User login
Cystic Scalp Lesion
The Diagnosis: Merkel Cell Carcinoma
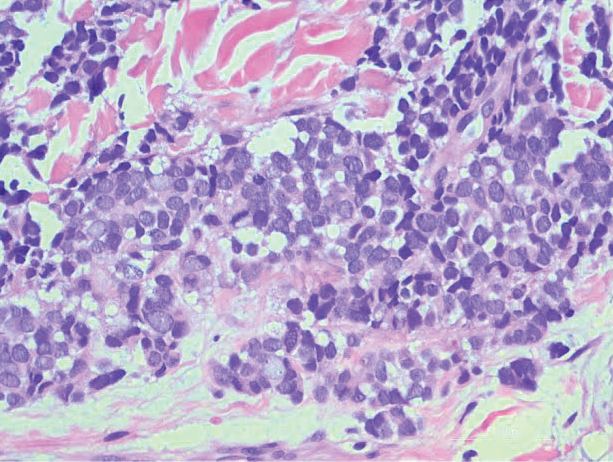
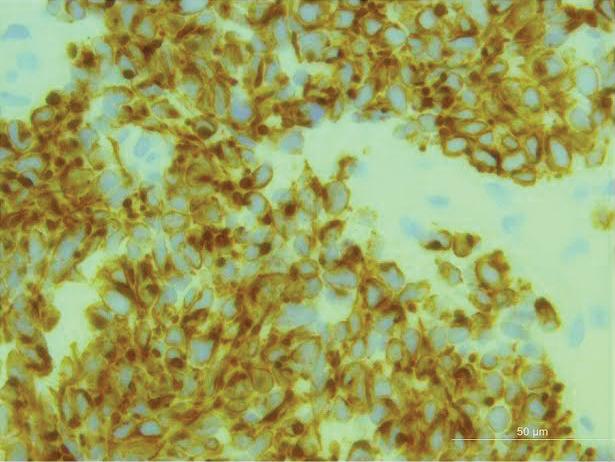
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
The Diagnosis: Merkel Cell Carcinoma
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
The Diagnosis: Merkel Cell Carcinoma
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
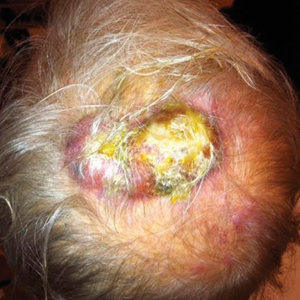
A frail 85-year-old man presented to the emergency department for treatment of a 4.0.2 ×2.5-cm, erythematous, tender nodule on the scalp. The area was increasingly painful with persistent throbbing, which led to sleep disruption. The nodule did not express any material and was not aspirated or surgically treated. The lesion had been present for 1 to 2 years and was small and stable in size until it grew rapidly in the 6 weeks prior to presentation. The patient initially presented to his general practitioner during this period of rapid growth and was diagnosed with an infected sebaceous cyst that was treated with a course of oral cephalexin without improvement. Bacterial or fungal cultures were not performed. No other similar lesions were present, but there was 1 palpable lymph node in the right posterior cervical chain. At the time of presentation to the emergency department, the patient felt well and denied weight loss, night sweats, or fevers. He was given a dose of intravenous cefazolin by the emergency physician and then was referred to surgery for management of an infected sebaceous cyst.