or local practice culture to guide their decision making,12 leading to variability in frequency, timing, and modality of imaging.
At the patient level, we found that children who received early imaging were more likely to receive multiple imaging studies. This finding supports our hypothesis that clinicians often order a second imaging study when the initial imaging study does not clearly demonstrate an abscess, and the child subsequently fails to demonstrate clear improvement after 24-48 hours of antibiotics.
Furthermore, early imaging was associated with overall increased utilization in our cohort, including increased likelihood of surgical drainage, 30-day readmission for lymphadenitis, as well as longer lengths of stay. Confounding may be one explanation for this finding. For instance, clinicians may pursue early imaging in children who present with longer duration of symptoms or more severe illness on presentation, as these factors may be associated with abscess formation.1,6,7 These clinical covariates are not available in PHIS. Thus, we used prior ED visits for lymphadenitis to approximate illness duration, and initial admission to ICU, receipt of IV analgesia, and receipt of broad-spectrum antibiotics to approximate illness severity in an attempt to mitigate confounding.
On the other hand, it is also possible that a proportion of children with a small fluid collection on imaging may have improved with antibiotics alone. There is a growing body of evidence in children with other head and neck infections (eg, retropharyngeal abscess and orbital cellulitis with periosteal abscess)13-15 that suggests that children with small abscesses often improve with antibiotic therapy alone. In children with cervical lymphadenitis who have small or developing abscesses identified via routine imaging on presentation, clinicians may be driven to pursue a surgical intervention with uncertain benefit. Deferring routine imaging in this population may provide an opportunity to improve the value of care in children with lymphadenitis without adversely affecting outcomes.
This study has several limitations given our use of an administrative database. Children with lymphadenitis may have been misclassified as these patients were identified using discharge diagnosis codes
Furthermore, we were unable to measure the exact time of imaging study in PHIS; we used imaging conducted on hospital day 0 as a proxy measure for imaging conducted within the first 24 hours of presentation. With this definition, some children who had early imaging were likely misclassified as not having received early imaging. For example, a patient who arrived in the ED at 9
Additionally, there may be a subset of children who underwent imaging prior to presentation at the PHIS hospital ED for further workup and admission. Imaging conducted outside a PHIS hospital was not captured in this database. Similarly, children who had a readmission at a different hospital than their index admission would not be captured using PHIS. Finally, PHIS captures data from children’s hospitals; practices at these hospitals may not be generalizable to practices in the community hospital setting.
CONCLUSION
In conclusion, we found that imaging practices in children hospitalized with cervical lymphadenitis were widely variable across hospitals. Children receiving early imaging had more resource utilization and intervention when compared with children who did not receive early imaging. Our findings may represent a cascade effect, in which routinely conducted early imaging prompts clinicians to pursue more testing and interventions in this population. Future studies should obtain more detailed patient level covariates to further characterize clinical factors that may impact decisions around imaging and clinical outcomes for children with cervical lymphadenitis.
Acknowledgments
The authors would like to acknowledge the following investigators for their contributions to data interpretation and review of the final manuscript: Angela Choe MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Margaret Rush MD, Children’s National Medical Center, Washington, DC; Ryosuke Takei MD, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Wallis Molchen DO, Texas Children’s Hospital, Houston, Texas; Stephanie Royer Moss MD, Cleveland Clinic, Cleveland, Ohio; Rebecca Dang, MD, Lucile Packard Children’s Hospital Stanford, Palo Alto, California; Joy Solano MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas; Nathaniel P. Goodrich MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Ngozi Eboh MD, Texas Tech University Health Sciences Center, Dallas, Texas; Ashley Jenkins MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Rebecca Steuart MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Sonya Tang Girdwood MD, PhD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Alissa McInerney MD, Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, New York; Sumeet Banker MD, MPH, New York Presbyterian Morgan Stanley Children’s Hospital, New York, New York; Corrie McDaniel DO, Seattle Children’s Hospital, Seattle, Washington; Christiane Lenzen MD, Rady Children’s Hospital, San Diego, California; Aleisha Nabower MD, Children’s Hospital & Medical Center, Omaha, Nebraska; Waheeda Samady MD, Ann & Robert H. Lurie Children’s Hospital, Chicago, Illinois; Jennifer Chen MD, Rady Children’s Hospital, San Diego, California; Marquita Genies MD, MPH, John’s Hopkins Children’s Center, Baltimore, Maryland; Justin Lockwood MD, Children’s Hospital Colorado, Aurora, Colorado; David Synhorst MD, Children’s Mercy Hospital Kansas, Overland Park, Kansas.
1. Sauer MW, Sharma S, Hirsh DA et al. Acute neck infections in children: who is likely to undergo surgical drainage? Am J Emerg Med. 2013;31(6):906-909. https://doi.org/10.1016/j.ajem.2013.02.043.
2. Sethia R, Mahida JB, Subbarayan RA, et al. Evaluation of an imaging protocol using ultrasound as the primary diagnostic modality in pediatric patients with superficial soft tissue infections of the face and neck. Int J Pediatr Otorhinolaryngol. 2017;96:89-93. https://doi.org/10.1016/j.ijporl.2017.02.027.
3. Neff L, Newland JG, Sykes KJ, Selvarangan R, Wei JL. Microbiology and antimicrobial treatment of pediatric cervical lymphadenitis requiring surgical intervention. Int J Pediatr Otorhinolaryngol. 2013;77(5):817-820. https://doi.org/10.1016/j.ijporl.2013.02.018.
4. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789-796. https://doi.org/10.1016/j.jpeds.2009.01.010.
6. Luu TM, Chevalier I, Gauthier M et al. Acute adenitis in children: clinical course and factors predictive of surgical drainage. J Paediatr Child Health. 2005;41(5-6):273-277. https://doi.org/10.1111/j.1440-1754.2005.00610.x.
7. Golriz F, Bisset GS, 3rd, D’Amico B, et al. A clinical decision rule for the use of ultrasound in children presenting with acute inflammatory neck masses. Pediatr Rad. 2017;47(4):422-428. https://doi.org/10.1007/s00247-016-3774-9.
8. Courtney MJ, Miteff A, Mahadevan M. Management of pediatric lateral neck infections: does the adage “… never let the sun go down on undrained pus …” hold true? Int J Pediatr Otorhinolaryngol. 2007;71(1):95-100. https://doi.org/10.1016/j.ijporl.2006.09.009.
9. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
10. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. https://doi.org/10.1001/jama.2018.1150.
11. Oren O, Kebebew E, Ioannidis JPA. Curbing unnecessary and wasted diagnostic imaging. JAMA. 2019;321(3):245-246. https://doi.org/10.1001/jama.2018.20295.
12. Palmer RH, Miller MR. Methodologic challenges in developing and implementing measures of quality for child health care. Ambul Pediatr Off J Ambul Pediatr Assoc. 2001;1(1):39-52. https://doi.org/10.1367/1539-4409(2001)001<0039:MCIDAI>2.0.CO;2.
13. Daya H, Lo S, Papsin BC, et al. Retropharyngeal and parapharyngeal infections in children: the Toronto experience. Int J Pediatr Otorhinolaryngol. 2005;69(1):81-86. https://doi.org/10.1016/j.ijporl.2004.08.010.
14. Wong SJ, Levi J. Management of pediatric orbital cellulitis: A systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006.
15. Wong DK, Brown C, Mills N, Spielmann P, Neeff M. To drain or not to drain-management of pediatric deep neck abscesses: a case-control study. Int J Pediatr Otorhinolaryngol. 2012;76(12):1810-1813. https://doi.org/10.1016/j.ijporl.2012.09.006.
1. Sauer MW, Sharma S, Hirsh DA et al. Acute neck infections in children: who is likely to undergo surgical drainage? Am J Emerg Med. 2013;31(6):906-909. https://doi.org/10.1016/j.ajem.2013.02.043.
2. Sethia R, Mahida JB, Subbarayan RA, et al. Evaluation of an imaging protocol using ultrasound as the primary diagnostic modality in pediatric patients with superficial soft tissue infections of the face and neck. Int J Pediatr Otorhinolaryngol. 2017;96:89-93. https://doi.org/10.1016/j.ijporl.2017.02.027.
3. Neff L, Newland JG, Sykes KJ, Selvarangan R, Wei JL. Microbiology and antimicrobial treatment of pediatric cervical lymphadenitis requiring surgical intervention. Int J Pediatr Otorhinolaryngol. 2013;77(5):817-820. https://doi.org/10.1016/j.ijporl.2013.02.018.
4. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
5. Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789-796. https://doi.org/10.1016/j.jpeds.2009.01.010.
6. Luu TM, Chevalier I, Gauthier M et al. Acute adenitis in children: clinical course and factors predictive of surgical drainage. J Paediatr Child Health. 2005;41(5-6):273-277. https://doi.org/10.1111/j.1440-1754.2005.00610.x.
7. Golriz F, Bisset GS, 3rd, D’Amico B, et al. A clinical decision rule for the use of ultrasound in children presenting with acute inflammatory neck masses. Pediatr Rad. 2017;47(4):422-428. https://doi.org/10.1007/s00247-016-3774-9.
8. Courtney MJ, Miteff A, Mahadevan M. Management of pediatric lateral neck infections: does the adage “… never let the sun go down on undrained pus …” hold true? Int J Pediatr Otorhinolaryngol. 2007;71(1):95-100. https://doi.org/10.1016/j.ijporl.2006.09.009.
9. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
10. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. https://doi.org/10.1001/jama.2018.1150.
11. Oren O, Kebebew E, Ioannidis JPA. Curbing unnecessary and wasted diagnostic imaging. JAMA. 2019;321(3):245-246. https://doi.org/10.1001/jama.2018.20295.
12. Palmer RH, Miller MR. Methodologic challenges in developing and implementing measures of quality for child health care. Ambul Pediatr Off J Ambul Pediatr Assoc. 2001;1(1):39-52. https://doi.org/10.1367/1539-4409(2001)001<0039:MCIDAI>2.0.CO;2.
13. Daya H, Lo S, Papsin BC, et al. Retropharyngeal and parapharyngeal infections in children: the Toronto experience. Int J Pediatr Otorhinolaryngol. 2005;69(1):81-86. https://doi.org/10.1016/j.ijporl.2004.08.010.
14. Wong SJ, Levi J. Management of pediatric orbital cellulitis: A systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006.
15. Wong DK, Brown C, Mills N, Spielmann P, Neeff M. To drain or not to drain-management of pediatric deep neck abscesses: a case-control study. Int J Pediatr Otorhinolaryngol. 2012;76(12):1810-1813. https://doi.org/10.1016/j.ijporl.2012.09.006.
© 2019 Society of Hospital Medicine