User login
Patch Testing for Adverse Drug Reactions
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
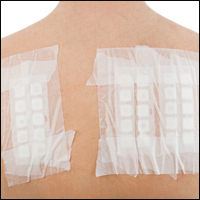
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
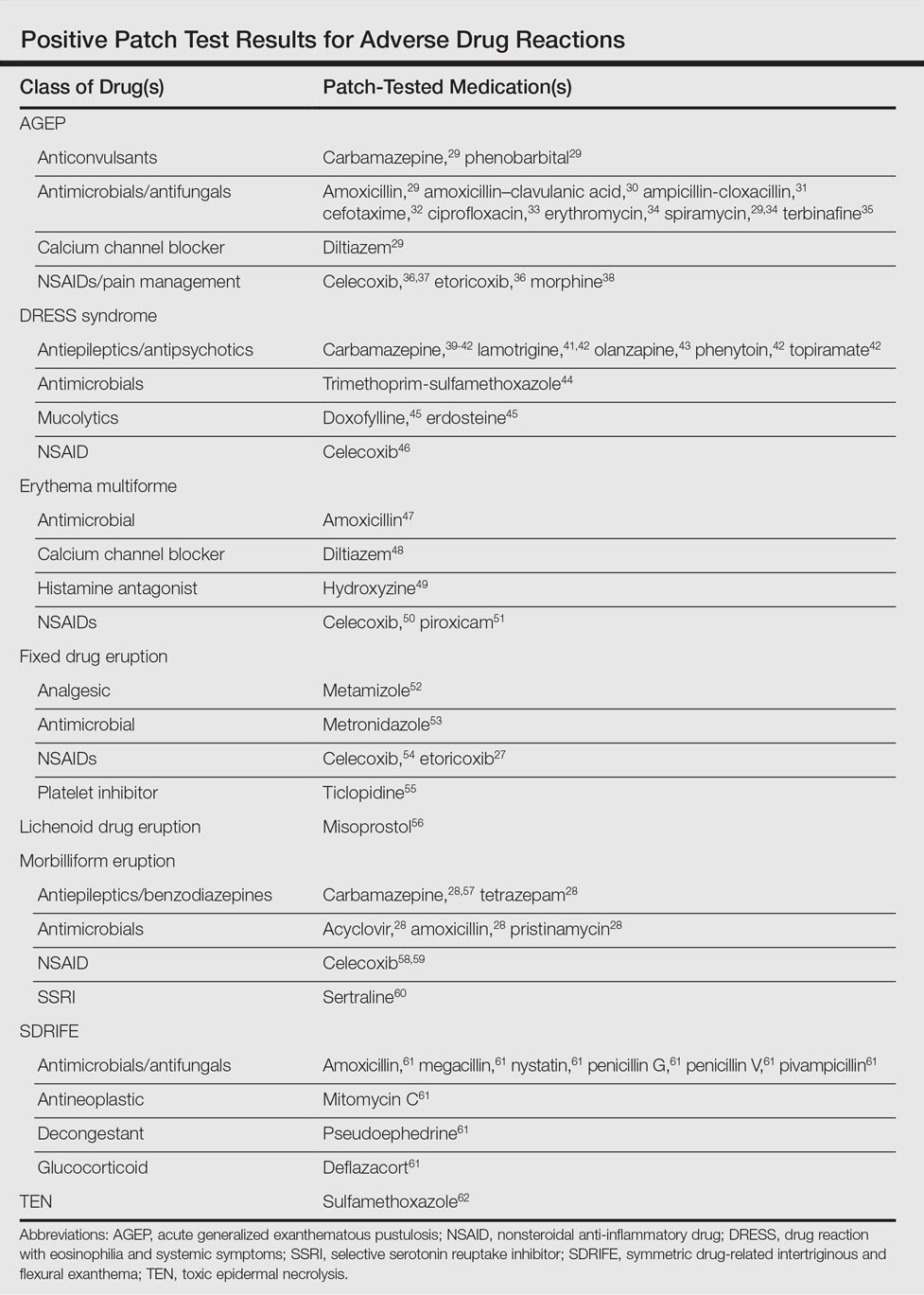
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.
Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
Adverse drug reactions account for 3% to 6% of hospital admissions in the United States and occur in 10% to 15% of hospitalized patients.1,2 The most common culprits are antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs).3-12 In most cases, diagnoses are made clinically without diagnostic testing. To identify drug allergies associated with diagnostic testing, one center selected patients with suspected cutaneous drug reactions (2006-2010) for further evaluation.13 Of 612 patients who were evaluated, 141 had a high suspicion of drug allergy and were included in the analysis. The excluded patients had pseudoallergic reactions, reactive exanthemas due to infection, histopathologic exclusion of drug allergy, angioedema, or other dermatological conditions such as contact dermatitis and eczema. Of the included patients, 107 were diagnosed with drug reactions, while the remainder had non–drug-related exanthemas or unknown etiology after testing. Identified culprit drugs were predominantly antibiotics (39.8%) and NSAIDs (21.2%); contrast media, anticoagulants, anticonvulsants, antimalarials, antifungals, glucocorticoids, antihypertensives, and proton pump inhibitors also were implicated. They were identified with skin prick, intradermal, and patch tests (62.6%); lymphocyte transformation test (17.7%); oral rechallenge (5.6%); or without skin testing (6.5%). One quarter of patients with a high suspicion for drug allergy did not have a confirmed drug eruption in this study. Another study found that 10% to 20% of patients with reported penicillin allergy had confirmation via skin prick testing.14 These findings suggest that confirmation of suspected drug allergy may require more than one diagnostic test.
Tests for Adverse Drug Reactions
The following tests have been shown to aid in the identification of cutaneous drug eruptions: (1) patch tests15-21; (2) intradermal tests14,15,19,20; (3) drug provocation tests15,20; and (4) lymphocyte transformation tests.20 Intradermal or skin prick tests are most useful in urticarial eruptions but can be considered in nonurticarial eruptions with delayed inspection of test sites up to 1 week after testing. Drug provocation tests are considered the gold standard but involve patient risk. Lymphocyte transformation tests use the principle that T lymphocytes proliferate in the presence of drugs to which the patient is sensitized. Patch tests will be discussed in greater detail below. Immunohistochemistry can determine immunologic mechanisms of eruptions but cannot identify causative agents.16,17,22
A retrospective study of patients referred for evaluation of adverse drug reactions between 1996 and 2006 found the collective negative predictive value (NPV)—the percentage of truly negative skin tests based on provocation or substitution testing—of cutaneous drug tests including patch, prick, and intradermal tests to be 89.6% (95% confidence interval, 85.9%-93.3%).23 The NPVs of each test were not reported. Patients with negative cutaneous tests had subsequent oral rechallenge or substitution testing with medication from the same drug class.23 Another study16 found the NPV of patch testing to be at least 79% after review of data from other studies using patch and provocation testing.16,24 These studies suggest that cutaneous testing can be useful, albeit imperfect, in the evaluation and diagnosis of drug allergy.
Review of the Patch Test
Patch tests can be helpful in diagnosis of delayed hypersensitivities.18 Patch testing is most commonly and effectively used to diagnose allergic contact dermatitis, but its utility in other applications, such as diagnosis of cutaneous drug eruptions, has not been extensively studied.
The development of patch tests to diagnose systemic drug allergies is inhibited by the uncertainty of percutaneous drug penetration, a dearth of studies to determine the best test concentrations of active drug in the patch test, and the potential for nonimmunologic contact urticaria upon skin exposure. Furthermore, cutaneous metabolism of many antigens is well documented, but correlation to systemic metabolism often is unknown, which can confound patch test results and lead to false-negative results when the skin’s metabolic capacity does not match the body’s capacity to generate antigens capable of eliciting immunogenic responses.21 Additionally, the method used to suspend and disperse drugs in patch test vehicles is unfamiliar to most pharmacists, and standardized concentrations and vehicles are available only for some medications.25 Studies sufficient to obtain US Food and Drug Administration approval of patch tests for systemic drug eruptions would be costly and therefore prohibitive to investigators. The majority of the literature consists of case reports and data extrapolated from reviews. Patch test results of many drugs have been reported in the literature, with the highest frequencies of positive results associated with anticonvulsants,26 antibiotics, corticosteroids, calcium channel blockers, and benzodiazepines.21
Patch test placement affects the diagnostic value of the test. Placing patch tests on previously involved sites of fixed drug eruptions improves yield over placement on uninvolved skin.27 Placing patch tests on previously involved sites of other drug eruptions such as toxic epidermal necrolysis also may aid in diagnosis, though the literature is sparse.25,26,28
Patch Testing in Drug Eruptions
Morbilliform eruptions account for 48% to 91% of patients with adverse drug reactions.4-6 Other drug eruptions include urticarial eruptions, acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid drug eruption, symmetric drug-related intertriginous and flexural exanthema (SDRIFE), erythema multiforme (EM), and systemic contact dermatitis. The Table summarizes reports of positive patch tests with various medications for these drug eruptions.
In general, antimicrobials and NSAIDs were the most implicated drugs with positive patch test results in AGEP, DRESS syndrome, EM, fixed drug eruptions, and morbilliform eruptions. In AGEP, positive results also were reported for other drugs, including terbinafine and morphine.29-38 In fixed drug eruptions, patch testing on involved skin showed positive results to NSAIDs, analgesics, platelet inhibitors, and antimicrobials.27,52-55 Patch testing in DRESS syndrome has shown many positive reactions to antiepileptics and antipsychotics.39-43 One study used patch tests in SDRIFE, reporting positive results with antimicrobials, antineoplastics, decongestants, and glucocorticoids.61 Nonsteroidal anti-inflammatory drugs, antimicrobials, calcium channel blockers, and histamine antagonists were implicated in EM.47-51 Positive patch tests were seen in morbilliform eruptions with selective serotonin reuptake inhibitors, antiepileptics/benzodiazepines, NSAIDs, and antimicrobials.28,57-60 In toxic epidermal necrolysis, diagnosis with patch testing was made using patches placed on previously involved skin with sulfamethoxazole.62
Systemic Contact Dermatitis
Drugs historically recognized as causing allergic contact dermatitis (eg, topical gentamycin) can cause systemic contact dermatitis, which can be patch tested. In these situations, systemic contact dermatitis may be due to either the active drug or excipients in the medication formulation. Excipients are inactive ingredients in medications that provide a suitable consistency, appearance, or form. Often overlooked as culprits of drug hypersensitivity because they are theoretically inert, excipients are increasingly implicated in drug allergy. Swerlick and Campbell63 described 11 cases in which chronic unexplained pruritus responded to medication changes to avoid coloring agents. The most common culprits were FD&C Blue No. 1 and FD&C Blue No. 2. Patch testing for allergies to dyes can be clinically useful, though a lack of commercially available patch tests makes diagnosis difficult.64
Other excipients can cause cutaneous reactions. Propylene glycol, commonly implicated in allergic contact dermatitis, also can cause cutaneous eruptions upon systemic exposure.65 Corticosteroid-induced systemic contact dermatitis has been reported, though it is less prevalent than allergic contact dermatitis.66 These reactions usually are due to nonmethylated and nonhalogenated corticosteroids including budesonide, cortisone, hydrocortisone, prednisolone, and methylprednisolone.67,68 Patch testing in these situations is complicated by the possibility of false-negative results due to the anti-inflammatory effects of the corticosteroids. Therefore, patch testing should be performed using standardized and not treatment concentrations.
In our clinic, we have anecdotally observed several patients with chronic dermatitis and suspected NSAID allergies have positive patch test results with propylene glycol and not the suspected drug. Excipients encountered in multiple drugs and foods are more likely to present as chronic dermatitis, while active drug ingredients started in hospital settings more often present as acute dermatitis.

Our Experience
We have patch tested a handful of patients with suspected drug eruptions (University Hospitals Cleveland Medical Center institutional review board #07-12-27). Medications, excipients, and their concentrations (in % weight per weight) and vehicles that were tested include ibuprofen (10% petrolatum), aspirin (10% petrolatum), hydrochlorothiazide (10% petrolatum), captopril (5% petrolatum), and propylene glycol (30% water or 5% petrolatum). Patch tests were read at 48 and 72 hours and scored according to the International Contact Dermatitis Research Group patch test scoring guidelines.69 Two patients tested for ibuprofen reacted positively only to propylene glycol; the 3 other patients did not react to aspirin, hydrochlorothiazide, and captopril. Overall, we observed no positive patch tests to medications and 2 positive tests to propylene glycol in 5 patients tested (unpublished data).
Areas of Uncertainty
Although tests for immediate-type hypersensitivity reactions to drugs exist as skin prick tests, diagnostic testing for the majority of drug reactions does not exist. Drug allergy diagnosis is made with history and temporality, potentially resulting in unnecessary avoidance of helpful medications. Ideal patch test concentrations and vehicles as well as the sensitivity and specificity of these tests are unknown.
Guidelines From Professional Societies
Drug allergy testing guidelines are available from the British Society for Allergy and Clinical Immunology70 and American Academy of Allergy, Asthma and Immunology.71 The guidelines recommend diagnosis by history and temporality, and it is stated that patch testing is potentially useful in maculopapular rashes, AGEP, fixed drug eruptions, and DRESS syndrome.
Conclusion
Case reports in the literature suggest the utility of patch testing in some drug allergies. We suggest testing excipients such as propylene glycol and benzoic acid to rule out systemic contact dermatitis when patch testing with active drugs to confirm cause of suspected adverse cutaneous reactions to medications.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
- Arndt KA, Jick H. Rates of cutaneous reactions to drugs. a report from the Boston Collaborative Drug Surveillance Program. JAMA. 1976;235:918-922.
- Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions. a report from the Boston Collaborative Drug Surveillance Program on 15,483 consecutive inpatients, 1975 to 1982. JAMA. 1986;256:3358-3363.
- Fiszenson-Albala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018-1022.
- Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90:342-347.
- Hunziker T, Kunzi UP, Braunschweig S, et al. Comprehensive hospital drug monitoring (CHDM): adverse skin reactions, a 20-year survey. Allergy. 1997;52:388-393.
- Swanbeck G, Dahlberg E. Cutaneous drug reactions. an attempt to quantitative estimation. Arch Dermatol Res. 1992;284:215-218.
- Naldi L, Conforti A, Venegoni M, et al. Cutaneous reactions to drugs. an analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. 1999;48:839-846.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:319-333.
- Vasconcelos C, Magina S, Quirino P, et al. Cutaneous drug reactions to piroxicam. Contact Dermatitis. 1998;39:145.
- Gerber D. Adverse reactions of piroxicam. Drug Intell Clin Pharm. 1987;21:707-710.
- Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia, JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:335-356.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-709.e9; quiz 718-720.
- Heinzerling LM, Tomsitz D, Anliker MD. Is drug allergy less prevalent than previously assumed? a 5-year analysis. Br J Dermatol. 2012;166:107-114.
- Salkind AR, Cuddy PG. Is this patient allergic to penicillin?: an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505.
- Torres MJ, Gomez F, Doña I, et al. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy. 2012;67:929-935.
- Cham PM, Warshaw EM. Patch testing for evaluating drug reactions due to systemic antibiotics. Dermatitis. 2007;18:63-77.
- Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions—a 20-year review. Contact Dermatitis. 2011;65:195-201.
- Romano A, Viola M, Gaeta F, et al. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66-74.
- Rosso R, Mattiacci G, Bernardi ML, et al. Very delayed reactions to beta-lactam antibiotics. Contact Dermatitis. 2000;42:293-295.
- Romano A, Torres MJ, Castells M, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 suppl):S67-S73.
- Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol. 2010;10:291-296.
- Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80-90.
- Waton J, Tréchot P, Loss-Ayay C, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160:786-794.
- Romano A, Viola M, Mondino C, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169-174.
- De Groot AC. Patch Testing. Test Concentrations and Vehicles for 4350 Chemicals. 3rd ed. Wapserveen, Netherlands: acdegroot publishing; 2008.
- Elzagallaai AA, Knowles SR, Rieder MJ, et al. Patch testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Drug Saf. 2009;32:391-408.
- Andrade P, Gonçalo M. Fixed drug eruption caused by etoricoxib—2 cases confirmed by patch testing. Contact Dermatitis. 2011;64:118-120.
- Barbaud A, Reichert-Penetrat S, Tréchot P, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49-58.
- Wolkenstein P, Chosidow O, Fléchet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Harries MJ, McIntyre SJ, Kingston TP. Co-amoxiclav-induced acute generalized exanthematous pustulosis confirmed by patch testing. Contact Dermatitis. 2006;55:372.
- Matsumoto Y, Okubo Y, Yamamoto T, et al. Case of acute generalized exanthematous pustulosis caused by ampicillin/cloxacillin sodium in a pregnant woman. J Dermatol. 2008;35:362-364.
- Chaabane A, Aouam K, Gassab L, et al. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429-432.
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280.
- Moreau A, Dompmartin A, Castel B, et al. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995;34:263-266.
- Kempinaire A, De Raevea L, Merckx M, et al. Terbinafine-induced acute generalized exanthematous pustulosis confirmed by a positive patch-test result. J Am Acad Dermatol. 1997;37:653-655.
- Mäkelä L, Lammintausta K. Etoricoxib-induced acute generalized exanthematous pustulosis. Acta Derm Venereol. 2008;88:200-201.
- Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004;103:555-557.
- Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(2 suppl):S21-S23.
- Inadomi T. Drug rash with eosinophilia and systemic symptoms (DRESS): changing carbamazepine to phenobarbital controlled epilepsy without the recurrence of DRESS. Eur J Dermatol. 2010;20:220-222.
- Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol. 2012;53:274-277.
- Aouam K, Ben Romdhane F, Loussaief C, et al. Hypersensitivity syndrome induced by anticonvulsants: possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488-1491.
- Santiago F, Gonçalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62:47-53.
- Prevost P, Bédry R, Lacoste D, et al. Hypersensitivity syndrome with olanzapine confirmed by patch tests. Eur J Dermatol. 2012;22:126-127.
- Hubiche T, Milpied B, Cazeau C, et al. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140-141.
- Song WJ, Shim EJ, Kang MG, et al. Severe drug hypersensitivity induced by erdosteine and doxofylline as confirmed by patch and lymphocyte transformation tests: a case report. J Investig Allergol Clin Immunol. 2012;22:230-232.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- González-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76-78.
- Gonzalo Garijo MA, Pérez Calderón R, de Argila Fernández-Durán D, et al. Cutaneous reactions due to diltiazem and cross reactivity with other calcium channel blockers. Allergol Immunopathol (Madr). 2005;33:238-240.
- Peña AL, Henriquezsantana A, Gonzalez-Seco E, et al. Exudative erythema multiforme induced by hydroxyzine. Eur J Dermatol. 2008;18:194-195.
- Arakawa Y, Nakai N, Katoh N. Celecoxib-induced erythema multiforme-type drug eruption with a positive patch test. J Dermatol. 2011;38:1185-1188.
- Prieto A, De barrio M, Pérez C, et al. Piroxicam-induced erythema multiforme. Contact Dermatitis. 2004;50:263.
- Dalmau J, Serra-baldrich E, Roé E, et al. Use of patch test in fixed drug eruption due to metamizole (Nolotil). Contact Dermatitis. 2006;54:127-128.
- Gastaminza G, Anda M, Audicana MT, et al. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36.
- Bellini V, Stingeni L, Lisi P. Multifocal fixed drug eruption due to celecoxib. Dermatitis. 2009;20:174-176.
- García CM, Carmena R, García R, et al. Fixed drug eruption from ticlopidine, with positive lesional patch test. Contact Dermatitis. 2001;44:40-41.
- Cruz MJ, Duarte AF, Baudrier T, et al. Lichenoid drug eruption induced by misoprostol. Contact Dermatitis. 2009;61:240-242.
- Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993;29:254-257.
- Grob M, Scheidegger P, Wüthrich B. Allergic skin reaction to celecoxib. Dermatology. 2000;201:383.
- Alonso JC, Ortega JD, Gonzalo MJ. Cutaneous reaction to oral celecoxib with positive patch test. Contact Dermatitis. 2004;50:48-49.
- Fernandes B, Brites M, Gonçalo M, et al. Maculopapular eruption from sertraline with positive patch tests. Contact Dermatitis. 2000;42:287.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Klein CE, Trautmann A, Zillikens D, et al. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1996;35:175-176.
- Swerlick RA, Campbell CF. Medication dyes as a source of drug allergy. J Drugs Dermatol. 2013;12:99-102.
- Guin JD. Patch testing to FD&C and D&C dyes. Contact Dermatitis. 2003;49:217-218.
- Lowther A, McCormick T, Nedorost S. Systemic contact dermatitis from propylene glycol. Dermatitis. 2008;19:105-108.
- Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012;67:1580-1585.
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45.
- Basedow S, Eigelshoven S, Homey B. Immediate and delayed hypersensitivity to corticosteroids. J Dtsch Dermatol Ges. 2011;9:885-888.
- Johansen JD, Aalto-korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- Mirakian R, Ewan PW, Durham SR, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43-61.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
Practice Points
- Consider patch testing in suspected eczematous drug rashes and fixed drug eruption.
- Patch test to inactive excipients as well as active ingredients.
- Caution patients that sensitivity of patch testing for systemic drug reactions is unknown and likely lower than specificity.