User login
Important Postdischarge Culture Results
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
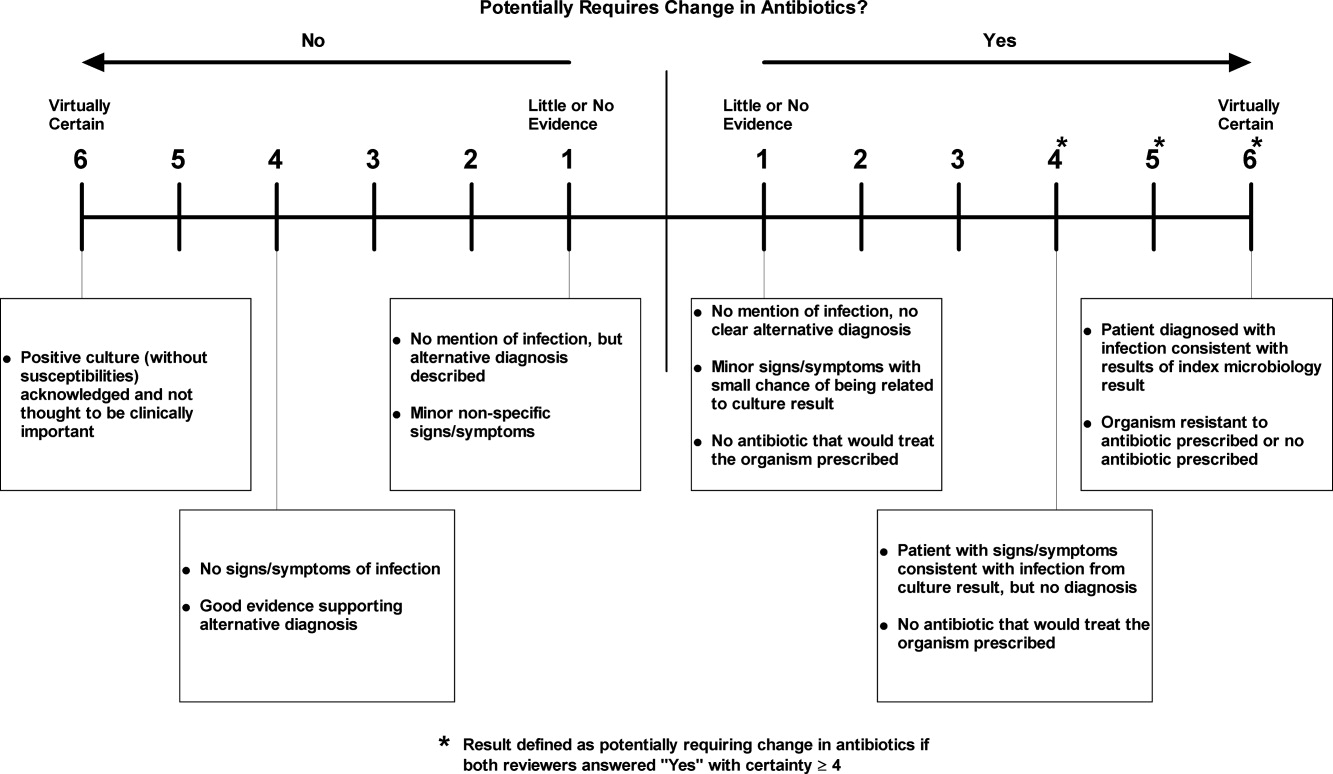
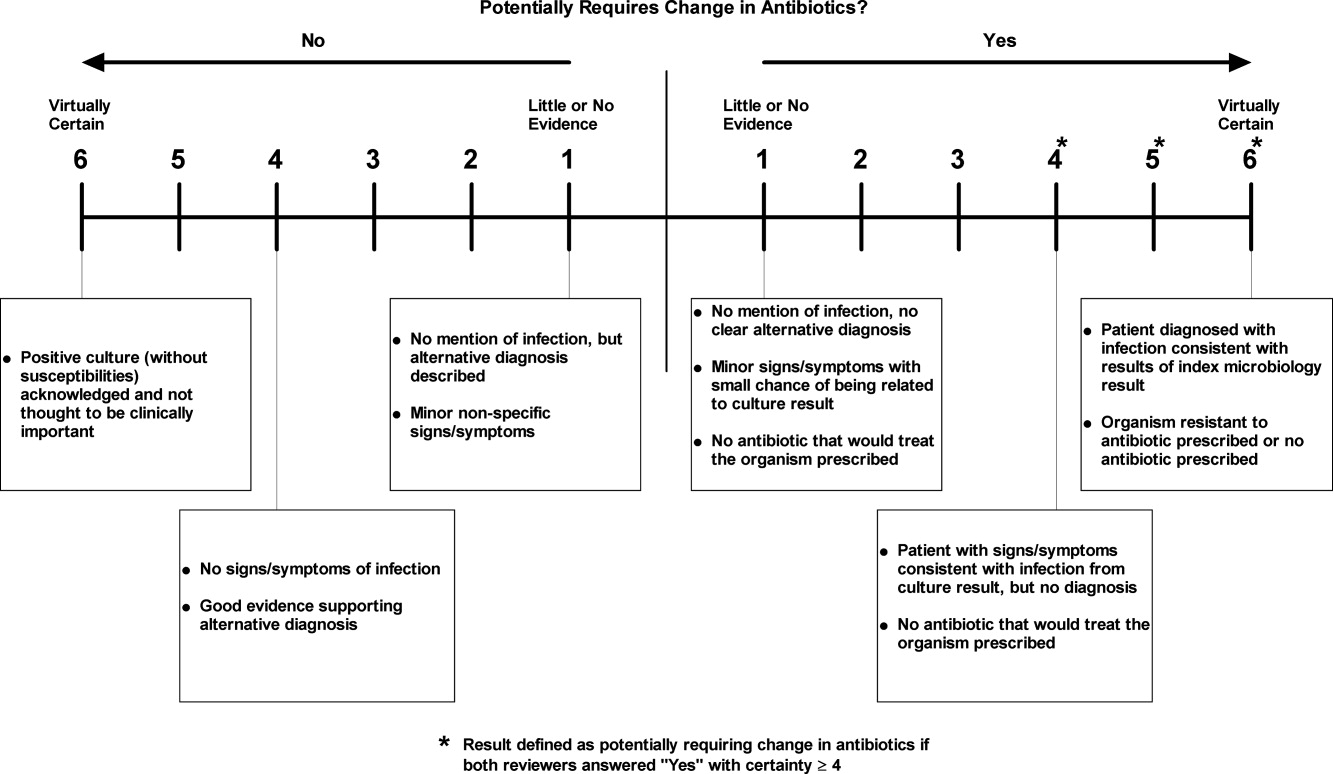
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.
Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
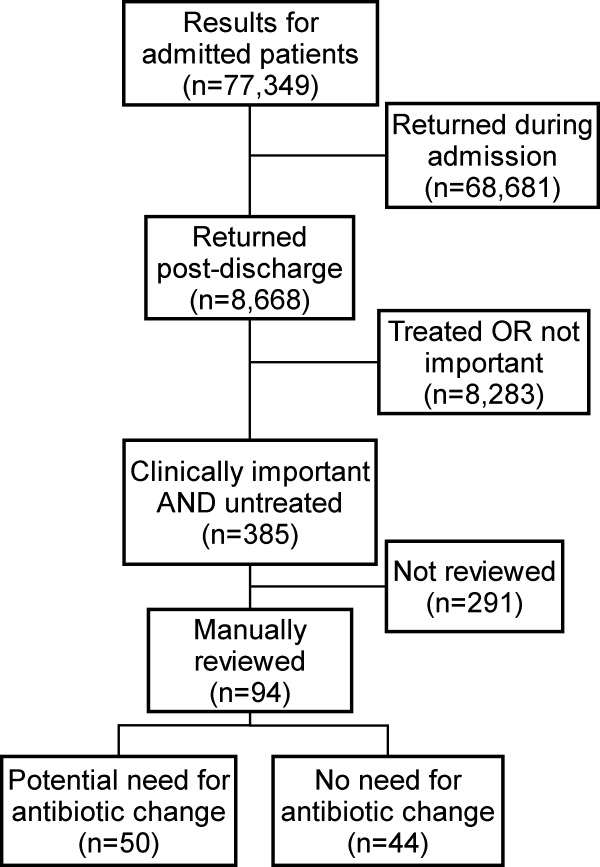
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.
Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.

Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.

Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
Many hospitalized patients have microbiology test results pending at the time of discharge.1, 2 Failure to follow up on these results in a timely fashion can lead to delays in diagnosis and adequate treatment of important infections. Prompt communication of the results of these pending tests to the responsible providers is crucial to minimize these delays.36 As hospitalized patients are increasingly cared for by clinicians other than their primary care providers,7 important information may be lost during the discharge process.8 This increasing fragmentation makes reliable communication of pending tests even more crucial.9, 10
Studies to date have primarily investigated tests from general medical services. In that setting, there is clearly room for improvement in test result communication. Discharge summaries often do not reach the outpatient providers at the time of the patients' follow‐up visits after hospitalization.11 When the discharge summaries are present, the majority of pending tests are not mentioned in them,2, 12, 13 and both inpatient and outpatient physicians are unaware of most of these results when they return.1 However, the specific characteristics of postdischarge microbiology results and the extent to which these results represent potential follow‐up errors in specialties other than general medicine have not been adequately studied.
We aimed to describe the issue of microbiology tests pending at the time of discharge from a hospital‐wide perspective. Specifically, we sought to determine: (1) frequency and characteristics of these results across all admitting services; and (2) how often these results potentially require a change in antimicrobial therapy.
Methods
Study Setting
We conducted our study at a 777‐bed, tertiary‐care academic hospital in Boston, MA with 13 medical and 18 surgical admitting specialties. The human research committee reviewed and approved the study design. For inpatient services, the hospital had well‐established computerized order entry and electronic discharge medication list systems, along with paper clinical notes. The affiliated outpatient practices used an internally developed electronic health record that could access the test results obtained during hospitalization.
Data Collection
We analyzed all 111,331 results of blood, urine, cerebrospinal fluid (CSF), and sputum cultures that were finalized by the hospital's microbiology laboratory in calendar year 2007. For each result, we determined the type of culture, the date of collection, the date of final result, and the identity and antibiotic susceptibility of any organisms isolated in the microbiology lab. For blood and CSF cultures, we also collected the date of preliminary susceptibilities. Preliminary susceptibilities are not reported for urine and sputum cultures at our institution. For cultures collected during hospital admission, we determined the dates of hospital admission and discharge, hospital service caring for the patient at the time of discharge, and the list of medications prescribed to the patient at discharge.
Case Selection Criteria
Our goal was to screen for postdischarge microbiology results that were likely to require action from the clinicians. To this end, we identified cases that were: (1) clinically important, which we defined as likely to represent a true infection or require further evaluation; and (2) were untreated at the time of discharge, which we defined as cases with no antibiotic or inadequate antibiotic therapy. We first excluded cultures obtained while patients were in the outpatient setting. We further excluded all cultures for which the preliminary susceptibilities or final results returned on or before the day of discharge from the hospital.
For each of the four culture types, we developed criteria to identify clinically important results. For blood cultures, we used a prediction model developed and validated at our institution that was based on the identity of the organism, time to first growth, and prior matching culture results.14 For the remaining three culture types, we defined clinical importance based on Centers for Disease Control and Prevention (CDC) definitions of nosocomial infections. These criteria were felt to be adequate to screen for both community‐acquired and nosocomial infections. For urine cultures, we required at least 100,000 colony‐forming units and growth of no more than two distinct organisms. For CSF, any growth was considered clinically important. For sputum, we required a positive culture as well as a discharge diagnosis of pneumonia based on International Classification of Diseases, Ninth Revision (ICD‐9) codes. The discharge diagnosis was included to incorporate the clinical interpretation required to separate true infections from contaminated samples or colonization.
To identify the untreated cultures, we compared the antibiotic susceptibility of the clinically important postdischarge results against the list of antibiotics prescribed to the patients at the time of hospital discharge. We considered the infections treated if there was at least one antibiotic on the discharge medication list to which the organism was found to be susceptible.
Manual Review
We manually reviewed a random sample of 94 of the clinically important and untreated postdischarge results to determine if the results potentially required a change in therapy and therefore required follow‐up. For each case, the electronic patient chart was reviewed by two internal medicine‐trained physicians on the study staff. Each reviewer was blinded to events that occurred after the cultures returned, and determined whether the results necessitated a potential change in antibiotic. The reviewer then indicated the level of certainty of that determination on a 6‐point Likert scale that had been previously used in reviews to identify adverse medical events15, 16: 1 = little or no evidence, 2 = slight evidence, 3 = not quite likely (<50:50 but close call), 4 = more likely than not (>50:50 but close call), 5 = strong evidence, and 6 = virtually certain evidence. To standardize the assignment of certainty for potential need for antibiotic change, we used a set of review guidelines developed by our study staff (Figure 1). A microbiology result was defined as potentially necessitating antibiotic change if both reviewers indicated as such and recorded a certainty with a score 4. Differences in assessments were resolved through discussion of the case between the reviewers.

Statistical Analysis
Using the 94 manually reviewed results, we examined how the proportion of clinically important and untreated microbiology results requiring follow‐up varied by type of culture and primary discharging service. We created a multivariable logistic regression model to predict which of the untreated, postdischarge results required follow‐up. The covariates in our model were selected a priori and included type of culture, hospital service at the time of discharge, patient age, sex, and insurance status. Type of culture and hospital service were included to determine how the distribution of untreated results varied across hospital specialties. Patient age, sex, and insurance status were included to account for differences in the prevalence of antibiotic‐resistant organisms and the clinician's choice of which empiric antimicrobial agent, if any, to initiate based on these patient‐level factors. We calculated a kappa statistic to measure the concordance of the assessments of the two reviewers prior to resolution of disagreements. All analyses were performed using SAS (version 9.2, Cary, NC).
Results
Of the 111,331 blood, urine, sputum, and CSF cultures analyzed, 77,349 (69%) were collected from hospitalized patients. The majority (63%) of the inpatient results were for blood cultures and one quarter (24%) were for urine cultures. Table 1 shows the distribution of the microbiology results across primary services responsible for the patients at the time of discharge. Half (49%) of the patients from whom the specimens were collected were female. The mean age of patients was 55 years. Most (68%) were white and most (86%) had either commercial insurance or Medicare (Table 1).
| Variable | Results for Admitted Patients (n = 77,349) | Results Finalized Postdischarge (n = 8,668) |
|---|---|---|
| ||
| Type of culture, n (%) | ||
| Urine | 18,746 (24) | 2,843 (33) |
| Blood | 48,546 (63) | 4,696 (54) |
| Sputum | 8,466 (11) | 1,059 (12) |
| CSF | 1,591 (2) | 70 (1) |
| Hospital service at discharge, n (%) | ||
| General Medicine | 15,997 (21) | 2,548 (29) |
| Oncology | 13,138 (17) | 1,341 (15) |
| Medical subspecialties | 20,846 (27) | 2,025 (23) |
| Surgery | 23,380 (30) | 2,031 (23) |
| Other | 3,988 (5) | 723 (8) |
| Patient characteristics | ||
| Female, n (%) | 38,125 (49) | 4,539 (52) |
| Age, n (SD) | 55 (21) | 56 (19) |
| Race, n (%) | ||
| White | 52,824 (68) | 5,669 (65) |
| Black | 9,319 (12) | 1,241 (14) |
| Asian | 1,565 (2) | 183 (2) |
| Hispanic | 5,116 (7) | 897 (10) |
| Other | 1,330 (2) | 146 (2) |
| Unavailable | 7,195 (9) | 532 (6) |
| Insurance, n (%) | ||
| Commercial | 35,893 (46) | 3,977 (46) |
| Medicare | 30,553 (40) | 3,473 (40) |
| Medicaid | 9,514 (12) | 1,034 (12) |
| Other | 1,389 (2) | 184 (2) |
Of the 77,349 microbiology tests obtained during hospital stays, 8668 (11%) of the inpatient microbiology results were reported after the patients were discharged from the hospital. Most (54%) of these postdischarge results were for blood cultures. The distribution of results across primary hospital service, patient sex, race, insurance, and mean patient age were similar to those for all inpatient results (Table 1). Of the 8668 postdischarge results, 385 (4%) met our screening criteria of being both clinically important and not treated by an antibiotic to which the organism was found susceptible at the time of discharge from the hospital. After manual review of a random subset of 94 of these screen‐positive cases, 50 (53%) required follow‐up (Figure 2). The interrater reliability for the reviewers was found to be kappa = 0.58 (P < 0.001). From our results, we estimated that 2.4% of the postdischarge microbiology results required follow‐up and potential change in therapy.

Potential need for antibiotic change was present in 30 of 45 (67%) urine cultures, 12 of 32 (38%) blood cultures, 8 of 16 (50%) sputum cultures, and 0 of 1 (0%) CSF cultures. By primary service, reviewers identified a potential need for antibiotic change in 19 of 25 (76%) of results from surgical services, 17 of 29 (59%) from general medicine, 6 of 16 (38%) from oncology, and 8 of 23 (35%) from medical subspecialties. Examples of cases that potentially required antibiotic change are shown in Table 2.
| Culture Type | Scenario |
|---|---|
| Urine | 42‐year‐old woman with dysuria after admission for hysterectomy; no empiric antibiotic treatment given; postdischarge urine culture grew Klebsiella pneumoniae |
| Blood | 81‐year‐old man with Crohn's disease on total parenteral nutrition (TPN) who was initially treated for sepsis from suspected line infection, but discharged without antibiotics, given negative cultures during admission; postdischarge blood culture grew Klebsiella pneumoniae |
| Sputum | 46‐year‐old woman prescribed levofloxacin for pneumonia; sputum culture returns postdischarge with Pseudomonas aeruginosa resistant to levofloxacin |
In our logistic regression model, both the type of culture and the primary hospital service were found to be significant predictors of a potential need for antibiotic change in the manually reviewed cases. Urine cultures were more likely than non‐urine cultures to potentially require antibiotic change (P = 0.03; OR 2.8, 95% CI 1.1‐7.2). Results from surgical services were most likely to potentially require antibiotic change, followed by general medicine, oncology, and medical subspecialties (Table 3).
| Variable | Results Potentially Requiring Change in Therapy (n = 50) | Results Not Requiring Change in Therapy (n = 44) | Odds Ratio (95% CI)* | Adjusted P‐value* |
|---|---|---|---|---|
| ||||
| Type of culture, n (%) | ||||
| Urine | 30 (60) | 15 (34) | 2.84 (1.13‐7.17) | 0.03 |
| Non‐urine | 20 (40) | 29 (66) | Ref | |
| Hospital service at discharge, n (%) | ||||
| General Medicine | 17 (34) | 12 (27) | Ref | |
| Oncology | 6 (12) | 10 (23) | 0.41 (0.11‐1.56) | 0.02 |
| Medical subspecialties | 8 (16) | 16 (36) | 0.34 (0.10‐1.16) | |
| Surgery | 19 (38) | 6 (14) | 2.40 (0.65‐8.89) | |
| Age, mean (SD) | 61 (20) | 59 (21) | 1.01 (0.98‐1.04) | 0.62 |
| Female, n (%) | 29 (58) | 21 (42) | 1.15 (0.44‐2.98) | 0.77 |
| Insurance, n (%) | ||||
| Commercial | 17 (34) | 19 (43) | Ref | |
| Medicare | 25 (50) | 19 (43) | 1.60 (0.42‐6.11) | 0.65 |
| Medicaid and other | 8 (16) | 6 (14) | 1.78 (0.43‐7.36) | |
Discussion
We performed a retrospective analysis of all blood, urine, sputum, and CSF cultures finalized at our institution in 2007 and found that many returned after patients were discharged. Overall, we estimated that 2.4% of these postdischarge results potentially required a change in antibiotic. This proportion varied by culture type and by primary hospital service at the time of discharge, with urine cultures and cultures from surgical services being most likely to potentially need change in antibiotic.
We speculate that postdischarge urine cultures may have been more likely to require antibiotic change in part due to different urgency that clinicians assign to different culture types. Urinary tract infections may present with more vague, transient, or minor complaints compared with bacteremia, pneumonia, and cerebrospinal fluid infections. For that reason, clinicians may be more likely to forego empiric antibiotics for pending urine cultures in favor of watchful waiting. Therefore, the postdischarge urine cultures with growth may include a higher proportion of untreated true infections compared with other culture types.
A similar difference in prescription of empiric antibiotics may help explain the differences seen across primary hospital specialties. For example, if patients on surgical services were less likely to receive empiric antibiotics, then the pool of postdischarge results would be more likely to include true infections that require antibiotic change. Furthermore, it is possible that surgical services may tend to order cultures for patients only if they already have convincing evidence of infections. It may be that selecting a group with higher likelihood of infection led to a higher proportion of true infections in surgical patients with cultures with growth.
Prior studies led by Roy and Were illustrated that pending microbiology results from general medicine services were often not communicated and followed up adequately.1, 2 For patients discharged with pending test results, between 47% and 89% of discharge summaries did not mention the pending tests.2, 12, 13, 17 These deficiencies in discharge summaries likely have a substantial impact on the proportion of tests followed up by outpatient clinicians. By extending the analysis hospital‐wide, our study suggests that pending microbiology results occur for a wide range of hospital services. While our study was not designed to determine whether these results were followed up appropriately, opportunities for miscommunication and missed follow‐up likely exist for all specialties.
The potential harms associated with inadequate test follow‐up have gained the attention of the patient safety community. In 2005, the Joint Commission underscored the importance of proper communication of critical lab results.3, 5, 18 Their recommendations included the development of systems to ensure adequate follow‐up of critical results in high‐risk scenarios including the postdischarge period.5 While many of the microbiology results do not fall into the criticalcategory, we feel that these results should be considered for inclusion in hospital efforts to track postdischarge results. These efforts should also address issues specific to microbiology results, such as preliminary status before antibiotic sensitivities are known.
Developing a comprehensive strategy for test result communication is challenging, and more so for results that return after transitions of care. Even defining the proper target of communication interventions can involve complex organizational and cultural issues. As these results span the inpatient and outpatient domains, there may be some ambiguity as to which provider is responsible when the results return. The inpatient clinicians ordering the microbiology cultures are in the best position to put the results into the patient's clinical context. However, these clinicians may no longer be on clinical duty when the results return, or they may not have a system to ensure that they are notified about these results. While the outpatient providers may be available, they have often not seen the patient in follow‐up at the time the results return and would need to repeat a clinical assessment to determine whether a change in antibiotics is required. While many feel that the ordering provider is a logical choice to perform the follow‐up of the result, not all agree and few institutions have developed clear policies on this issue. To avoid this ambiguity, future work will require institutions to clearly outline which party is responsible for test result follow‐up during transitions of care.
Potential solutions to improve communication of these results must be tailored to the local infrastructure of the institution. In hospitals that do not have extensive electronic systems, a solution might involve a registered nurse, nurse practitioner, or lab technician whose responsibilities include identifying postdischarge results and communicating them to the ordering clinician, primary care provider, and patient. In settings with more advanced electronic infrastructure, solutions could be designed to automatically notify the responsible providers electronically, as well as post the results to a patient portal. Regardless of the level of technical sophistication, it is vital to create a system that has is highly reliable to prevent these important results from falling through the cracks.
Our study did have some limitations. First, we evaluated results from only one institution. It is unclear how substantially differences in practice patterns or patient populations would affect the number of postdischarge microbiology results in other settings. Second, we did not assess whether these results were actually followed up or whether treatment regimens were altered. As this study was retrospective in nature, we could not expect clinicians to recall the clinical scenarios surrounding each result and decided that documentation in clinical notes would be an unreliable indicator of whether any follow‐up action had been taken. Even without this information, however, we would submit that our findings represent a substantial near‐miss rate and threat to patient safety (approximately one potentially actionable, postdischarge microbiology result every other day for our hospital), and call for a fail‐safe system to ensure appropriate actions are taken.
In conclusion, microbiology results are often pending at the time patients are discharged from the hospital and roughly 2.4% of these results potentially require a change in therapy. This proportion was highest for urine cultures and cultures drawn from surgical patients. Our results suggest that a hospital‐wide system is warranted to ensure adequate communication of postdischarge microbiology results. Further research is required to evaluate the impact of such a system on the follow‐up rates of pending microbiology tests.
Acknowledgements
The authors thank Deborah Williams from the Brigham and Women's Division of General Medicine for her programming assistance.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,.Doing better with critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,66–67.
- .Fumbled handoffs: one dropped ball after another.Ann Intern Med.2005;142(5):352–358.
- ,,,.Communicating critical test results: safe practice recommendations.Jt Comm J Qual Patient Saf.2005;31(2):68–80.
- .Introduction: Communicating critical test results.Jt Comm J Qual Patient Saf.2005;31(2):61,63–65.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320(7237):791–794.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,, et al.Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals.J Hosp Med.2009;4(8):E28–33.
- , , , , .Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care.J Gen Intern Med.2010;26(4):393–398.
- ,,,,,.Using electronic data to predict the probability of true bacteremia from positive blood cultures.Proc AMIA Symp.2000:893–897.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,, et al.Incidence and types of adverse events and negligent care in Utah and Colorado.Med Care.2000;38(3):261–271.
- ,,,.General practitioner‐hospital communications: a review of discharge summaries.J Qual Clin Pract.2001;21(4):104–108.
- ,,,,,.Failure to recognize and act on abnormal test results: the case of screening bone densitometry.Jt Comm J Qual Patient Saf.2005;31(2):90–97.
Copyright © 2010 Society of Hospital Medicine